Abstract
The breast cancer susceptibility gene 1 (BRCA1) plays a key role in mammary tumorigenesis. However, the reasons why silencing the Brca1 gene leads to tumorigenesis are not clearly understood. We report here that BRCA1-deficiency activates the AKT oncogenic pathway, one of the most common alterations associated with human malignancy. Mutation of Brca1 gene increases the phosphorylation and the kinase activity of AKT. The BRCA1-BRCT domains bind to phosphorylated AKT (pAKT) and lead to its ubiquitination towards protein degradation. BRCA1 mutant cells lacking the BRCT repeats accumulate nuclear pAKT and consequently inactivate the transcription functions of FOXO3a, a main nuclear target of pAKT. Our results demonstrate that BRCA1 is a negative regulator of the AKT pathway and imply the significance of BRCA1/AKT pathway in tumorigenesis.
Keywords: BRCA1, PKB/AKT, Phosphorylation, Ubiquitination
Introduction
The breast cancer susceptibility gene 1 (Brca1) has been shown to play a key role in both hereditary and sporadic mammary tumorigenesis (1–3). BRCA1 has been implicated in DNA damage repair, cell cycle checkpoint control, transcriptional regulation and maintenance of genomic stability, which are key factors in tumorigenesis (1,2). However, the extent to which BRCA1-activated molecular pathways contribute to its tumor suppressor activity remains unclear. The BRCA1 BRCT domains are phospho-protein binding motifs which are important for the tumor-suppressor function of BRCA1 (4–6). Most BRCA1 mutations cause truncated BRCA1 gene products that lack one or both C-terminal BRCT domains. Clinically relevant missense mutations identified at the C-terminus of BRCA1 abolish the BRCT structure (1,2) and BRCA1-deficiency leads to tumor formation in mice (5).
The AKT/PKB kinase is a well-characterized effector of phosphoinositide 3-kinase (PI3K), and its deregulation plays an important role in the pathogenesis of human cancers. Increased AKT kinase activity has been reported in about 40% of breast and ovarian cancers (7). Many oncoproteins and tumor suppressors intersect in the AKT pathway, finely regulating cellular functions at the interface of signal transduction and classical metabolic regulation (7,8). A number of nonexclusive mechanisms contribute to AKT hyperactivation in human cancer (9). PTEN-deficiency, PIK3CA and Ras mutations lead to AKT activation and occur frequently in human cancers. PML and PHLPP also regulate the AKT pathway in tumorigenesis (10,11). Thus, it appears that AKT activation plays a pivotal role in the genesis of cancer.
In spite of extensive efforts studying BRCA1 and AKT individually, an interaction of BRCA1 and AKT has not been reported. However, several lines of evidence suggest a role for BRCA1 in the regulation of AKT. AKT can phosphorylate BRCA1 (12), implying that AKT may bind to BRCA1 directly. Both AKT and BRCA1 regulate the cell-cycle and genomic stability through the CHK1 pathway (2,13), and BRCA1 regulates estrogen receptor-alpha (ER) activity in a PI3K/AKT dependent manner (14), strongly supporting the notion that the AKT pathway contributes to BRCA1-mediated tumorigenesis.
Materials and Methods
Cell culture
Primary embryonic fibroblasts were generated from Brca1+/+ and Brca1tr/tr embryos at embryonic day 13.5. Early passage primary mouse embryonic fibroblasts (MEFs) were immortalized by transfection with a plasmid expressing the SV40 large T antigen.
RNAi
siRNA AKT1 and BRCA1 were designed in 3’ UTR region by Oligoengine RNAi software and synthesized by Dharmacon. Lentiviral BRCA1-shRNAs were from Sigma.
In vitro AKT kinase assay
AKT kinase activity from cell extracts was analyzed by IP/Kinase assay (AKT Kinase Assay Kit, Cell Signaling Technology).
In vitro protein interactions
GST fusion BRCA1 proteins were produced in Escherichia coli and purified according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Indirect immunofluorescence
Images were captured and pseudocolored with IPLab image analysis software. At least 100 cells were analyzed for each experiment. The experiments were repeated at least three times.
Ubiquitination assay
Cells were transfected with indicated plasmids and lysates were analyzed by IP and WB with the indicated antibodies.
The details of materials and methods were described in Supplemental data.
Results and Discussion
BRCA1 is a negative regulator of AKT pathway
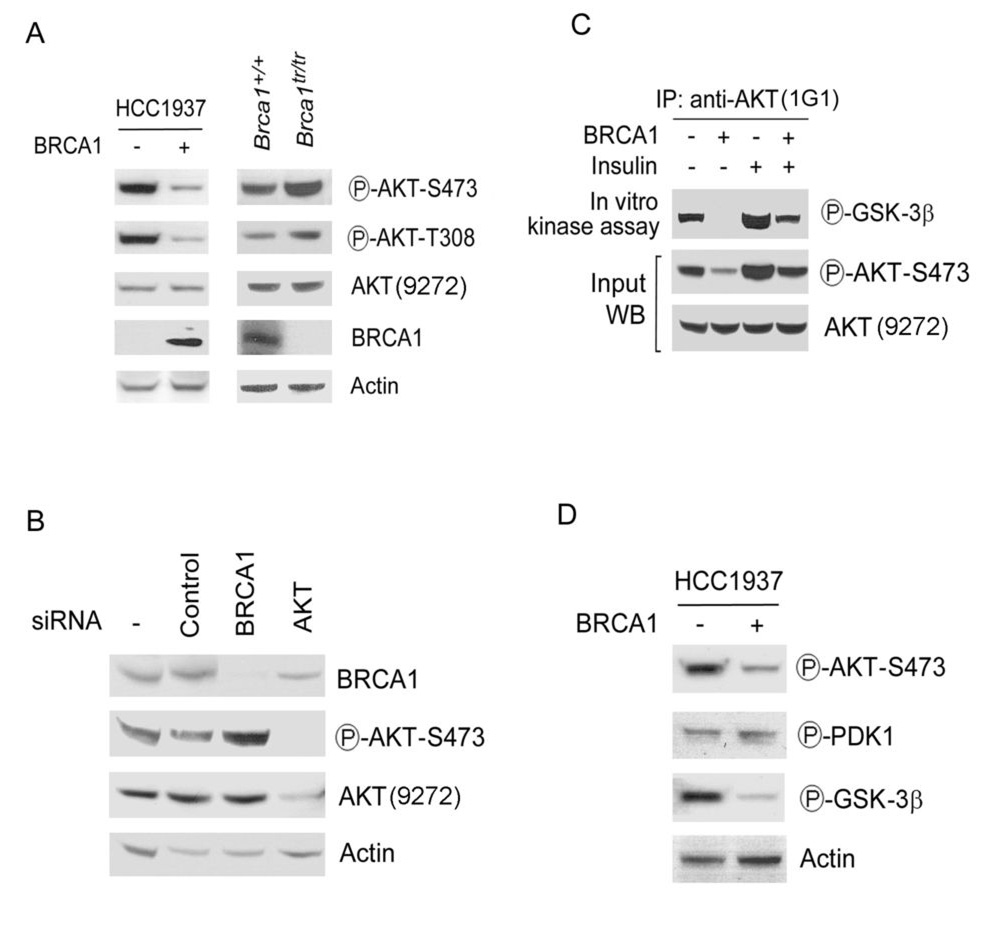
Here, we reasoned that BRCA1 might be involved in the AKT signal transduction. To examine the role of BRCA1 in the AKT pathway, BRCA1 was stably expressed in the breast cancer cell line HCC1937, which is hemizygous with respect to the BRCA1 mutant allele 5382insC expressing a BRCA1 protein lacking the C-terminal BRCT repeats. This mutation is associated with an increased risk of cancer and eliminates the activity of BRCA1 in the repair of DNA damage and maintenance of genomic stability (1,2). Phosphorylation of AKT on Thr-308 and Ser-473 is required for its full activation (7). Using antibodies specific for phospho-Ser-473 and Thr-308 AKT, we tested the phosphorylation status of AKT in HCC1937 cells with or without exogenously expressing wild-type BRCA1. AKT was phosphorylated on both Thr-308 and Ser-473 in HCC1937 cells (Fig. 1A). Expression of BRCA1 reduced phosphorylation levels of both Thr-308 and Ser-473 of AKT by ~80% (Fig. 1A). Expression of AKT recognized by an antibody (Cell Signaling, #9272) showed the same level by insulin treatment when pAKT increased 2~3 folds (Fig. 1C), suggesting that this AKT antibody mainly recognizes unphosphorylated form of AKT. These data are consistent with published reports for using the same antibody (10,11). Expression of BRCA1 in HCC1937 cells had no detectable effect on levels of unphosphorylated AKT, detected by this antibody. Furthermore, immortalized mouse embryonic fibroblasts (MEFs) expressing a truncated Brca1 allele (Brca1tr/tr) (5) showed higher levels of pAKT, compared with Brca1+/+ MEFs, confirming that BRCA1 negatively regulates the phosphorylation of AKT (Fig. 1).
Fig. 1. BRCA1 is a negative regulator of the AKT kinase pathway.
A. BRCA1 negatively regulates the phosphorylation of AKT. Left panel: Lysates were prepared from HCC1937 cells (BRCA1 inactive) that stably expressed BRCA1 (+) or vector only (−) and analyzed by Western blotting (WB) with the indicated antibodies. Right panel: Lysates from Brca1+/+or Brca1tr/tr MEFs were analyzed by WB. B. Increased phosphorylation of AKT in BRCA1 knockdown cells. siRNAs corresponding to the genes for the indicated proteins were transferred into MCF-7 breast cancer cells. After 2 days, lysates were prepared and analyzed by WB with the indicated antibodies. C. BRCA1 negatively regulates the AKT kinase activity. Whole-cell lysates prepared from HCC1937 cells expressing BRCA1 (+) or vector only (−) were subjected to immunoprecipitation (IP) with the anti-AKT antibody (AKT kinase kit, Cell Signaling) and analyzed by WB with the indicated antibodies. Insulin is a specific activator of the AKT pathway. D. BRCA1 negatively regulates the AKT pathway. Lysates from HCC1937 cells were analyzed by WB with the indicated antibodies. PDK1 is a kinase of AKT and GSK-3β is a downstream substrate of AKT.
Because the basal levels of pAKT are negatively regulated by PTEN, which is also deleted in HCC1937 cells, we verified that pAKT1 was regulated by BRCA1 in MCF-7 breast cancer cell line (with wild-type BRCA1 and PTEN). Knockdown of BRCA1 expression by BRCA1 small interfering RNA (siRNA) and short hairpin RNA (shRNA) enhanced the amounts of pAKT (Fig. 1B and Fig. S1A). Expression of total PTEN and phosphorylated PTEN displayed the same level in these cells (Fig. S1B), suggesting that regulation of pAKT by BRCA1 is independent on PTEN. Knockdown of BRCA1 in MEFs with or without Pten gene increased levels of pAKT (Fig. S1C), supporting that BRCA1 independently regulates pAKT.
To detect effect of BRCA1 on AKT kinase activity, we performed an in vitro AKT kinase assay by using GSK-3 fusion protein as a substrate. Insulin is a specific activator of the AKT pathway. As expected, insulin treatment could increase AKT kinase activity (Fig. 1C). Expression of BRCA1 decreased the AKT kinase activity, correlated with a reduction of pAKT levels. Overall, these results indicate that BRCA1 negatively regulates the phosphorylation and activation of AKT.
PDK1 is a protein kinase that phosphorylates Thr-308 of AKT. The phosphorylation status of PDK1 in HCC1937 cells expressing BRCA1 was identical to that of control cells (Fig. 1D), suggesting that BRCA1 may regulate the AKT phosphorylation and activity by acting on factors downstream of PDK1. Furthermore, expression of BRCA1 decreased pAKT levels, which correlated with decreased phosphorylation of its substrate GSK-3β (Fig. 1D), implying that BRCA1 regulates AKT kinase.
BRCA1 interacts with AKT
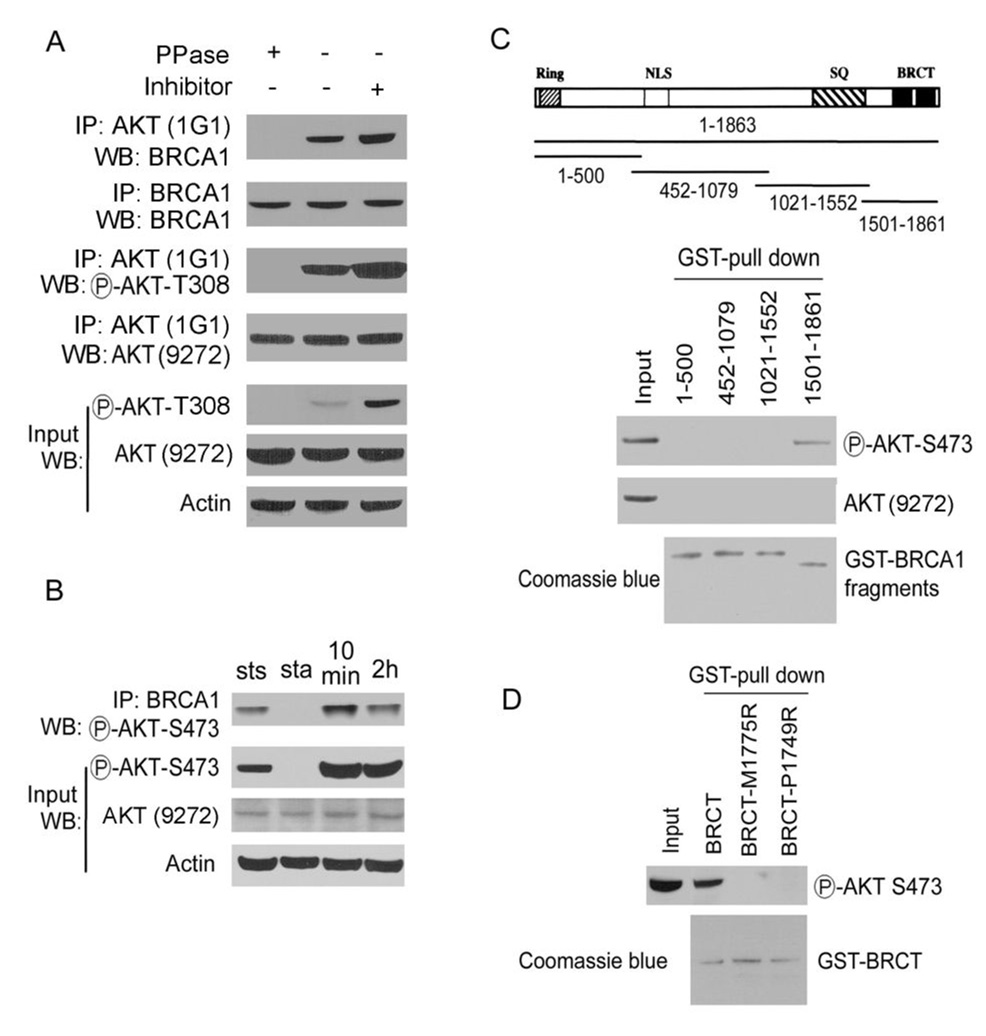
To detect that the interaction of BRCA1 and AKT is phospho-specific in cells, a coimmunoprecipitation assay was performed. Lysates that had first been treated with protein phosphatase (PPase) abolished this interaction, while inclusion of phosphatase inhibitors in lysis buffer increased the interaction of BRCA1 with AKT (Fig. 2A). These results suggest that the BRCA1-AKT interaction depends on the phosphorylation of AKT. Immunoprecipitates by pull-down with the AKT antibody (1G1) included both phosphorylated and un-phosphorylated forms of AKT (Fig. 2A, pAKT T308 and AKT #9272). Because the AKT antibody (1G1) did not work for Western blot analysis (see the manufacture and data not shown), we used the AKT antibody (#9272, recognize un-phosphorylated form of AKT) to verify the immunoprecipitation assay (Fig. 2A). The results showed that the un-phosphorylated form of AKT was unchanged in lysates with protein phosphatase or phosphatase inhibitor treatment. Furthermore, pAKT, but not unphosphorylated AKT, was detected in BRCA1 immunoprecipitates in MCF7 cells (Fig. S2, left panel). Also, BRCA1 was detected in pAKT immunoprecipitates in MCF7 cells (Fig. S2, right panel), confirming the existence of the BRCA1/pAKT complex in cells. Association of pAKT with BRCA1 was increased after serum stimulation (Fig. 2B), further confirming that the BRCA1-AKT interaction depends on the phosphorylation of AKT.
Fig. 2. BRCA1 interacts with pAKT.
A. BRCA1 interacts with pAKT in cells. Lysates from MCF7 cells were treated with or without λ phosphatase (PPase) or phosphatase inhibitor (10 mM NaF and 50 mM β-glycerophosphate) and then subjected to IP, followed by WB with the indicated antibodies. 20% input was loaded as a control. B. MCF7 cells were prepared at steady state (sts), starvation (sta) and serum stimulation for times indicated. Lysates were subjected to IP with the anti-BRCA1 antibody, followed by WB with the indicated antibodies. C. BRCA1 interacts with pAKT in vitro. Lysates from MCF7 cells were incubated with various GST-BRCA1 fragments immobilized on Sepharose beads were eluted and analyzed by WB with antibody against the phosphorylated or unphosporylated form of AKT (#9272). 20% input was loaded as a control. Equal loading of GST proteins was confirmed by coomassie blue staining. D. BRCA1-BRCT domains interact with pAKT. Lysates were incubated with GST-BRCA1-BRCT wild-type, M1775R and P1749R mutant proteins immobilized on Sepharose beads. The complex was eluted and analyzed by WB with anti-pAKT antibody. Equal loading of GST proteins was confirmed by coomassie blue staining.
Next, we investigated the binding domains of BRCA1 with AKT using an in-vitro binding assay. A series of four overlapping glutathione S-transferase (GST) fusion proteins, spanning the entire coding region of BRCA1, were used to define regions of BRCA1 that interact with AKT. Purified GST-BRCA1 fusion proteins were added to MCF7 cell-free extracts, and GST-BRCA1-AKT complexes were isolated with glutathione beads. pAKT, but not unphosphorylated AKT, was found to bind fragment 1501–1861 of BRCA1 as detected by Western blotting (Fig. 2C). BRCA1 fragment 1501–1861 contains the BRCT domains, which are phospho-protein binding domains that are important for the tumor-suppressor function of BRCA1. The recombinant BRCA1-BRCT domain (residues 1599–1863) fused to GST bound endogenous pAKT from a MCF7 cell lysate (Fig. 2D). The cancer-associated missense mutations M1775R and P1749R in the BRCT domains of the carboxyl terminus of BRCA1 ablate the functions of BRCA1 (2). We tested binding ability of these BRCA1 mutants to pAKT and both failed to interact with pAKT (Fig. 2D), suggesting that the intact structure of the tandem BRCA1-BRCT domains is essential for its interaction with pAKT.
BRCA1 ubiquitinates the pAKT leading to its degradation
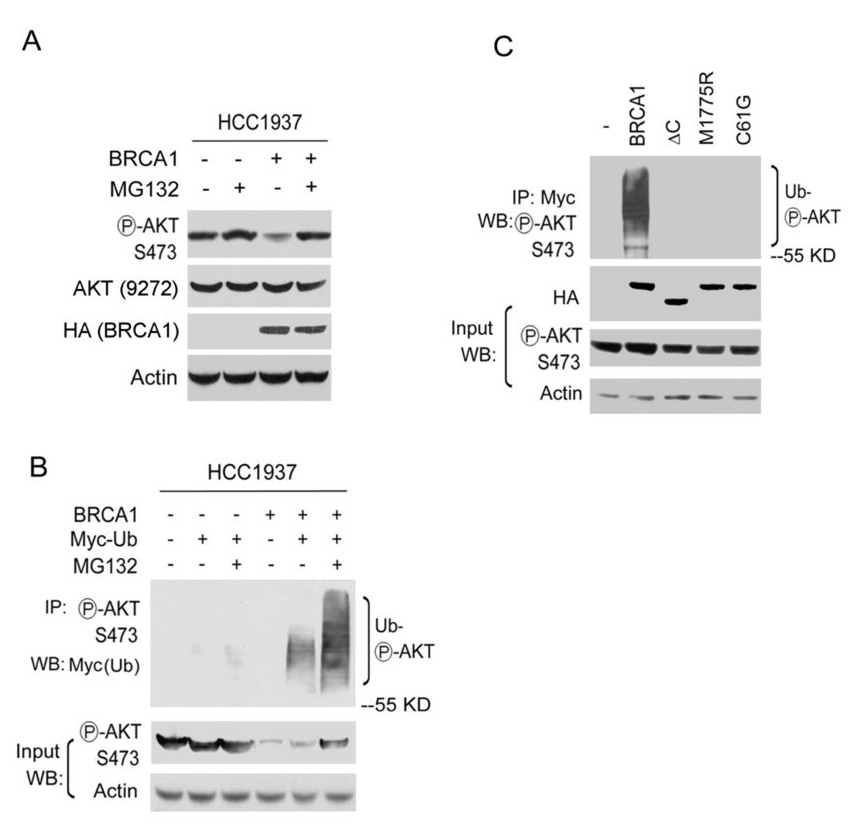
Recent reports indicate that BRCA1 and its partner BARD1 are enzymatic mediators of protein ubiquitination (15,16). The potent ubiquitin E3 ligase activity of the BRCA1/BARD1 heterodimer may be responsible for the inactivation of pAKT by BRCA1. To test this possibility, we used MG132, a proteasome inhibitor. pAKT levels increased in HCC1937 cells expressing BRCA1 or in Brca1+/+ MEFs when treated with MG132 (Fig. 3A and Fig. S3), suggesting that the BRCA1-dependent ubiquitination may cause the degradation of pAKT. To further investigate this possibility, we transfected an ubiquitin expression plasmid in HCC1937 cells and found that BRCA1 stimulated the ubiquitination of pAKT in HCC1937 cells expressing BRCA1 (Fig. 3B).
Fig. 3. BRCA1 ubiquitinates pAKT.
A. Lysates were prepared from HCC1937 cells expressed BRCA1 or vector with or without 50 µM MG132 treatment for two hours, and analyzed by WB with the indicated antibodies. B. HCC1937 cells expressing BRCA1 or empty vector were transfected with a Myc-tagged ubquitin plasmid in the absence or presence of 50 µM MG132 for two hours. Lysates were analyzed by IP and WB with the indicated antibodies. C. 293T cells transfected with constructs expressing Myc-ubiquitin, HA-BRCA1, HA-BRCA1-mutants were treated with 50 µM MG132 for two hours. Lysates were analyzed by IP and WB with the indicated antibodies. ΔC, C-terminal deletion of BRCA1. C61G, inactive E3 ubiquitin ligase of BRCA1. M1775R, point mutation in BRCT domains.
Polyubiquitination often serves as a signal for the protein degradation. To characterize the potential biological consequences of the ubiquitinated pAKT, we compared the stability of pAKT in cells treated with MG132. Treatment with MG132 stabilized the ubiquitinated pAKT and resulted in longer chains of ubiquitin appended on pAKT (Fig. 3B), suggesting that BRCA1-dependent ubiquitination causes the degradation of pAKT. It has been shown that expression of BRCA1 in cells stimulates the ubiquitination of phosphorylated RNA polymerase II (17). Using this system, BRCA1 and ubiquitin expression constructs were transfected into 293T cells. Transfected wild-type BRCA1 stimulated the ubiquitination of pAKT, but BRCA1 constructs lacking the BRCT repeats (ΔC) or encoding disease-associated missense mutation (M1775R) did not. To further detect effect of BRCA1/BARD E3 ubiquitin ligase activity on pAKT ubiquitination, a C61G mutant of BRCA1 that is inactive E3 activity of BRCA1 was tested. Cells expressing the C61G mutant did not show the ubiquitination signals of pAKT (Fig. 3C), indicating that the BRCA1 associated E3 ubiquitin ligase activity is required for the ubiquitination of pAKT. Furthermore, the C-terminal deletion (ΔC) or M1775R mutation disrupted BRCA1 binding to pAKT, as analyzed by co-immunoprecipitation assays (Fig. S4). Thus, intact BRCT domains are required for the binding of pAKT to BRCA1 for its degradation in cells, consistent with our results from the in vitro binding assays.
BRCA1 deficiency leads to an increased nuclear localization of pAKT
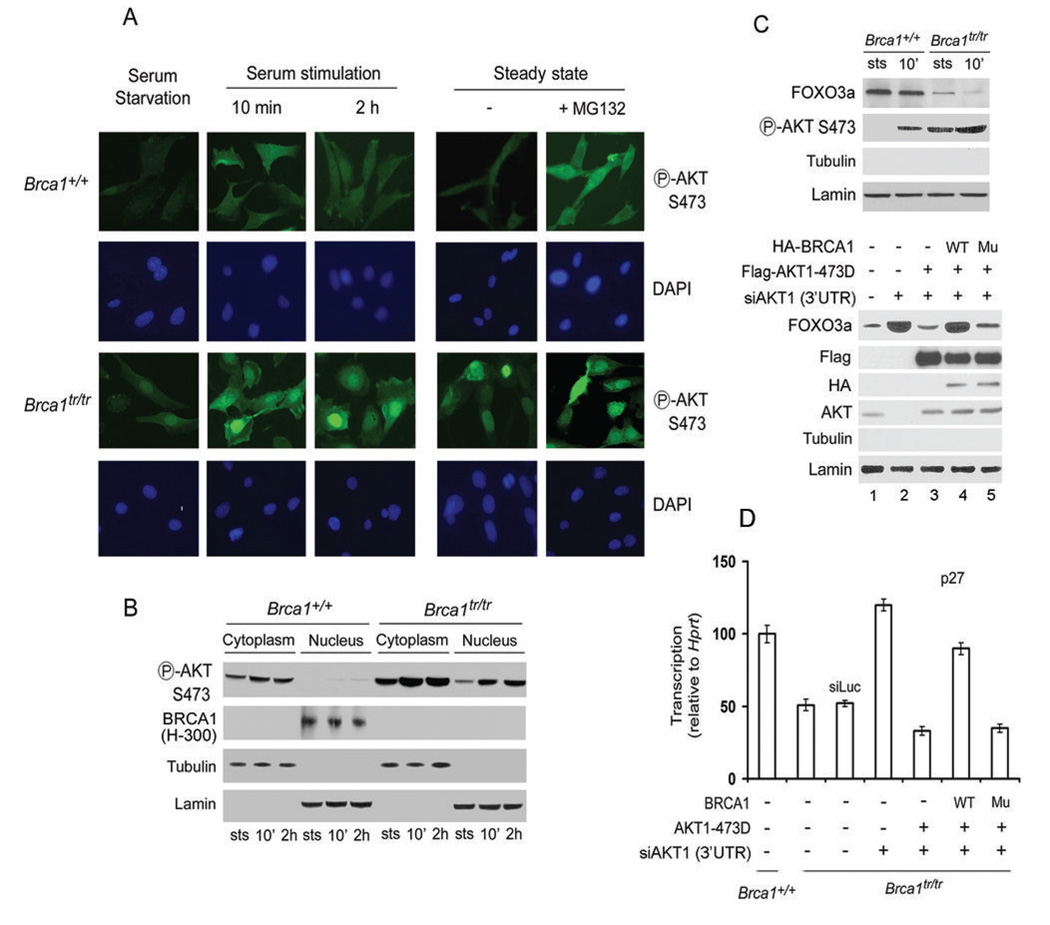
After phosphorylation and activation on the membrane pAKT is released into the cytoplasm and translocated to the nucleus (7). Furthermore, BRCA1 is a nuclear-cytoplasmic shuttling protein and cancer-associated mutations in the BRCT domains alter subcellular localization of BRCA1 (18). To test whether BRCA1 regulates the localization of pAKT, we used Brca1+/+ and Brca1tr/tr MEFs. First, we determined AKT status in Brca1+/+ and Brca1tr/tr MEFs by subjecting them to serum starvation and stimulation. Brca1tr/tr cells showed stronger pAKT staining in both cytoplasm and nuclei at steady-state and serum stimulation (Fig. 4A). Treatment with MG132 increased pAKT staining in Brca1+/+ MEFs, consistent with the observations by Western blotting (Fig. 3). To confirm these observations, cytoplasmic and nuclear fractions were prepared from these cells and analyzed by Western blotting. Increased pAKT levels were found in the cytoplasm and nuclei of Brca1tr/tr cells compared to Brca1+/+ cells (Fig. 4B). These observations indicate that the absence of full length BRCA1 protein leads to an increased nuclear localization of pAKT.
Fig. 4. BRCA1 deficiency leads to increased nuclear localization of pAKT.
Brca1+/+ and Brca1tr/tr MEFs were prepared at steady state (sts), starvation and serum stimulation for times indicated. Immunofluorescence staining showed in A and WB analysis in B. Nuclei were stained with DAPI. C. The expression levels of FOXO3a were decreased in nuclear extracts from Brca1tr/tr MEFs, analyzed by WB (the upper panel). Expressing the AKT1-S473D mutant resulted in the decrease of FOXO3a in Brca1tr/tr MEFs (the lower panel). Nuclear extracts from Brca1tr/tr MEFs transfected with indicated plasmids were analyzed by WB. AKT1 siRNA targeted on 3’ UTR region of AKT1 and only decreased expression of endogenous AKT1, but not affect expression of the transfected flag-AKT1. Lanes 3–5 with the AKT antibody showed the transfected flag-AKT1. WT: wild-type of Brca1 plasmid, Mu: Brca1-BRCT mutant (M1775R, point mutation in BRCT domains). D. BRCA1 affects FOXO3a activity via the AKT pathway. mRNA levels of p27kip1 in Brca1tr/tr MEFs transfected with indicated plasmids or siRNAs were measured by real-time PCR. Error bars are s.d. of triplicates. WT: wild-type of Brca1 plasmid, Mu: Brca1-BRCT mutant (M1775R, point mutation in BRCT domains).
Interaction of AKT-BRCA1 mediates the AKT1-FOXO pathway
The forkhead transcription factor FOXO3a is a direct nuclear target of AKT, as AKT-mediated phosphorylation causes FOXO3a inactivation and nuclear export (19,20). To test whether BRCA1-deficiency enhances the nuclear function of pAKT, we next examined effects of BRCA1-pAKT interaction on FOXO3a inactivation and nuclear export. We found that the levels of FOXO3a decreased in nuclear extracts from Brca1tr/tr MEFs (Fig. 4C, the upper panel), suggesting that BRCA1-deficiency causes FOXO3a nuclear export by activating the AKT pathway. An AKT1-S473D mutant, only bound to wild-type BRCA1 protein but not to the BRCA1-BRCT mutant (Fig. S5), was expressed in Brca1tr/tr MEFs resulted in the decrease of FOXO3a (Fig. 4C, the lower panel). Co-transfected wild-type BRCA1, but not BRCA1-BRCT mutant, abolished the effect of AKT1-S473D, confirming that BRCA1 regulates the pAKT-mediated FOXO3a nuclear export. FOXO3a exerts some of its tumor-suppressive functions by inducing the transcription of p27kip1 (19). As quantified by real-time PCR, overexpression of AKT1-S473D decreased p27kip1 mRNA levels in Brca1tr/tr MEFs (Fig. 4D). Co-transfected wild-type BRCA1 could block the effect of AKT1-S473D, whereas the BRCA1-BRCT mutant could not, due to the lack of interaction with pAKT. Thus, we demonstrate that interaction of pAKT-BRCA1 regulates the transcription function of FOXO3a.
The role of the BRCA1-AKT interaction in tumorigenesis is a novel finding. Establishment of this novel BRCA1-AKT pathway and the elucidation of its precise molecular functions are expected to improve our understanding of hereditary as well as sporadic mammary tumorigenesis. Targeting the BRCA1/AKT pathway may lead to the improved design of highly specific molecular targeted therapies with efficacy and reduced toxicity for BRCA1-deficient cancers.
Acknowledgments
We thank Tanya T. Paul and Stephen J. Elledge for providing the GST-BRCA1 constructs, Junjie Chen for providing the wild-type and mutant GST-BRCA1-BRCT constructs, Natsuko Chiba and Jeffrey D. Parwin for providing the Myc-ubiquitin and wild-type and mutant BRCA1 constructs, and Bing-Hua Jiang for providing AKT1-473D construct. This work is supported in part by grants from the Susan G. Komen Foundation (QY), the Ovarian Cancer Research Fund (QY), Siteman Cancer Center Award (QY), NIH CA 10445 and CA 123232 (TKP).
References
- 1.Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res. 2005;3:531–539. doi: 10.1158/1541-7786.MCR-05-0192. [DOI] [PubMed] [Google Scholar]
- 2.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 3.McCoy ML, Mueller CR, Roskelley CD. The role of the breast cancer susceptibility gene 1 (BRCA1) in sporadic epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1:72. doi: 10.1186/1477-7827-1-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu X, Chini CC, He M, Mer G, Chen J. The BRCT domain is a phospho-protein binding domain. Science. 2003;302:639–642. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig T, Fisher P, Ganesan S, Efstratiadis A. Tumorigenesis in mice carrying a truncating Brca1 mutation. Genes Dev. 2001;15:1188–1193. doi: 10.1101/gad.879201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manke IA, Lowery DM, Nguyen A, Yaffe MB. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–639. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Kumar CC, Di CA, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 8.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 9.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miralem T, Avraham HK. Extracellular matrix enhances heregulin-dependent BRCA1 phosphorylation and suppresses BRCA1 expression through its C terminus. Mol Cell Biol. 2003;23:579–593. doi: 10.1128/MCB.23.2.579-593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shtivelman E, Sussman J, Stokoe D. A role for PI 3-kinase and PKB activity in the G2/M phase of the cell cycle. Curr Biol. 2002;12:919–924. doi: 10.1016/s0960-9822(02)00843-6. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Hu C, Riegel AT, Fan S, Rosen EM. Growth factor signaling pathways modulate BRCA1 repression of estrogen receptor-{alpha} activity. Mol Endocrinol. 2007 doi: 10.1210/me.2006-0397. [DOI] [PubMed] [Google Scholar]
- 15.Starita LM, Parvin JD. Substrates of the BRCA1-Dependent Ubiquitin Ligase. Cancer Biol Ther. 2006;5:137–141. doi: 10.4161/cbt.5.2.2479. [DOI] [PubMed] [Google Scholar]
- 16.Baer R, Ludwig T. The BRCA1/BARD1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr Opin Genet Dev. 2002;12:86–91. doi: 10.1016/s0959-437x(01)00269-6. [DOI] [PubMed] [Google Scholar]
- 17.Starita LM, Horwitz AA, Keogh MC, Ishioka C, Parvin JD, Chiba N. BRCA1/BARD1 ubiquitinate phosphorylated RNA polymerase II. J Biol Chem. 2005;280:24498–24505. doi: 10.1074/jbc.M414020200. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez JA, Au WW, Henderson BR. Cytoplasmic mislocalization of BRCA1 caused by cancer-associated mutations in the BRCT domain. Exp Cell Res. 2004;293:14–21. doi: 10.1016/j.yexcr.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 20.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]