Abstract
Purpose
To demonstrate the CT imaging, conformal irradiation and treatment planning capabilities of a small animal radiation research platform (SARRP).
Methods
The SARRP employs a dual-focal spot, constant voltage x-ray source mounted on a gantry with a source-to-isocenter distance of 35 cm. Gantry rotation is limited to 120° from vertical. Eighty to 100 kVp x-rays from the smaller 0.4 mm focal spot are used for imaging. Both 0.4 mm and 3.0 mm focal spots operate at 225 kVp for irradiation. Robotic translate/rotate stages are used to position the animal. Cone-beam (CB) CT imaging is achieved by rotating the horizontal animal between the stationary x-ray source and a flat-panel detector. Radiation beams range from 0.5 mm in diameter to (60 × 60) mm2. Dosimetry is measured with radio-chromic films. Monte Carlo dose calculations are employed for treatment planning. The combination of gantry and robotic stage motions facilitate conformal irradiation.
Results
The SARRP spans 3 ft × 4 ft × 6 ft (WxLxH). Depending on filtration, the isocenter dose outputs at 1 cm depth in water range from 22 to 375 cGy/min from the smallest to the largest radiation fields. The 20% to 80% dose fall-off spans 0.16 mm. CBCT with (0.6 × 0.6 × 0.6) mm3 voxel resolution is acquired with less than 1 cGy. Treatment planning is performed at sub-mm resolution.
Conclusions
The capability of the SARRP to deliver highly focal beams to multiple animal model systems provides new research opportunities that more realistically bridge laboratory research and clinical translation.
Keywords: Small animal, radiation research, focal laboratory irradiation
Introduction
In cancer research, small animals, such as mice, rats, and rabbits are used extensively to evaluate the effectiveness of novel treatment as well as treatment related toxicity. At present, simple single beam/single fraction techniques are commonly used to irradiate laboratory animals. This technology is far removed from the advanced three-dimensional (3D) imaging, planning and computer-controlled delivery technologies that are used for human treatment. The uniform dose distributions delivered to research animals bear little resemblance to the highly non-uniform dose distributions commonly employed in clinical conformal radiation therapy. Traditional understanding of tumor control probability and normal tissue complication probability based on animal radiation research has become increasingly less suitable to model response and predict outcome of modern radiation therapy. The technological disparity between animal irradiation and human treatment also presents a difficult hurdle in the development of novel treatment methods that combine conformal irradiation and other therapeutic agents, such as drugs, biomarkers and molecularly targeted therapeutics. There is clearly a pressing need to bridge the technological gap between laboratory radiation research and human treatment methods.
Several groups, including ours, have initiated efforts to overcome the disparity (1–7). DesRosiers et al (2) noted that small animal irradiation requires an accuracy within 1 mm. They demonstrated that a Gamma Knife system [Elekta Instrument AB, Stockholm, Sweden] can achieve an accuracy of ±0.5 mm for small animal research. The disadvantage with the approach is the inherent non-local energy deposition associated with the cobalt-60 gamma rays. Stojadinovic et al (3,7) recently reported on a “micro-radiation” system where a small, high-activity 192Ir source in combination with custom-fabricated tungsten collimators was used to deliver beams from four orthogonal angles. Apertures ranging from 5 mm to 15 mm in diameter are available. The system is attractive in the simplicity of its design, but is challenged by the rapid decrease of its dose rate with distance, the requisite high source activity and the appreciable dimension of its smallest 5mm diameter beam. In an attempt to attain smaller field dimensions, Graves et al (6) are exploring a novel iris-collimating system that can be mounted to a small animal micro-CT system. Preliminary results indicate that beam with diameters ranging from 8.5 cm to 0.6 mm in diameter can be produced. The system awaits dosimetric characterization as the final x-ray source has not been determined.
This paper provides a “proof of concept” for our design and construction of a small animal radiation research platform (SARRP) that mimics the isocentric external-beam treatment machine used to deliver image guided radiation therapy for humans (1,5). . Our present efforts were directed to demonstrate our concept and the functionality of the component parts of the system for image guidance, irradiation and treatment planning. In-depth system characterizations of these capabilities and their integrated operations are on-going, although various functions of the system are already being incorporated in several laboratory animal investigations.
Methods and Materials
Design Consideration
The design specifications of the SARRP include (1) a gantry that supports isocentric and non-coplanar conformal irradiation; (2) on-board CT guidance to facilitate accurate repositioning for fractionated irradiation schemes and co-registration with other small animal imaging modalities; and (3) 3D conformal treatment planning to provide quantitative dosimetry.
None of the specified features for the SARRP are available in present laboratory animal irradiation systems, but are necessary to mimic human treatment methods. However, these technologies are available as individual component parts, at least for humans. The challenge is to downsize and integrate them into a common platform for small animal irradiation experiments. For CT imaging, we consider 0.25 mm-cubed voxel resolution as a reasonable specification that can be achieved at an acceptable level of imaging dose. It follows that the precision of the mechanical motions for positioning the small animals and the dose calculation grid would be of similar magnitude, i.e. 0.25 mm, or less. In addition, we would like to achieve accurate in vivo dosimetry that is in agreement with measurement to within 5% to 10%.
The x-ray source
The SARRP employs a common x-ray source for both imaging and irradiation purposes. A small focal spot producing energies from 50 kVp to 120 kVp is desirable for imaging purposes. On the other hand, higher kVp x-rays are more suitable for irradiation of laboratory animals such as mice, rats and rabbits. A larger focal spot will also allow higher dose output. Monte Carlo simulations were performed for a non-diverging 1 mm × 10 mm slit x-ray beam to examine the choice of energy that would provide adequate penetration, and ensure local energy deposition thus sharp dose fall-off in water. Based on these considerations, an industrial Seifert ISOVOLT 225M2 x-ray source [Seifert X-ray Corp., Lewistown, PA, USA] was selected. This constant potential x-ray source employs 2 focal spots, 0.4 mm and 3.0 mm per IEC 336 specifications, to produce x-rays from 50 kVp to 225 kVp.
The SARRP Platform
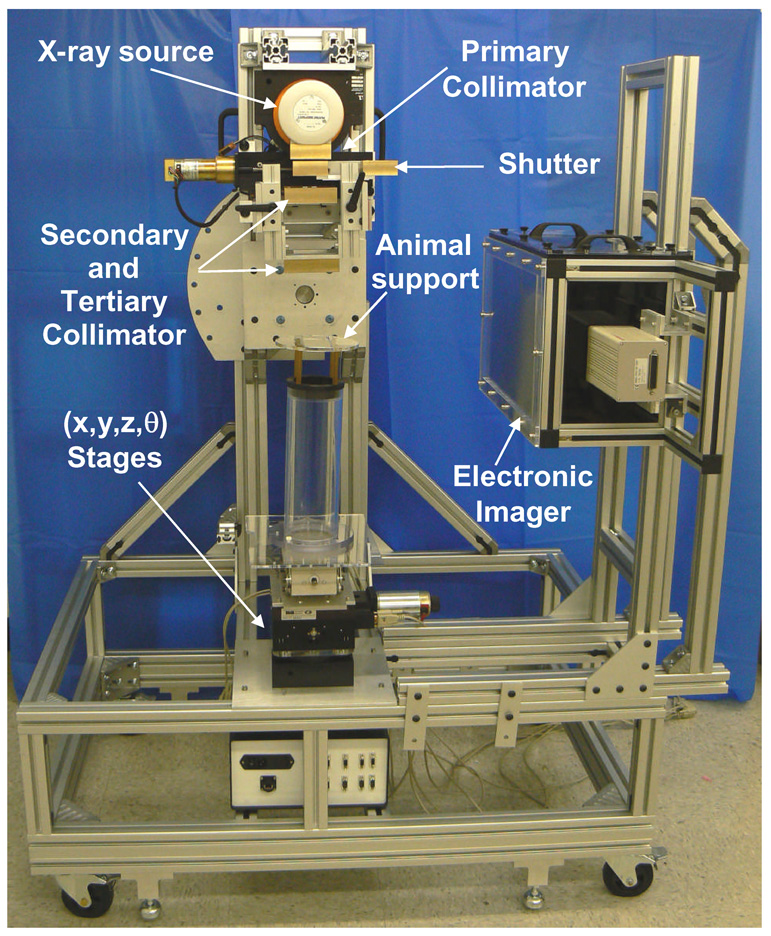
Figure 1 shows the physical appearance and functionality of the SARRP. The structural frame of the SARRP is made of aluminum profiles and measures 3 ft wide, 4 ft long and 6 ft tall at the largest extent of each dimension. The Seifert x-ray source is mounted on an isocentric gantry that can be rotated manually from 0° at vertical to 120° below horizon in 15° counter-clockwise increments. The gantry isocenter is set at 35 cm from the x-ray focal spot. At present, the SARRP is enclosed in a temporary shielding arrangement comprised of hanging lead-blankets that ensure compliance with radiation safety requirements. The platform is equipped with wheels for ease of transport, but rests firmly on stops for stationary use. Portability is desirable as the system will be relocated for closer access with the animal research laboratories.
Figure 1.
A picture of the small animal radiation research platform (SARRP) with conformal irradiation and cone beam CT guidance capabilities. The maximum extent of the system measures 3 ft in width, 4 ft in length, and 6 ft in height. It is equipped with wheels for ease of transport, but rests on stops for stationary use. The camera-based electronic imaging system has since been replaced with a 21cm × 21 cm flat panel amorphous silicon detector, 9 months prior to this writing.
An animal subject immobilized in its customized support is to be placed on the plastic cylindrical tower structure as shown in Figure 1. Computer controlled robotic translation and rotation stages underneath the tower are used for accurate and reproducible positioning of the animal. At the top level, the x-y translation stage has a range of motion of ± 50 mm with an accuracy of 0.065 mm (per axis) and a bidirectional repeatability of 0.006 mm [XY-6060, Danaher Motion, Salem, USA]. In the middle, the z-vertical stage has a range of 38 mm with a bi-directional repeatability of ± 0.125 mm [Servo Systems Co. Montville, NJ, USA]. On the bottom, the rotation stage is configured to provide 360° rotation (and ± 5° over-travel) with an accuracy of 0.05° and a bidirectional repeatability of ± 0.007° [RTR-6, Danaher Motion, Salem, USA]. The rotation stage is set at the bottom to ensure a single axis of rotation for imaging and irradiation. The maximum rotational speed is 5 rpm. The combination of gantry orientation and robotic stage motion facilitates the delivery of complex conformal dose distributions. The overall design is chosen to simplify the placement and construction of the robotic animal positioning system.
The Collimation System
As shown in Figure 1, the SARRP is equipped with a custom-built multi-stage collimation system to limit the girth of the collimator required to block down the 41° cone angle of the exiting beam, and to limit the width of the geometric penumbra. The collimation stages are fabricated from 1 inch thick brass. The first “primary” collimation stage is permanently mounted to yield a “native” field size of 20 cm × 20 cm at isocenter, which would be used for imaging. A shutter system consisting of two sliding brass blocks is attached to the primary collimator. The intent is to have the shutter closed until the tube ramps up to the specified kVp and the current is stabilized. Plates of 1 mm thick aluminum (Al) and/or 0.5 mm thick copper (Cu) can be mounted as part of the primary collimator assembly as added filtration to achieve the desirable beam “hardness’ and output.
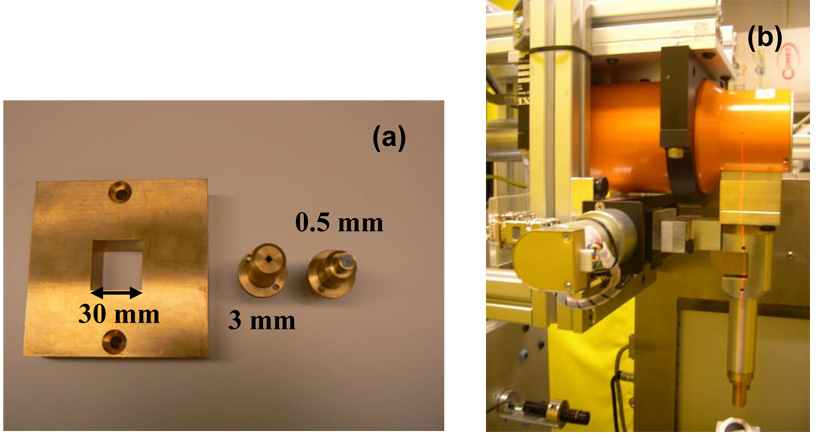
The secondary and tertiary collimation stages of Figure 1 accommodate a range of square and circular brass collimation inserts from 60 mm × 60 mm to 0.5 mm in diameter. Figure 2a shows inserts of 30 mm × 30 mm, 3 mm × 3mm and 0.5 mm diameter. When small irradiation fields are used, a collimating “nozzle” system is employed to achieve rigid alignment with the x-ray source, as shown in Figure 2b. The extension of the end-face of all collimators is adjustable but is presently set at 50 mm from isocenter to accommodate irradiation of a larger animal such as a rat. This separation also maintains the theoretical geometric penumbra broadening of less than 0.08 mm and 0.6 mm at 6 cm downstream from the collimator surface for the 0.4 mm and 3 mm focal spot respectively.
Figure 2.
(a) A picture of the tertiary brass collimation inserts with dimensions of 30 mm × 30 mm, 3 mm × 3mm and 0.5 mm diameter for the SARRP. (b) A picture of the radiosurgery-like collimating cone assembly attached to primary collimator of the SARRP for small field irradiations (< 5mm × 5mm in dimensions).
The Imaging System
The SARRP is equipped with detectors at two fixed positions for imaging. Figure 3 shows the CAD drawing of the novel configuration for CBCT imaging where the x-ray source and a flat panel amorphous silicon detector [Perkin Elmer, Fremont, CA, USA] are fixed at opposite horizontal positions of 90° and 270° respectively. The approach is chosen as the most direct means to accommodate the robotic stages for positioning the animal for complex irradiation arrangements. The 21 cm × 21 cm flat panel detector (which has replaced camera-based system in Figure 1, 6 months prior to the time of writing) is placed at 17.5 cm from isocenter to attain an image magnification factor of 1.5. The necessary multiple projection images are obtained by rotating the animal immobilized in a prone position identical to that intended for irradiation. The second imaging system consists of a 20 cm × 25 cm film-screen cassette installed on top of the translation stage. Simple setup verification is achieved by acquiring orthogonal projection images (posterioanterior and lateral) in conjunction with the flat panel detector.
Figure 3.
A CAD drawing of the novel configuration for cone-beam CT imaging on the SARRP. The x-ray source and a flat panel amorphous silicon detector are fixed at opposite horizontal positions of 90° and 270° respectively. The animal subject is rotated by the robotic stage. The 21 cm × 21 cm flat panel amorphous silicon detector has replaced the camera-based electronic imaging system of Figure 1.
In several imaging studies, mouse projection images were acquired to demonstrate feasibility of the novel rotating setup for CBCT imaging. Each mouse was anesthetized and held prone on a Styrofoam support. The uncollimated primary beam, 20 cm × 20 cm at isocenter, is used for imaging. X-rays of 100 kVp emitted from the 0.4 mm focal spot and filtered by 0.5 mm thick Cu were employed. Images were acquired at a current of 0.5 mA, with “continuous” beam-on as well as “continuous” stage rotation. Three hundred and sixty (360) projections were acquired at approximately 1 degree angular increments. For comparison, the projection images for CBCT were also acquired in the “stop and capture” mode, where the stage was “stopped” at a stationary position during rotation during which a projection image was acquired. Each projection image was corrected for “dead” pixels. An adequate number (> 50) of “dark” and “flood” images were acquired prior to animal imaging to correct for background and flood field non-uniformity. The Feldkamp (FDK) CBCT algorithm was used for reconstruction (8). The imaging dose to the animal was estimated using Gafchromic EBT films [International Specialty Products, Inc., Wayne, New Jersey, USA] placed in a small cylindrical plastic-water phantom and irradiated by the CBCT imaging procedure. The plastic water phantom material purports to be water equivalent for kV photon energies [Standard Plastic Water, CIRS Inc., Norfolk, Virginia].
Treatment Planning
A research Pinnacle3 radiation treatment planning system [Philips Radiation Oncology Systems, Madison, WI, USA] is employed to plan beam placement and visualize dosimetry of an animal experiment in 3D. Dose calculation is performed using one of two Monte Carlo packages; a research release of the Pinnacle3 RTP system and the BEAMnrc (EGSnrc) code [National Research Council of Canada, Ottawa, Canada]. The latter runs on a separate, dedicated LINUX workstation, using exported beam geometry from Pinnacle3 as input. The BEAMnrc calculation employs pre-computed phase space files for the Seifert tube and scores dose deposition in voxelized density/material matrices. The resultant 3D dose matrix is reformatted and imported into Pinnacle3 for display, overlaid on the planning CT. A similar hybrid approach using BEAMnrc and Pinnacle3 was recently reported by Chow and Leung (9). In the case of Pinnacle3 Monte Carlo calculations, x-rays were sampled from the phase space file generated from BEAMnrc and modeled as emanating from a Gaussian focal spot. All beam modifiers, collimators and media downstream of the x-ray source were modeled in the Monte Carlo calculations; except for the on-going modeling efforts for the smaller nozzle collimators.
Dose in air and in a solid water phantom were measured using Gaf-chromic EBT films, using optical density calibration data exposed with 6MV x-ray beams. For all of our EBT film dosimetry, we employed a protocol described by Devic et al (10). A maximum dose of 6 Gy to 8 Gy was delivered to each EBT film measurement. The response of EBT film has been demonstrated to be highly energy-independent and therefore desirable for our 225 kVp or lower energy xrays (11). Cross-beam profiles were measured at depth in plastic water for a range of field sizes formed by the tertiary collimators or nozzle-collimators. Sheets of EBT film were sandwiched between the horizontal slabs of a plastic water phantom at different depths and irradiated. The added filtration consisted primarily of 4 mm Al or 0.5 mm Cu. The latter was chosen to harden the beam by reducing the fluence of the lower energy photons of the spectrum. The SSD was nominally set at 35 cm, although variations occurred between experimental runs. Two-dimensional dose distributions in water were calculated with both Pinnacle3 and EGSnrc Monte Carlo codes for the square collimators and compared with EBT film measurements. The specialized nozzle collimators have yet to be modeled at this time.
For our feasibility studies, planning CT for Monte Carlo calculations was obtained from a clinical 16-slice helical CT scanner [Brilliance Big Bore, Philips Medical Systems, Highland Heights, OH, USA], although the minimal voxel dimensions of approximately 0.8 mm-cubed afforded by the human CT scanner were clearly too large. Notwithstanding, the CT numbers were converted to the nominal photon interaction parameters for 225 kVp x-rays based on a standard CT-number-to-material mapping utility in the research Pinnacle3 system for dose calculations. The dose calculation and display resolutions were downsized to 0.2 mm-cubed accordingly. The validation of the accuracy of the Pinnacle-Monte Carlo dose calculations in heterogeneous media based on either phantom models or CT data is part of a larger on-going in-depth investigation. The results will be reported separately.
Animal Irradiation Studies
Two single fraction exposures of the mouse brains were performed as feasibility studies. All mice were anesthetized by Katamine injection. CT scans were acquired and used for planning with our research Pinnacle3 system, as described above. The first experiment involved the delivery of fifteen 1 mm beam to a target region inside the brains of 2 mice. For simplicity, the 15 beams were arranged at equal angular spacing in a horizontal arc as facilitated by rotating the stage, as shown by the surface-rendered display of Pinnacle3 in Figure 4. The patchy artifacts seen outside the mouse are due to the presence of surface irregularities of the animal support in the significantly downsized CT grid comprised of 0.2 mm × 0.2 mm × 0.2 mm voxels. One mouse received 20 Gy, and the other 33 Gy. For both mice, T2-weighted MRI scans were acquired pre-irradiation and on day 1 and day 35 post-irradiation, respectively, with an 11.7 Tesla research MRI scanner [Bruker Biospin Gmbh, Silberstreifen, Germany] to examine possible radiation induced changes; although it was unclear as to what MRI scanning technique would be most appropriate.
Figure 4.
A surface-rendered display of the Pinnacle3 3D treatment planning system showing the irradiation of a mouse brain using 15 beams equally spaced in a coronal arc arrangement. Each beam is of 1 mm in diameter.
The second irradiation experiment involved a single vertical 3 mm × 3 mm beam directed posteriorily to the right hemisphere of the mouse. γ-H2AX phosphorylation was investigated for correspondence with radiation-induced DNA strand breaks (12). Doses of 12 Gy and 20 Gy were delivered to each of 2 mice respectively. Each mouse was sacrificed after irradiation. The brain was removed within 30 minutes, rinsed in 1X PBS, and fixed in 4% paraformaldehyde for 24 hours. The fixed brains were embedded in paraffin and stained as previously described using a monoclonal anti-phospho-H2AX (Ser139) antibody [Upstate Biotechnology, Lake Placid, NY] (12). After washing, slides were incubated at room temperature for 1 hour with goat anti-mouse antibody conjugated with Alexa Fluor 488 [Molecular Probes, Leiden, Netherlands] and visualized with a Zeiss fluorescence microscope [Carl Zeiss, Inc., Peabody, MA, USA].
Results
The flat panel detector consists of 512 × 512 pixels, each of 400 µm-squared, and would provide CBCT images with a limiting voxel resolution of 0.27 mm × 0.27 mm × 0.27 mm at an image magnification of 1.5. For routine application, the CBCT volume was reconstructed as a 256 × 256 × 256 matrix at a voxel resolution of 0.55mm × 0.55mm × 0.55mm. From the initiation of image acquisition to the completion of CBCT reconstruction, the continuous rotate-acquisition procedure took 4 minutes, whereas the “stop and capture” procedure took about 7 minutes. Differences in the reconstructed CBCT quality were insignificant, if at all noticeable. In the continuous rotate-acquisition mode, a complete revolution of the stage took 65 sec, resulting in the animal receiving 0.85 cGy in mid-volume as measured with EBT film in the cylindrical solid water phantom. The surface dose was similar.
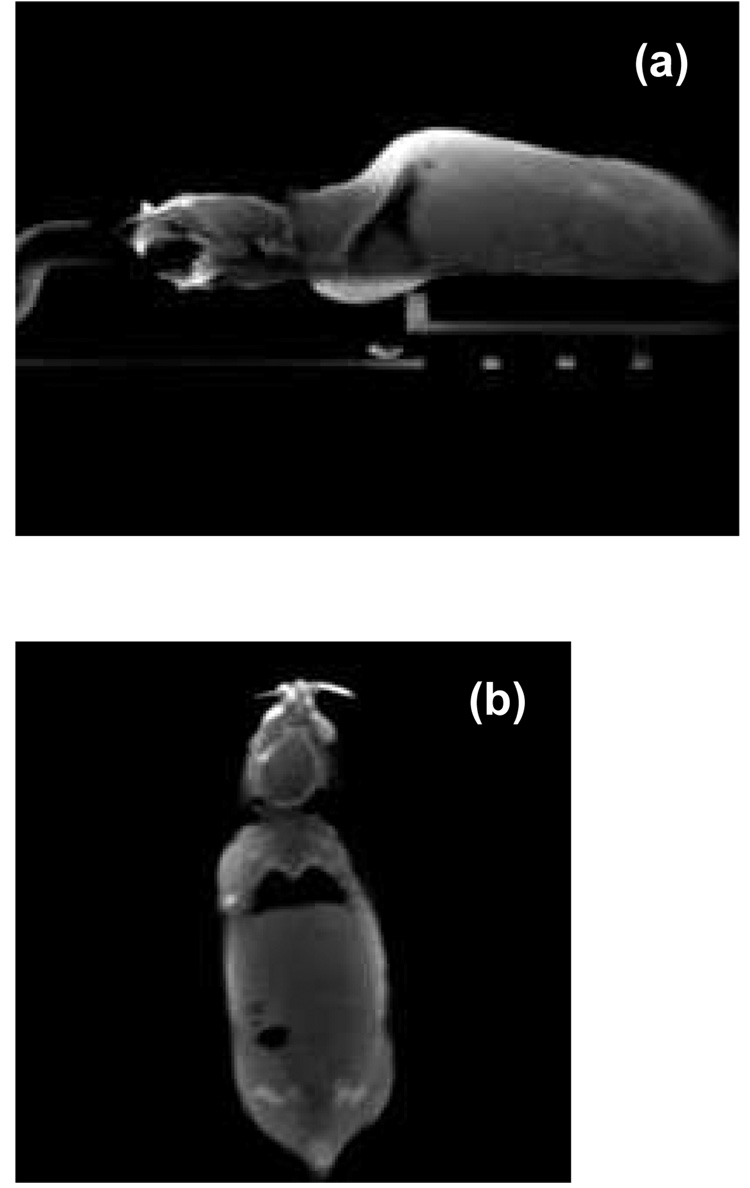
Figure 5a shows a sagittal slice of the CBCT of an anesthetized mouse scanned in the prone position. Figure 5b shows a coronal slice of the same mouse. The mouse was immobilized with a custom head holder equipped with ear-pins and bite-block.
Figure 5.
(a) A sagittal slice of cone-beam CT of an anesthetized mouse scanned in the prone position. (b) A coronal CBCT slice of the same mouse. The mouse was immobilized with a custom head holder equipped with ear-pins and bite-block. The projection images were acquired with the flat panel detector.
Table 1 lists the dose outputs at 1 cm or 0.5 cm depth in solid water measured with EBT films for different field sizes, and different added filtration. When 4 mm Al was used for added filtration, the output at 1 cm depth was high; about 200 cGy/min for a 1.3 mm diameter beam and 375 cGy/min for a 60 mm × 60 mm beam. The outputs were approximately halved when the aluminum filtration was replaced with 0.5 mm Cu. Addition of 2 mm Al to the 0.5 mm Cu did not alter the output significantly.
Table 1.
Radiation output of the SARRP in cGy/min for a range of field dimensions at 35 cm SSD. The Seifert tube was operated at 225 kVp, 13 mA using the larger (3 mm) focal spot, except when noted, with either Al and/or Cu added filtration. A tube current of 3 mA using the smaller (0.4 mm) focal spot was employed for smallest “nozzle” collimator. The output is defined as the dose in water at 1 cm depth.
| Field dimensions | Added Filtration | Dose output (cGy/min) |
|---|---|---|
| Tertiary Collimators | ||
| 60 mm × 60 mm* | 4 mm Al | 378 |
| 30 mm × 30 mm* | 4 mm Al | 334 |
| 1.3 mm diameter* | 4 mm Al | 206 |
| 10 mm × 10 mm | 0.5 mm Cu + 2 mm Al | 131 |
| 5 mm × 5 mm | 0.5 mm Cu + 2 mm Al | 124 |
| 1 mm diameter | 0.5 mm Cu + 2 mm Al | 102 |
| “Nozzle” Collimators | ||
| 5 mm × 5 mm | 0.5 mm Cu | 146 |
| 3 mm × 3 mm | 0.5 mm Cu | 122 |
| 1 mm diameter | 0.5 mm Cu | 92 |
| 0.5 mm diameter with 0.4 mm focus | 0.5 mm Cu | 22 |
Adjusted for the 33.5 cm SSD of the measurements by applying an inverse-square correction.
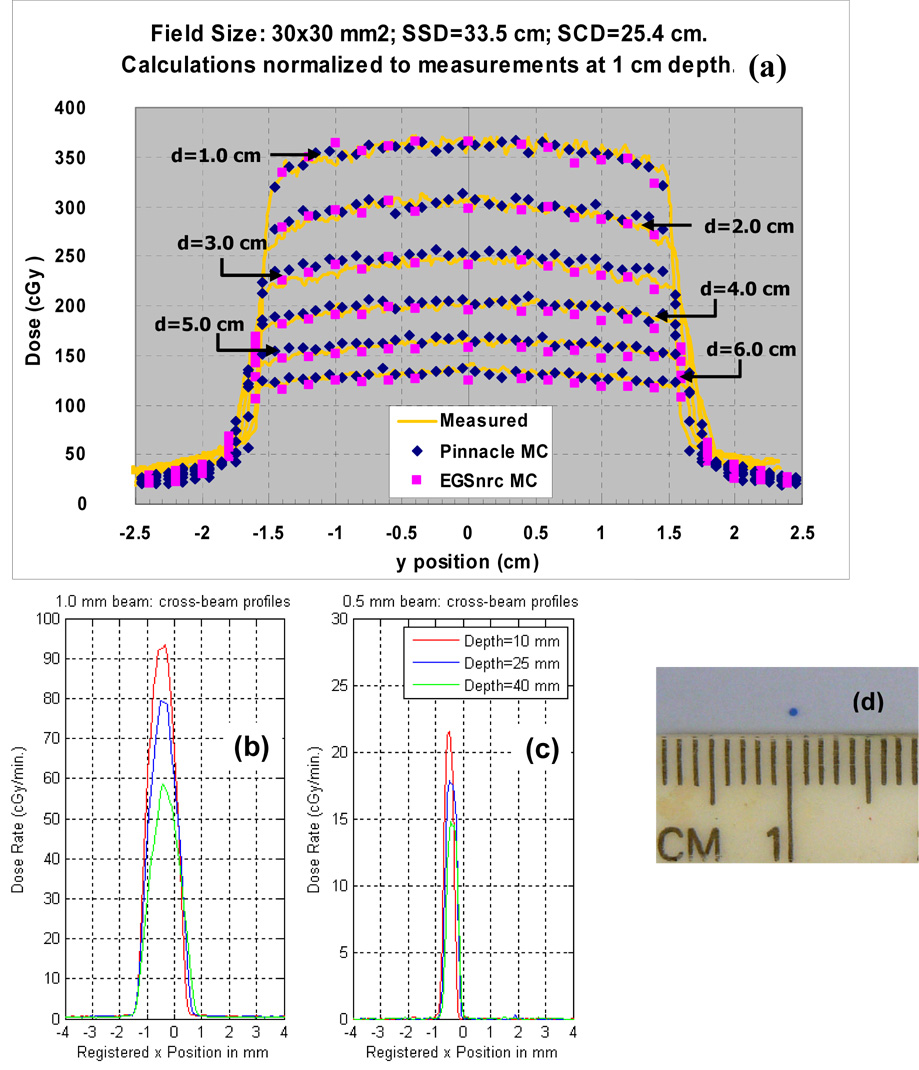
Figure 6a shows cross-beam profiles measured at different depths in solid water for a 30 mm × 30 mm beam at a SSD of 33.5 cm, in comparison with the Monte Carlo calculations provided by the Pinnacle3 research and EGSnrc codes respectively. The added filtration was 4 mm Al. The Monte Carlo calculations were performed to achieve 2% uncertainty. The agreement is generally good. The largest discrepancies of about 10% occur in the low dose region outside the geometric beam edges where contributions from scattered photons predominate. The measured cross-beam profiles for the smallest beams of 1 mm and 0.5 mm diameters, respectively, at depths of 1, 2 and 4 cm in a solid water phantom are shown in Figures 6b and c, respectively. The added filtration of 0.5 mm Cu was employed for these measurements. The 1 mm diameter beam was produced by the 3mm focal spot, while the 0.5 mm diameter beam by the 0.4 mm focal spot. A picture of the EBT film at 1 cm exposed by the latter beam is shown in Figure 6d. The span of the 80% to 20% penumbra at 1 cm depth was 0.16 mm for the 0.5 mm beam; and about 0.3 mm for the 1 mm beam. While both x-ray beams were expected to pass through x=0 cm on the graphs, the profiles suggest that the beams were shifted by about 0.5 mm.
Figure 6.
(a) Measured cross-beam profiles at depths in plastic water for a 30 mm × 30 mm beam at a SSD of 33.5 cm, in comparison with the Monte Carlo calculations by the Pinnacle3 research (blue) and EGSnrc (pink) codes respectively. The added filtration was 4 mm Al. (b) and (c) Measured cross-beam profiles for the smallest beams of 1 mm and 0.5 mm diameters formed by “nozzle” collimators, respectively. The added filtrations were 0.5 mm Cu. Note that the beams are shifted from the expected central axis beam path by about 0.5mm. A picture of one of the exposed EBT film is shown in (d).
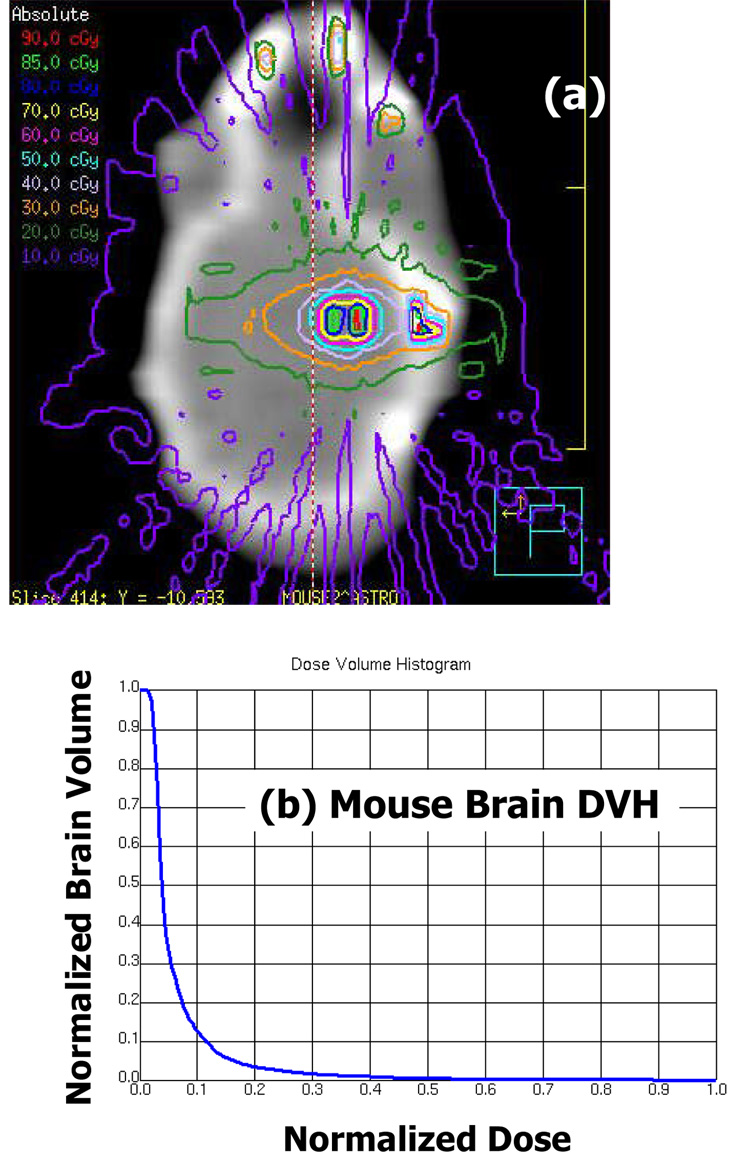
Figure 7a shows the EGSnrc generated isodose distributions overlaid on a coronal CT slice of one of the mice irradiated by the fifteen 1 mm diameter beams arrangement as shown in Figure 4. The Monte Carlo calculations were performed to attain 5% statistical uncertainty. The added filtration was 4 mm Al. The presence of dose in air is due to the presence of non-zero density (noisy) voxels encountered by the EGSnrc calculations. The 50% isodose line circumscribes an elliptical region that spans less than 3 mm in its long axis. Dose enhancement is observed in the thicker portion of the skull as photon-electric interactions are more appreciable at 225 kVp than at the megavoltage energies used for human treatment. This observation also motivated the use of 0.5 mm Cu filtration to further harden the beam. Figure 7b shows the corresponding dose volume histogram of the irradiated mouse brain, contoured as the soft tissue density volume encased by the skull. No more than 3% of the brain volume received more than 50% of the prescribed dose. No difference was observed between the T2 weighted MRI scans of the irradiated region acquired prior to irradiation, 1 day post- and 35 days post-irradiation.
Figure 7.
(a) The EGSnrc generated isodose distributions overlaid on a coronal CT slice of one of the mouse brains irradiated by the fifteen 1 mm diameter beams arrangement as shown in Figure 4. The “dose in air” artifacts are due to non-zero density CT numbers in the calculation grid. The added filtration was 4 mm Al. (b) The corresponding dose volume histogram of the irradiated mouse brain, contoured as the soft tissue density volume encased by the skull.
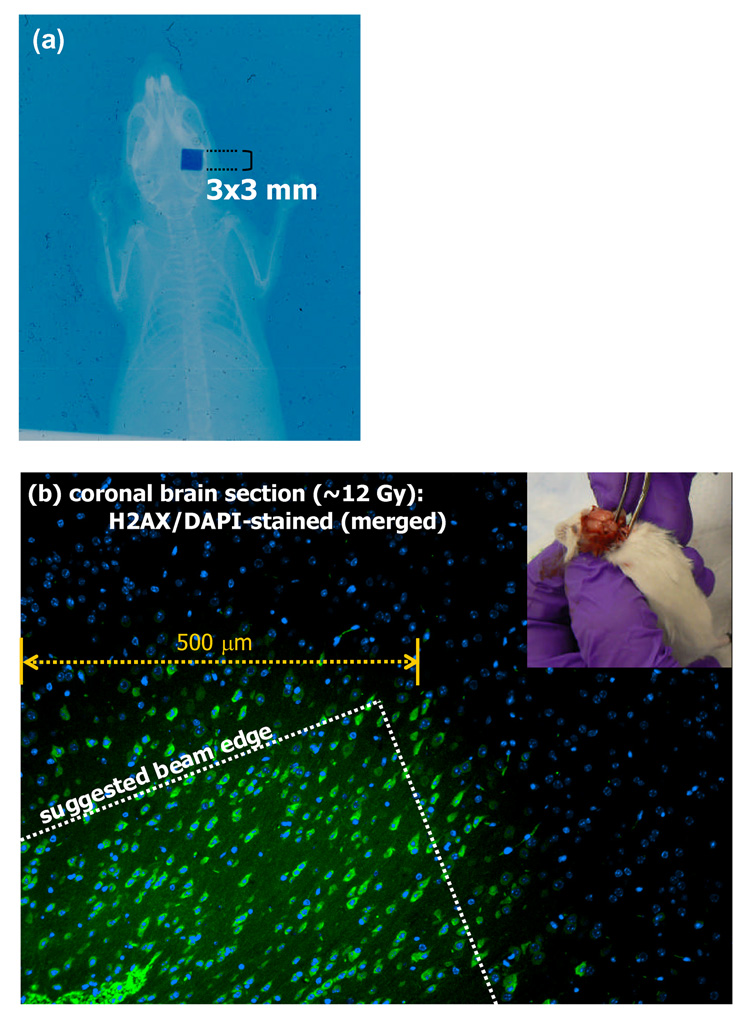
Figure 8a shows a double-exposed EBT film of the mouse brain irradiated in our second feasibility experiments where a single 3 mm × 3mm beam was directed posteriorily to the right hemisphere of mouse. Figure 8b shows a merged image of the sectioned mouse brain that was stained with DAPI for cell nuclei and with antibody against γ-H2AX for correspondence with radiation-induced DNA strand breaks. As expected, the entire section shows DAPI staining. However, there is an apparent sharp demarcation of a region that also shows γ-H2AX staining. This image supports the focal radiation damage resulting from the sharp dose fall-off of the 225 kVp beam. However, the evidence is circumstantial as the experiment did not include rigorous geometric registration that would validate the coincidence of the irradiation and γ-H2AX regions. As such, a beam edge was suggested on the figure. Nevertheless, no other γ-H2AX regions were observed from the sectioned sample.
Figure 8.
(a) A double-exposed EBT film of the mouse irradiation where a single 3 mm × 3mm beam was directed posteriorily to the right hemisphere. (b) A merged image of the sectioned mouse brain that was stained with DAPI for cell nuclei and with antibody against γ-H2AX for correspondence with radiation-induced DNA strand breaks. The entire section shows DAPI staining, while there is an apparent sharp demarcation of a region that also shows γ-H2AX staining. A beam edge is suggested on the image as the experiment did not include geometric validation of the coincidence of the irradiation and γ-H2AX regions. The inset in the figure shows the extraction of the irradiated mouse brain for staining.
Discussions
During the past decade, advanced imaging and delivery technologies have enabled the radiation therapy community to reduce treatment margins, escalate dose to the tumors while sparing normal tissues. During this period, great strides have also been made in the laboratory that have significantly improved our understanding of the molecular mechanisms contributing to radiation response, which in turn, spurs the development of novel approaches of molecular therapy. In appears that the synergistic integration of radiation technology and biology holds potential to greatly improve the outcome of future cancer treatment.
A significant hurdle to translate laboratory discoveries into radiation treatment is the technological disparity in the irradiation methods used for human treatment and animal research. Our results demonstrate clearly that the SARRP can provide advanced irradiation, imaging and planning capabilities, suitably downsized for small animal radiation research. Radiation dose deposition can be confined to within 1 mm. When the 3 mm focal spot is employed with 4 mm Al added filtration, the dose outputs at 1 cm depth in water and 35 cm SSD are high; about 200 cGy/min for the 1 mm diameter beam and 375 cGy/min for the 60 mm × 60 mm beam. However, this beam quality may be susceptible to greater attenuation by bony material. The use of 0.5 mm Cu filtration to harden the beam results in the reduction of dose output by a factor of 2. In the limit of using the smallest 0.5 mm diameter beam, the smaller 0.4 mm focal spot and Cu filtration, the output decreases to 22 cGy/min; and may not be acceptable for practical reason. We suspect that improving the accuracy of collimator and source alignment will alleviate the output reduction. On the other hand, the reduced dose output for the 0.5 mm beam can be readily remedied by using the larger 3mm focal spot or by reducing the SSD. Nevertheless, it is highly encouraging that the 80% to 20% penumbrae widths are of the order of 0.2 to 0.3 mm and that the leakage dose is negligible (see Figures 6b and c); thereby ensuring highly localized dose deposition.
It is clear that the voxel resolution of the human CT systems at about 1mm-cubed is too coarse for planning 3D conformal irradiation experiments of small animals. The voxel resolution of better than 0.3mm-cubed from our on-board cone-beam CT, or from other small animal CT systems, is more suitable. However, for dose calculation purposes, the relationships of CT numbers with tissues from these systems are likely to require more involved calibration than those from traditional planning CT. The heightened heterogeneity effects associated with the use of 225 kVp x-rays must also be addressed. The uncertainty in CT-based dose calculations for the SARRP is an important area that needs further investigation and validation.
Efforts are on-going to rigorously characterize and to refine the capabilities of the system. Prior to its deployment for laboratory research, a critical task is to establish the limits of integrated operation of the SARRP. Our initial experience suggests that accurate repeat positioning of an animal is challenging. The spatial accuracy and precision with which a small conformal radiation beam can be planned and delivered to an intended target volume in a small animal, in the setting of single or multiple irradiation fractions, must be quantified. Such knowledge is needed to establish an irradiation margin that ensures coverage of the intended target volume. Several non-trivial tasks are involved and represent a significant investigation that is on-going at our institute (13).
The pending availability of our system generates considerable excitement for new collaborations between laboratory and translational research scientists at our institution. Already, several exploratory studies are being conducted that takes advantage of the platform’s ability to focally irradiate a specific anatomic region or target in a mouse subject. These include studies of the response of normal tissue stem cells of different organs and tumors (14,15) to focal radiation injuries; the development of positron-emission-tomography markers for early assessment of radiation induced toxicity in the lungs (16); and the study of molecularly targeted therapy in combination with radiation in both pancreatic and prostate tumor models (17, 18). Efforts are also needed to develop MRI sequences to quantify radiation response in different organ systems. We are hopeful that our SARRP, and similar initiatives from other laboratories, will serve to provide the timely and powerful technology to greatly transform future cancer treatment.
Acknowledgements
We wish to thank Drs. Alvaro Martinez, Peter Corry, David Jaffray, Jeff Siewerdsen and Mark Oldham for the many stimulating discussions when the project was first conceived at William Beaumont Hospital, Royal Oak, MI. We also wish to thank Mr. Nick Grupido and Dr. Licai Jiang at OSMIC, Inc. MI, and Dr. Randy Gu at Oakland University, MI for their early involvement in the project. This project is supported in part by a grant, R01 CA108449 from the NCI as a Bioengineering Research Partnership.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Conflict of Interest
There are no conflicts of interest to be reported for all listed authors.
References
- 1.Wong J, Armour E, Oldham M, et al. An Image Guided Small Animal Radiation Research Platform. Proceedings of European Society for Therapeutic Radiology and Oncology (ESTRO 21) Praha, Rad. Oncol. 2002;64(S1):S61. [Google Scholar]
- 2.DesRosiers C, Mendonca M, Tyree C, et al. Use of the Leksell gamma knife for localized small field lens irradiation in rodents. Tech. in Cancer Res. Treat. 2003;2:449–454. doi: 10.1177/153303460300200510. [DOI] [PubMed] [Google Scholar]
- 3.Stojadinovic S, Low DA, Vicic M, et al. Progress toward a microradiation therapy small animal conformal irradiator. Med Phys. 2006;33(10):3834–3845. doi: 10.1118/1.2349693. [DOI] [PubMed] [Google Scholar]
- 4.Jaffray D, Moseley J, Chow, et al. Proceedings of the American Association of Physicists in Medicine, 48th Annual Meeting. Orlando, FL: 2006. An image-guided irradiator for pre-clinical radiation therapy studies. [Google Scholar]
- 5.Deng H, Kennedy CW, Armour E, et al. The small-animal radiation research platform (SARRP): dosimetry of a focused lens system. Phys Med Biol. 2007;52(10):2729–2740. doi: 10.1088/0031-9155/52/10/007. [DOI] [PubMed] [Google Scholar]
- 6.Graves EE, Zhou H, Chatterjee R, et al. Design and evaluation of a variable aperture collimator for conformal radiotherapy of small animals using a microCT scanner. Med Phys. 2007;34(11):4359–4367. doi: 10.1118/1.2789498. [DOI] [PubMed] [Google Scholar]
- 7.Stojadinovic S, Low DA, Hope AJ, et al. MicroRT—small animal conformal irradiator. Med. Phys. 2007;34:4706–4716. doi: 10.1118/1.2799887. [DOI] [PubMed] [Google Scholar]
- 8.Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J. Opt. Soc. Am. A. 1984;1:612–619. [Google Scholar]
- 9.Chow JC, Leung MK. Treatment planning for a small animal using Monte Carlo simulation. Med Phys. 2007;34:4810–4817. doi: 10.1118/1.2805254. [DOI] [PubMed] [Google Scholar]
- 10.Devic S, Seuntjens J, Sham E, et al. Precise radiochromic film dosimetry using a flat-bed document scanner. Med. Phys. 2005;32:2245–2253. doi: 10.1118/1.1929253. [DOI] [PubMed] [Google Scholar]
- 11.Chiu-Tsao ST, Ho Y, Shankar R, et al. Energy dependence of response of new high sensitivity radiochromic films for megavoltage and kilovoltage radiation energies. Med Phys. 2005 Nov;32(11):3350–3354. doi: 10.1118/1.2065467. [DOI] [PubMed] [Google Scholar]
- 12.Nowak E, Etienne O, Millet P, et al. Radiation-induced H2AX phosphorylation and neural precursor apoptosis in the developing brain of mice. Radiat Res. 2006;165(2):155–164. doi: 10.1667/rr3496.1. [DOI] [PubMed] [Google Scholar]
- 13.Matinfar M, Grey O, Iordachita I, et al. Proceedings of the 10th International Conference on Medical Image Computing and Computer Assisted Intervention. Brisbane, Australia: 2007. Oct, Small Animal Radiation Research Platform: Imaging, Mechanics, Control and Calibration. [DOI] [PubMed] [Google Scholar]
- 14.Quiñones-Hinojosa A, Chaichana K. The human subventricular zone: A source of new cells and a potential source of brain tumors. Experimental Neurology. 2007 doi: 10.1016/j.expneurol.2007.03.016. in press. [DOI] [PubMed] [Google Scholar]
- 15.Collis SJ, Neutzel S, Thompson TL, et al. Hematopoietic Progenitor Stem Cell Homing in Mice Lethally Irradiated with Differing Dose Rates of Ionizing Radiation. Radiation Res. 2004;162:48–55. doi: 10.1667/rr3197. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Armour E, Ford E, et al. Proceedings of the American Association of Physicists in Medicine, 48th Annual Meeting. Minneapolis, MN: 2007. Noninvasive evaluation of early radiation-induced pulmonary inflammation via [11C]-PK11195 based PET imaging. [Google Scholar]
- 17.Karikari C, Roy I, Tryggestad E, et al. Targeting the Apoptotic Machinery in Pancreatic Cancers using Small Molecule Antagonists of the X-linked Inhibitor of Apoptosis (XIAP) Protein. Mol Cancer Ther. 2007;6(3):957–966. doi: 10.1158/1535-7163.MCT-06-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collis SJ, DeWeese TL. Enhanced Radiation Response Through Directed Molecular Targeting Approaches. Cancer Metastasis Rev. 2004;23:277–292. doi: 10.1023/B:CANC.0000031767.30730.d1. [DOI] [PubMed] [Google Scholar]