Abstract
Perlecan is a ubiquitous pericellular proteoglycan ideally placed to mediate cell signaling events controlling migration, proliferation and differentiation. Its control of growth factor signaling usually involves interactions with the heparan sulfate chains covalently coupled to the protein core’s N-terminus. However, this modular protein core also binds with relatively high affinity to a number of growth factors and surface receptors, thereby stabilizing cell-matrix links. This review will focus on perlecan/growth factor interactions and describe recent advances in our understanding of this highly-conserved proteoglycan during development, cancer growth and angiogenesis. The pro-angiogenic capacities of perlecan that involve proliferative and migratory signals in response to bound growth factors will be explored, as well as the anti-angiogenic signals resulting from interactions between the C-terminal domain known as endorepellin and integrins that control cell adhesion to the extracellular matrix. These two somewhat diametrically-opposed roles will be discussed in light of new data emerging from various fields which converge on perlecan as a key regulator of cell growth and angiogenesis.
Perlecan was originally isolated in 1980 by Hassell and coworkers from the Engelbreth-Holm-Swarm sarcoma, a basement membrane-secreting tumor, and was soon demonstrated to be expressed also at the cell surface of human colon carcinoma cells (1). In spite of their differential expression, the two molecules were shown to have biosynthetic and immunological similarities. Because of its large size —the mRNA encoding perlecan is ~15 kb—it took over a decade of efforts to complete the cDNA cloning of the full-length mouse perlecan, followed by the complete structure of the human counterpart, its chromosomal mapping, and its genomic organization (2). The eponym “perlecan” derives from its ultrastructural appearance of “beads on a string”, a feature attributable to the various globular domains interspersed among more linear structures (1).
Perlecan is composed of five distinct domains with homology to growth factors and to protein modules involved in lipid metabolism, cell adhesion, and homotypic and heterotypic interactions (3). Notably, the N-terminal domain I contains three attachment sites for the heparan sulfate side chains with additional attachment sites in the carboxyl terminus domain V (Figure 1). Interestingly, the other two main HSPGs from basement membranes, collagen XVIII and agrin, do not share much structural homology with exception of agrin domain V. Collagen XVIII is a member of the subfamily of collagens, also known as multiplexins. These collagens, which include collagen XV, harbor a central triple-helical domain that is interrupted and flanked by non-collagenous regions (4). The C-terminal, non-collagenous domain of collagen XVIII contains the angiogenesis inhibitor endostatin. Agrin is also a modular HSPG that is best known for its ability to organize postsynaptic differentiation at the neuromuscular junction but is also involved in muscle and renal homeostasis (5). The N-terminal and central region of agrin are quite unique. However, the C-terminal domain has a structural organization similar to domain V of perlecan with three laminin-like globular domains interspersed by EGF-like repeats (see below).
FIGURE 1.
Schematic diagram of human perlecan depicting the various domains (Roman numerals) and the abbreviations for each module (top). The bottom panel shows a list of perlecan interactive partners relative to each protein core domain.
Perlecan is a ubiquitous macromolecule that is predominantly a basement membrane/ extracellular matrix proteoglycan with an intrinsic ability to self-assembly into dimers and oligomers. It is often secreted into the pericellular space where it is ideally situated to mediate the action of signaling molecules that are either secreted by the cells themselves in response to environmental cues or secreted by other cells in a paracrine fashion (3). Perlecan’s modular protein core interacts with a number of extracellular matrix constituents, receptors and growth factors (Figure 1 and Table 1). By surrounding the cell, perlecan may act to control the pericellular concentration of mitogens and morphogens. Its widespread expression across species suggests that it may be performing this role for many different types of cells that are responding to different stimuli at the same time. This hypothesis was supported when the effects on embryonic development were studied in perlecan knock-out mice. These mice demonstrated a complex series of phenotypes which was not confined to one tissue or organ system (6,7). Most of the mice survived the early stages of embryonic development quite successfully, but then approximately half of them died around embryonic day 11.5 because of either cardiac system failure from intra-pericardial hemorrhage due to malformed and transposed major blood vessels or failure of the neural system to develop (7). Those mice that progressed to birth died soon after from respiratory failure most probably due to major skeletal abnormalities present in the ribs and diaphragm region (6). Histological examination of these mice showed a marked disorganization in the structure and architecture of the developing cartilage tissue (6) which may have been caused by disturbed signaling gradients. Other skeletal changes included shortened long bones and a dwarf-like phenotype similar to that seen in human Schwartz-Jampel Syndrome —a condition shown to be due to a mutation in the perlecan gene (1). A complication with these types of studies is the possibility of an up-regulation of other heparan sulfate proteoglycans (HSPG)1 in the basement membranes and extracellular matrix that may perform similar functions leading to compensation of the phenotype in some animals. This is particularly relevant because the growth signaling molecules bind to the HS chains which may be very similar among HSPGs. This may have been the case in some of the perlecan-deficient mice where an increase in type XVIII collagen and/or agrin could have provided enough HS with the appropriate structure to replace the roles of perlecan (8). The presence of HS is absolutely required for successful embryonic development because zygotes totally lacking the ability to synthesize any did not proceed past the early gastrulation phase of development. It would be hypothesized that a total lack of HS would lead to a loss of all mitogen/morphogen gradients, and whilst the cells could grow to the multicellular blastula stage, the diffusion of cytokines away from the cells would cause a failure in the formation of a tube critical to gastrulation (9). Mice that specifically lack type XVIII collagen have abnormalities in eye development and some effects on angiogenesis (4), whereas animals lacking agrin have defective neuromuscular junctions due to the inability of the synapses to localize the acetylcholine receptors correctly (5). Although it is tempting to suggest that agrin is specific for neural tissue, it has been shown to be produced by chondrocytes and to be localized to basement membranes in the kidney similar to collagen XVIII (5).
Table 1.
Interactions of Perlecan with Growth Factors and Morphogens: Activation of Multiple Signaling Pathways
| Growth Factor | Interacting Perlecan Moiety | Effect/Activity |
|---|---|---|
| FGF2 | Heparan sulfate chains Domain I | Mostly activation of FGF receptor function. Required for receptor activation. Enhanced growth, angiogenesis and chondrogenesis |
| FGF7 | Domain III and Domain V/Endorepellin | Required for receptor activation and mitogenic activity in colon carcinoma cells and keratinocytes |
| FGF10 | Heparan sulfate chains, specific microdomains | Stimulates FGFR2b activity and branching morphogenesis in the submandibular salivary gland |
| FGF18 | Domain III | Regulation of growth plate chondrocyte proliferation, hypertrophy and vascularization |
| VEGF | Heparan sulfate chains; co-localization with perlecan in tumor angiogenic vessels | Enhanced tumor angiogenesis and survival |
| PDGF | HS chains and Domains III and IV | Storage of PDGF and modulation of PDGF receptor activity |
| Progranulin | Domain V/Endorepellin | Promotes cell migration and invasion of bladder cancer cells |
| Hedgehog | Domain V/Endorepellin of Trol in Drosophila | Activation of neural stem cell division in Drosophila. Required for receptor activity. Control of neurogenesis in the developing telencephalon |
| TGFβ/Wnt | Protein core/UNC-52 Trol in Drosophila | Migration of gonadal leader cells in C. elegans |
| IL-2 | Heparan sulfate chains | Neuroblast proliferation in Drosophila Enhanced proliferation of IL-2-dependent Cells |
The important role of HS and the fact that type XVIII collagen can compensate for the lack of perlecan were also demonstrated when mice that produced HS-deficient perlecan were bred with mice deficient in collagen type XVIII. This resulted in mice that displayed an ocular phenotype that was more severe than in those animals expressing the HS-deficient perlecan (8).
Mutations of the C. elegans perlecan ortholog, UNC-52, cause defects in the formation and maintenance of the muscle myofilament lattice. Notably, perlecan/UNC-52 affects gonadal leader cell migration by modulating the bioactivity of several growth factors including FGF, TGFβ, and Wnt (10). In Drosophila, perlecan/Trol stimulates neuroblast proliferation (11) and modulates FGF and Hedgehog signaling, and this interaction is mitogenic for neural stem cells (12). Perlecan also potentiates cell cycle progression and neuronal differentiation in the murine cerebral hemispheres and regulates Sonic Hedgehog availability in the floor plate (13). Thus, it is likely that perlecan may play multiple developmental roles by concentrating growth factors and morphogens near the cell surface and by restricting their subsequent diffusion (10).
PERLECAN SIGNALING AND FGFs
Perlecan binds to many growth factors, particularly those from the fibroblast growth factor family, known regulators of neovascularization. It has been shown that the HS chains are responsible for the binding to FGF1, 2, 7, 9, 10, 18 (14) and such interactions may lead to enhanced angiogenesis and chondrogenesis. Most of the research performed on the binding of perlecan to growth factors has concentrated on FGFs because of the availability of good research tools including recombinant growth factors and their receptors, antibodies, and the Baf32 cell line that has been transfected with the genes for the various FGFR isoforms (14). The other point about FGFs is that they are produced by many cell types and in most cases signal cells to proliferate. These results suggest that FGF bioactivity is strongly influenced by the contextual environment in which the growth factor is presented, and expand the repertoire of subtle variations and sometimes paradoxical effects perlecan can display in particular pericellular environments.
Perlecan around the chondrocyte has been shown to localize FGF2 and act as a mechanotransducer by causing proliferation via activation of the MAP kinases Erk1 and Erk2 (15), both major signaling molecules involved in the response to FGFs. In addition to FGF2, smooth muscle cells also express FGFR1, which has been shown to be important in proliferation and, in particular, during neointimal formation that causes re-stenosis following angioplasty of atherosclerotic lesions within blood vessels (16). The role of HS in smooth muscle cell proliferation has been shown to be important; when it is removed by a combination of bacterial heparinase digestions, the cells at a site of injury no longer respond to introduced FGF2 (17).
Perlecan is also involved in the binding and bioactivity of FGF7, also known as keratinocyte growth factor. Colon carcinoma cells, in which the perlecan gene is disrupted by targeted homologous recombination, grow slowly, fail to respond to exogenous FGF7 with or without heparin and are less tumorigenic when injected in immunocompromised mice (18). In an engineered human skin model, perlecan-deficient keratinocytes form a poorly-organized epidermis which is partially restored by exogenous FGF7 (19). Finally, the protein core of perlecan binds to FGF18, a key factor for chondrogenesis, and alters the mitogenic effect of FGF18 on growth plate chondrocytes (20). This finding is also supported by the similarity in cartilage phenotype between perlecan null and the FGF18 null mice which both exhibit a defect in endochondral ossification (1).
Perlecan HS is also involved in branching morphogenesis of the submandibular salivary gland by specifically interacting with FGF10 (21). In this ex vivo model, heparanase co-localizes with perlecan in the glandular basement membrane and liberates FGF10 bound to the heparan sulfate chains. This leads to a signaling cascade which activates MAPK, stimulates the formation of epithelial clefts and ultimately enhances branching morphogenesis. The specificity of this interaction was demonstrated by surface plasmon resonance studies showing that FGF10 and FGF10/FGFR2b complexes bound to HS chains on perlecan, and that these complexes could be liberated by heparanase (21).
The role of HS and heparin has been modeled and indicated that at some concentrations and in solution they are capable of signaling through their cognate receptors, whereas at higher concentrations they can inhibit growth. Perlecan’s HS has been shown to signal through FGF2 and FGFR1 in solution, but these results have been obtained using perlecan derived from cultured endothelial cells and the genetically-manipulated Baf32 cell line (22).
Perlecan is a key component of a basement membrane-like structure surrounding chondrocytes (23) and, together with dystroglycan, promotes basement membrane differentiation and maintenance of cell polarity in Drosophila follicle cell epithelium (24). Perlecan can be substituted not only with HS but also with chondroitin sulfate. Interestingly, chondroitin sulfate perlecan enhances collagen fibrillogenesis in cartilage (25), thereby providing a plausible explanation for the chondrodysplasia observed in the perlecan-null mice. Moreover, the chondroitin sulfate moiety in perlecan inhibits FGF2 delivery to its cognate receptor, FGFR3, in cartilage growth plate (26).
All of these results need to be confirmed in vivo but support the hypothesis that perlecan is an inactive sink for FGFs; this would partly explain the reason why cells that are surrounded by perlecan and produce FGF do not proliferate out of control. Instead, they remain in a quiescent state unresponsive to many mitogenic signals. Whereas HS chains favor FGF/FGFR interaction, chondroitin sulfate chains in perlecan could act as “negative” regulators of FGF/FGFR activity, primarily by physically constraining the FGFs from contacting their cognate receptors. It would be of interest to determine the structure of the HS attached to the different perlecan species in order to determine the specific microdomain structures that are responsible for mediating these various signals.
PERLECAN AND OTHER GROWTH FACTOR SIGNALING
Some families of growth factors have been shown to demonstrate differential binding to perlecan HS with one such example being the VEGFs. One of the longer isoforms, VEGF189, which contains exon 6 that encodes a basic stretch of amino acids and which has been shown to be responsible for matrix localization, binds to perlecan HS derived from endothelial cells whereas the shorter and more highly expressed VEGF165 does not (Whitelock and Stringer, unpublished). Interestingly, a fraction that included both the secreted and cell surface HSPGs from fibroblasts was shown to bind VEGF165 (27). This would support the idea that perlecan localizes the larger forms of VEGF to the matrix but does not sequester the shorter forms, enabling them to diffuse through the pericellular matrix and bind to the cell surface HSPGs where they can signal the cell through either neuropilin or the VEGF tyrosine kinase receptors displayed on the cell surface (28). This hypothesis is supported by an elegant study in zebrafish where the localization of VEGF in the matrix was disturbed by knocking down the expression of the enzyme 6-O-sulfotransferase which affects the levels of sulfation present within HSPGs (29). Cell proliferation occurs satisfactorily but the process of branching morphogenesis is severely retarded. One would speculate that the perlecan produced by endothelial cells undergoing angiogenesis would have low amounts of 6-O-sulfate and if it produced a perlecan that had a high proportion of these sulfate groups, it would prevent angiogenesis by hindering the diffusion of VEGF. This may be a process that cells use to modulate the response of the endothelial cells to VEGFs produced in the pericellular environment. Interestingly, this may also be a factor in PDGF signaling. PDGF has been shown to contain an alternatively spliced exon that contains “heparin-binding” or matrix localization sequences. Both PDGF homodimers bind to perlecan HS derived from endothelial cells (30), and the inhibition of smooth muscle cell growth by perlecan may involve the inhibition of PDGF signaling which has downstream effects on FGF2 signaling. Finally, the LDL repeats in perlecan domain II, a module predicted to interact with lipids (31), are involved in uptake of LDL and VLDL (32).
Thus, perlecan might be indirectly involved in the complex interplay among these signaling pathways during cartilage development and differentiation.
PRO-ANGIOGENIC ACTIVITY
Perlecan is highly expressed in the stroma of various types of solid tumors. It is often associated with the microvasculature which provides nutrients and oxygen to the growing neoplastic cells , and its expression correlates with a more aggressive phenotype. In 1994 we reported the first evidence that perlecan could be involved in angiogenesis. We found that in tumor xenografts composed of human-derived prostate carcinoma cells and mouse-derived stromal elements, perlecan secreted by the human prostate cancer cells was deposited along the newly-formed (angiogenic) vessels of the tumor xenografts. Thus, we hypothesized that perlecan might directly contribute to the scaffolding of angiogenic blood vessels (3). Nearly concurrently, it was demonstrated that perlecan is the major co-factor for the activity of FGF2, a powerful angiogenic factor, and for the specific interaction with its cognate receptor leading to enhanced mitogenesis and angiogenesis. Notably, antisense targeting of endogenous perlecan in a variety of transformed cells including colon carcinoma and melanoma cells causes a significant inhibition of tumor growth and angiogenesis (3). Seemingly, colon carcinoma cells with a somatic cell mutation leading to a perlecan null phenotype show growth retardation and minimal angiogenesis in tumor xenografts (18). The central role of perlecan in angiogenesis is further confirmed by genetic manipulation leading to complete ablation of the perlecan gene (6,7). A significant proportion of perlecan-null mice develop numerous vascular anomalies including transposition of the great arteries and abnormal coronary arteries (1). In an animal model expressing a mutated form of perlecan lacking the canonical glycosaminoglycan attachment site, and thus lacking HS side chains, there is impaired angiogenesis and retarded tumor growth (33), whereas perlecan is required to inhibit thrombosis in an animal model of deep vascular injury (16). A recent study adds a new dimension to these results because it demonstrates that regulation of perlecan gene expression is regulated by a mechano-transduction pathway in endothelial cells and that this is a key mechanism through which endothelial cells inhibit vascular smooth muscle cell proliferation in response to changes in mechanical environment (34).
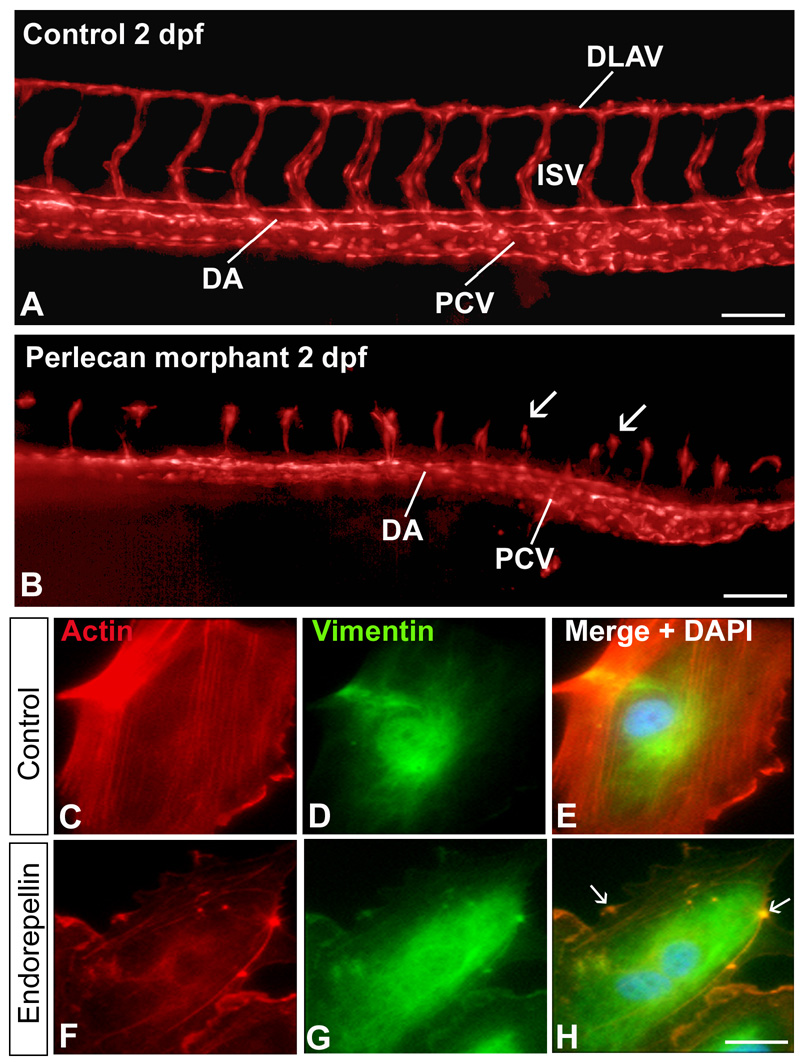
A central function for perlecan in cardiovascular development and angiogenesis has been recently demonstrated in the zebrafish Danio rerio. Morpholino-mediated knockdown targeting three separate regions of the perlecan mRNA showed relatively normal development of axial vessels, dorsal aorta and posterior cardinal vein, but a blunted and anomalous development of the angiogenic vessels, intersegmental and dorsal longitudinal anastomotic vessels (Figure 2 A,B.) (35). Notably, the perlecan morphant phenotype could be rescued by microinjecting human perlecan into single-cell embryos. The overall phenotype of the perlecan morphants is similar to that evoked by null mutations or knockdown of VEGFR2, phospholipase Cγ-1, a major downstream target of VEGF/VEGFR angiogenic signaling, VEGFR2 receptor blockade by the small molecule SU5416, or by antisense knockdown of VEGFA. Thus, it is possible that perlecan is required for the proper targeting of VEGF to its cognate receptor during developmental angiogenesis.
FIGURE 2.
Pro- and anti-angiogenic activity of perlecan and endorepellin, respectively. (A,B) Effects of perlecan knockdown on the development of zebrafish embryonic vasculature. The vasculature is visualized in red (pseudocolor) and derives from the transgenic expression of GFP driven by the fli1 promoter specific for endothelial cells (A) Epifluorescence microscopy with 3D deconvolution of the trunk vessels from a zebrafish control embryo at 2 days post-fertilization (dpf). Note the correct formation of the dorsal aorta (DA), posterior cardinal vein (PCV), intersegmental vessels (ISV) and dorsal longitudinal anastomotic vessels (DLAV). (B) In contrast, the perlecan morphants exhibit relatively normal axial vessels, but significantly blunted ISVs and no DLAVs (arrows). Bar = 100 µm. (C–H) Double immunofluorescent micrographs of human endothelial cells following a 30 min-exposure to endorepellin using rhodamine-phalloidin to label actin (red) or antibodies against vimentin (green). Notice the endorepellin-evoked disruption of the actin cytoskeleton (F–H) in contrast to the preservation of the actin microfilaments in untreated endothelial cells (C–E). The arrows in panel H show bundles of actin filaments collapsed onto the plasmalemma, Bar = 5 µm.
In hepatoblastoma xenografts, VEGF is deposited in the same perivascular pattern as tumor-derived perlecan (36) and the vascular recovery following VEGF blockade by systemic delivery of soluble VEGFR1 and VEGFR2 is mediated by enhanced expression of perlecan at such locations. Concurrently, there is an increase in heparanase in the perivascular zones. Perlecan-bound VEGF might be dynamically regulated by heparanase-mediated release from the HS chains of perlecan and/or by proteolytic processing of perlecan protein core with ultimate release of domain I-associated HS/VEGF complexes in a similar way to that shown previously for domain I-associated FGF complexes (37). Thus, sequestration and release of perlecan-bound VEGF in the tumor microenvironment represents a mechanism for continuous vessel growth and tumor progression. The net result is a protracted activation of VEGFR2 which caused a sustained activation of the Akt pathway promoting survival and angiogenesis (36).
Interestingly, HSPGs can also act across cells or “in trans” (9), and specifically can potentiate in trans VEGFR-mediated angiogenesis (38). Arteries and arterioles are surrounded by mural cells, either vascular smooth muscle cells for large arteries and veins or pericytes for capillaries. Mural cell HSPGs, most likely including perlecan which is a major product of smooth muscle cells/pericytes, can transactivate VEGFR2 on endothelial cells by enhancing signal transduction and by facilitating the formation of receptor-ligand complexes on endothelial cells (38). Thus, perlecan occupies a central role in angiogenesis because it can potentially mediate not only the VEGF/VEGFR axis but also the transactivation of smooth muscle cells/pericytes during angiogenesis.
While the overwhelming majority of the reports supports a pro-angiogenic activity of the parent perlecan proteoglycan, other studies suggest the possibility that perlecan might inhibit tumor growth and angiogenesis (39). These apparently contradicting data could be reconciled by considering the fact that perlecan acts in a cell context-specific manner. In the vast majority of epithelial tumors (i.e., cancers), perlecan may be required for presenting FGF2 and VEGF to the expanding tumor vasculature, whereas in sarcomas perlecan might be inhibitory via the liberation of cryptic anti-angiogenic fragments (see next section).
ANTI-ANGIOGENIC PROPERTIES: CRYPTIC C-TERMINAL FRAGMENTS
During a search for perlecan binding partners using the yeast two-hybrid system and domain V of perlecan as the bait, we isolated a highly interactive cDNA clone which encoded the NC1 domain of collagen type XVIII (40) comprising the powerful anti-angiogenic fragment named endostatin. It was soon realized that domain V of the perlecan protein core harbored a powerful angiostatic activity as demonstrated by various in vitro and in vivo angiogenic assays; this region was renamed endorepellin to designate its intrinsic anti-endothelial activity (40). Endorepellin is composed of three laminin-like globular domains (LG1–LG3) interspersed by four EGF-like modules (Figure 1), and interacts specifically with the α2β1 integrin, an established receptor for collagen I, in platelets (41) and endothelial cells (42). In the latter, endorepellin triggers a signaling cascade that leads to disruption of the endothelial actin cytoskeleton (Figure 2 C–H) and thus to cytostasis (3,42–44). Using a proteomic approach, several key proteins involved in angiogenesis including β-actin were significantly down-regulated by exposing endothelial cells to recombinant endorepellin (45). Importantly, systemic delivery of human recombinant endorepellin to tumor xenograft-bearing mice causes a marked suppression of tumor growth and metabolic rate mediated by a sustained down-regulation of the tumor angiogenic network (46). Genetic analysis using siRNA-mediated block of endogenous α2β1 integrin or animals lacking the α2β1 integrin receptor have definitively shown that this is a key receptor for endorepellin —and thus for perlecan protein core— and have further demonstrated that endorepellin targets the tumor xenograft vasculature in an α2β1 integrin-dependent manner (47). Endorepellin might represent a member of the family of cryptic domains residing within larger parent molecules of the extracellular microenvironment that acts in a dominant negative manner.
Notably, the last laminin-like globular domain, LG3 (Figure 3), possesses most of the biological activity (42) and can be released from the parent molecule by BMP-1/Tolloid-like metalloproteinases (48) which recognize an ND dipeptide that is highly conserved across species including human, mouse, Drosophila and zebrafish (35). This highly-conserved region within the perlecan protein core together with the high conservation of BMP-1/Tolloid-like metalloproteinases suggests that liberation of LG3 might be of physiological importance. Mutations in LG3 molecules displaying lower or no affinity for calcium (48) disrupt LG3 angiostatic activity (Figure 3). It is noteworthy that the proximal two globular domains of endorepellin, LG1 and LG2, might be occupied by a number of high-affinity ligands such as dystroglycan and endostatin within basement membranes and on cell surfaces. In contrast, LG3 might be relatively accessible and thus likely to be released by partial proteolysis, a process that is common to most LG domains of laminin. Indeed, LG3 fragments with identical N-terminal residues (i.e., cleaved by BMP-1/Tolloid-like metalloproteinases) have been found in the urine of patients with end-stage renal failure (49) and chronic allograft nephropathy (50), and in the amniotic fluid of pregnant women with a marked increase in women with symptoms of premature rupture of fetal membranes (51) and those carrying trisomy 21, Down syndrome, fetuses (52). In addition, endorepellin fragments have been found in the media conditioned by apoptotic endothelial cells (53). In this case, the secreted LG3 interacts with the α2β1 integrin receptor of fibroblasts and triggers a signaling cascade that leads to activation of an anti-apoptotic pathway and potentially to a fibrogenic response (53).
FIGURE 3.
Endorepellin is cleaved by the BMP-1/Tolloid proteases, liberating the antiangiogenic LG3 domain. The illustration depicts a graphic illustration of endorepellin and a comparative model of LG3. Highlighted in magenta is a calcium ion shown to be vital for function. To the right are immunofluorescence images of endothelial cells stained for actin (red) and depicting the effects of LG3 (notice the disruption of actin cytoskeleton) and of an LG3 mutant which exhibits reduced affinity for calcium. Nuclei are stained in blue by DAPI.
We hypothesize that endorepellin/LG3 is liberated via partial proteolysis during tissue remodeling and cancer growth thereby representing an additional layer of control for angiogenesis, which also depends on the cellular context and specific integrin expression. In line with this fine tuning, circulating LG3 levels have been shown to be reduced in patients with breast cancer (54) suggesting that reduced titers might be a useful biomarker for cancer progression and invasion.
A COMMON THEME: RELEASE OF BIOACTIVE FRAGMENTS AND THEIR FINE BALANCE
A common theme is emerging from an increasing body of literature. The main postulate is that processing of extracellular matrix proteins is not a random event but is a guided and focused biological process that can affect either positively or negatively the growth of cells and, in particular, angiogenesis. For example, cathepsin L, a cysteine protease of the papain superfamily, cleaves collagen XVIII in the hinge region of the NC1 domain, thereby liberating endostatin, a strong anti-angiogenic factor (4). Efficient endostatin generation requires a moderately acidic pH, a typical feature of the tumor microenvironment. Interestingly, apoptotic endothelial cells secrete cathepsin L which, in turn, cleaves endorepellin near its C-terminal region thereby liberating endorepellin’s angiostatic LG3 domain (55). Thus, cathepsin L and BMP1/Tolloid-like proteases acting in concert could liberate LG3 from the perlecan associated with the cell surface or embedded within the basement membrane. Finally, cathepsin L has been recently shown to be a key enzyme required for the conversion of proheparanase into an active heparanase by specifically cleaving multiple sites within the linker region (56). Thus, differential expression of cathepsin L may have opposite effects on angiogenesis by generating either anti-angiogenic factors (endostatin and endorepellin’s LG3) or pro-angiogenic factors (FGF, VEGF, PDGF etc.) via heparanase-mediated cleavage of the HS chains of perlecan and collagen XVIII. The molecular understanding of this fine balance between pro- and anti-angiogenic activities will undoubtedly lead to a better treatment of cancer and other diseases where angiogenesis is prevalent.
AN ENDOREPELLIN-LIKE STRUCTURE IN AGRIN
Agrin, another basement membrane and synaptic HSPG, has an endorepellin-like domain at its C-terminus. This domain comprises three LG modules interspersed by three EGF-like repeats (5). Notably, endorepellin-like and LG3 fragments are generated from agrin by a specific serine protease, neurotrypsin (57). Neurotrypsin cleaves agrin at two homologous sites liberating a 90-kDa fragment and the C-terminal globular domain, LG3 (57). The release of cryptic fragments within agrin could promote interactions with other proteins and receptors that were inaccessible to full-length agrin. While there is no evidence that any of these modules affect angiogenesis, there is ample evidence that they play important biological roles and can also mediate signaling events propagated from surface receptors. For example, the endorepellin-like region of agrin is involved in binding to dystroglycan and integrins (5). In addition, the LG3 module of agrin signals through a synaptic receptor that has been recently identified as the Na+-K+-ATPase (58). Agrin LG3 inhibitory activity evokes membrane depolarization and increases action potential in neurons by interacting with the α3 subunit of the Na+-K+-ATPase, a member of the family of ion pumps (58). Elegant work by Rüegg and collaborators (59) has shown that a mini-agrin gene composed of the N-terminus (which binds to laminin γ1) and the C-terminal endorepellin-like structure (which binds to α-dystroglycan) can function as a biological linker and can ameliorate muscular dystrophy caused by mutation in the laminin α2 gene (59). Interestingly, chimeric proteins composed of the N-terminus of agrin and the mouse endorepellin can also have similar linker/stabilizing activity and rescue the dystrophic phenotype (59). Thus, it is conceivable that modules containing LG domains interspersed by EGF-like repeats, such as those present in perlecan and agrin, might have developed unique cell- and tissue-specific functions. Not surprisingly, these modules have been conserved for over 500 million years of evolution. Moreover, utilization of endorepellin-like structures which bind with high affinity to surface receptors such α-dystroglycan and α2β1 integrin might be utilized in the future for the treatment of diseases in which these two receptors are involved.
ADDITIONAL BIOLOGICAL PROPERTIES OF ENDOREPELLIN/LG3
In a different pathological setting, LG3 is released by apoptotic endothelial cells induced by serum starvation which leads to enhanced proteolytic processing of extracellular matrix constituents, including perlecan (53). Released LG3 causes a α2β1 integrin-dependent anti-apoptotic pathway in fibroblasts, which express high levels of this receptor, and this could have a potential effect on abnormal fibrogenic healing (53). This is another example of cell-specific context in which cryptic perlecan fragments might show a diverse effect. Indeed, in various fibrotic diseases, apoptosis of endothelial cells precedes the recruitment of fibroblasts, and thus release of LG3 could affect not only angiogenesis, but also the production of collagen and the overall fibrotic response.
Quantitative proteomic analysis of the pancreatic cancer secretome has shown that a fragment of perlecan encompassing endorepellin is increased approximately five fold relative to the secretome of normal pancreatic ductal cells (60), suggesting that endorepellin/LG3 might serve as a potential biomarker for pancreatic cancer. We have found LG3 abundantly secreted by human colon carcinoma cells (48) and a variety of transformed cells (unpublished observation). Thus, endorepellin/LG3 could be used as a biomarker for pancreatic cancer and other forms of cancer since LG3 is soluble, circulating in the blood and can be secreted into several biological fluids including urine and amniotic fluid. This makes detection of LG3 a feasible procedure with potential diagnostic and prognostic value.
A WORKING MODEL OF PERLECAN’S ROLE IN TUMOR ANGIOGENESIS
Extracellular matrix-derived signals control vascular morphogenesis and remodeling. Growth factors such as FGF and VEGF require not only their receptors for full biological activity, but they also require essential co-factors such as HS and, indirectly, the protein core of perlecan and other extracellular HSPGs. Thus, perlecan functions in targeting, storage and delivery of growth factors to their functional receptors. It has been recently proposed that complex molecules such as perlecan, which reaches 100–200 nm in length, could serve to cluster various ectodomains of transmembrane proteins, stabilize their interactions and thus create a stable signaling complex. During tumor progression, the vascular basement membrane undergoes constant remodeling and when heparanase is preponderant it could release growth factors from the HS chains of perlecan (Figure 4). These increased levels of growth factors together with the cofactor HS would activate their respective cognate receptors which, in turn, would activate the pro-survival activity of Akt and ultimately promote angiogenesis and tumor progression. Obviously, marked proteolysis would also generate a large number of growth factors and cytokines that are bound to the protein core including PDGF, FGF7, and FGF2. When proteolysis is somewhat “limited”, endorepellin and LG3 could be liberated in the tumor microenvironment to counteract the FGF/FGFR and VEGF/VEGFR2 axes: endorepellin interacts with the α2β1 integrin receptor and triggers a signaling cascade that leads to disruption of the endothelial cell actin cytoskeleton, inhibition of cell motility, and ultimately inhibition of angiogenesis and concurrent tumor suppression (Figure 4). This conceptual framework could be easily applied to other endogenous inhibitors of angiogenesis associated with the basement membrane, such as those derived from various basement membrane collagens.
FIGURE 4.
Working model showing the potential dual role of perlecan in tumor angiogenesis. Heparanase-mediated release of growth factors, primarily FGF2 and VEGF, bound to the heparan sulfate chains of perlecan would lead to sustained activation of FGF/FGFR and VEGFR2/Akt pathways, with consequent enhanced survival of endothelial cells and increased tumor angiogenesis and tumor progression. On the other hand, limited proteolysis of perlecan protein core could liberate endorepellin and LG3; this evokes a α2β1 integrin-dependent disruption of endothelial cell actin cytoskeleton, inhibition of endothelial cell migration, reduced angiogenesis and reduced tumor growth.
Understanding the balance between pro- and anti-angiogenic cues would be of great therapeutic potential in the future. Would blocking heparanase, for instance, be a suitable treatment for certain forms of highly vascularized cancers? What protease inhibitors would be most beneficial for tilting the balance toward a less vascularized or avascular condition? Should heparin mimetics be used in tumor therapy to cause the diffusion of growth factors away from the tumor cells? Would combination therapy work? These important questions can conceivably be answered in the near future after we elucidate the role each component exerts in the complex processes of vascular generation, regression and remodeling that occur during cancer evolution.
ACKNOWLEDGEMENT
We thank Angela McQuillan for help with the graphics, Jason Zoeller for providing the zebrafish figure, Charles Reed for providing the LG3 model, and Chris C. Clark for critical evaluation of this review. We apologize for not citing original work because of editorial restrictions regarding the number of references.
Footnotes
This work was supported in part by NIH grants RO1 CA39481, RO1 CA47282, and RO1 CA120975 (R.V.I.), NH&MRC Project Grant 512167 (J.M. & J.W.) and ARC Discovery Project Grant DP0557863 & ARC Linkage Grants LP0455407 and LX0667295 (J.W.)
Abbreviations: HS, heparan sulfate; HSPG, HS proteoglycan; FGF, fibroblast growth factor; FGFR, FGF receptor; VEGF, vascular endothelial growth factor; VEGFR1 and VEGFR2, VEGF receptor 1 and 2; PDGF, platelet-derived growth factor
REFERENCES
- 1.Hassell JR, Yamada Y, Arikawa-Hirasawa E. Role of perlecan in skeletal development and diseases. Glycoconj. J. 2003;19:263–267. doi: 10.1023/A:1025340215261. [DOI] [PubMed] [Google Scholar]
- 2.Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- 3.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nature Rev. Mol. Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 4.Marneros AG, Olsen BR. Physiological role of collagen XVIII and endostatin. FASEB J. 2005;19:716–728. doi: 10.1096/fj.04-2134rev. [DOI] [PubMed] [Google Scholar]
- 5.Bezakova G, Rüegg MA. New insights into the roles of agrin. Nature Rev. Mol. Cell Biol. 2003;4:295–308. doi: 10.1038/nrm1074. [DOI] [PubMed] [Google Scholar]
- 6.Arikawa-Hirasawa E, Watanabe E, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nature Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 7.Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 10.Merz DC, Alves G, Kawano T, Zheng H, Culotti JG. UNC-52/perlecan affects gonadal leader cell migrations in C.elegans hermaphrodites through alterations in growth factor signaling. Dev. Biol. 2003;256:173–186. doi: 10.1016/s0012-1606(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 11.Voigt A, Pflanz R, Schafer U, Jackle H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev. Dyn. 2002;224:403–412. doi: 10.1002/dvdy.10120. [DOI] [PubMed] [Google Scholar]
- 12.Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, Welsh CJR, McDermott S, Datta S. Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev. Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 13.Girós A, Morante J, Gil-Sanz C, Fairén A, Costell M. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev. Biol. 2007;7:29. doi: 10.1186/1471-213X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ornitz DM. FGFs,heparan sulfate and FGFRs: complex interactions essential for development. BioEssays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage. 2007;15:752–763. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc. Natl. Acad. Sci. USA. 2000;97:6722–6727. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinsella MG, Tran P-K, Weiser-Evens MCM, Reidy M, Majack RA, Wight TN. Changes in perlecan expression during vascular injury. Role in the inhibition of smooth muscle cell proliferaton in the late lesion. Arterioscler. Thromb. Vasc. Biol. 2003;23:608–614. doi: 10.1161/01.ATV.0000063109.94810.EE. [DOI] [PubMed] [Google Scholar]
- 18.Ghiselli G, Eichstetter I, Iozzo RV. A role for the perlecan protein core in the activation of the keratinocyte growth factor receptor. Biochem. J. 2001;359:153–163. doi: 10.1042/0264-6021:3590153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sher I, Zisman-Rozen S, Eliahu L, Whitelock JM, Maas-Szabowski N, Yamada Y, Breitkreutz D, Fusenig NE, Arikawa-Hirasawa E, Iozzo RV, Bergman R, Ron D. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J. Biol. Chem. 2006;281:5178–5187. doi: 10.1074/jbc.M509500200. [DOI] [PubMed] [Google Scholar]
- 20.Smith SML, West LA, Hassell JR. The core protein of growth plate perlecan binds FGF-18 and alters its mitogenic effect on chondrocytes. Arch. Biochem. Biophys. 2007;468:244–251. doi: 10.1016/j.abb.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, Whitelock J, Elkin M, Vlodavsky I, Hoffman MP. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development. 2007;134:4177–4186. doi: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- 22.Knox S, Merry C, Stringer S, Melrose J, Whitelock J. Not all perlecans are created equal. Interactions with fibroblast growth factor (FGF) 2 and FGF receptors. J. Biol. Chem. 2002;277:14657–14665. doi: 10.1074/jbc.M111826200. [DOI] [PubMed] [Google Scholar]
- 23.Kvist AJ, Nyström A, Hultenby K, Sasaki T, Talts JF, Aspberg A. The major basement membrane components localize to the chondrocyte pericellular matrix-a cartilage basement membrane equivalent? Matrix Biol. 2008;27:22–33. doi: 10.1016/j.matbio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng W-M, Baumgartner S. Perlecan and dystroglycan act at the basal side of the drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kvist AJ, Johnson AE, Mörgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszódi A, Fässler R, Sasaki T, Timpl R, Aspberg A. Chondroitin sulfate perlecan enhances collagen fibril formation. J. Biol. Chem. 2006;281:33127–33139. doi: 10.1074/jbc.M607892200. [DOI] [PubMed] [Google Scholar]
- 26.Smith SML, West LA, Govindraj P, Zhang X, Ornitz DM, Hassell JR. Heparan and chondroitin sulfate on growth plate perlecan mediate binding and delivery of FGF-2 to FGF receptors. Matrix Biol. 2007;26:175–184. doi: 10.1016/j.matbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Robinson CJ, Mulloy B, Gallagher JT, Stringer SE. VEGF165-binding sites within heparan sulfate encompass two highly sulfated domains and can be liberated by K5 lyase. J. Biol. Chem. 2006;281:1731–1740. doi: 10.1074/jbc.M510760200. [DOI] [PubMed] [Google Scholar]
- 28.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 29.Chen E, Stringer SE, Rusch MA, Selleck SB, Ekker SC. A unique role for 6-O sulfation modification in zebrafish vascular development. Dev. Biol. 2005;284:364–367. doi: 10.1016/j.ydbio.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Whitelock JM, Iozzo RV. Heparan sulfate: a complex polymer charged with biological activity. Chem. Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- 31.Fuki I, Iozzo RV, Williams KJ. Perlecan heparan sulfate proteoglycan. A novel receptor that mediates a distinct pathway for ligand catabolism. J. Biol. Chem. 2000;275:25742–25750. doi: 10.1074/jbc.M909173199. [DOI] [PubMed] [Google Scholar]
- 32.Hummel S, Osanger A, Bajari TM, Balasubramani M, Halfter W, Nimpf J, Schneider WJ. Extracellular matrices of the avian ovarian follicle. Molecular characterization of chicken perlecan. J. Biol. Chem. 2004;279:23486–23494. doi: 10.1074/jbc.M312694200. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, Liu B, Cao Y, Tryggvason K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- 34.Baker AB, Ettenson DS, Jonas M, Nugent MA, Iozzo RV, Edelman ER. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-β signaling pathway. Circ. Res. 2008;103:289–297. doi: 10.1161/CIRCRESAHA.108.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoeller JJ, McQuillan A, Whitelock J, Ho S-Y, Iozzo RV. A central function for perlecan in skeletal muscle and cardiovascular development. J. Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadenhe-Chiweshe A, Papa J, McCrudden KW, Frischer J, Bae J-O, Huang J, Fisher J, Lefkowitch JH, Feirt N, Rudge J, Holash J, Yancopoulos GD, Kandel JJ, Yamashiro DJ. Sustained VEGF blockade results in microenvironmental sequestration of VEGF by tumors and persistent VEGF receptor-2 activation. Mol. Cancer. Res. 2008;6:1–9. doi: 10.1158/1541-7786.MCR-07-0101. [DOI] [PubMed] [Google Scholar]
- 37.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin and heparanases. J. Biol. Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 38.Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellén L, Claesson-Welsh L. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev. Cell. 2006;10:625–634. doi: 10.1016/j.devcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Mathiak M, Yenisey C, Grant DS, Sharma B, Iozzo RV. A role for perlecan in the suppression of growth and invasion in fibrosarcoma cells. Cancer Res. 1997;57:2130–2136. [PubMed] [Google Scholar]
- 40.Mongiat M, Sweeney S, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 41.Bix G, Iozzo RA, Woodall B, Burrows M, McQuillan A, Campbell S, Fields GB, Iozzo RV. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the α2β1 integrin receptor. Blood. 2007;109:3745–3748. doi: 10.1182/blood-2006-08-039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bix G, Fu J, Gonzalez E, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Höök M, Reed CC, Iozzo RV. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J. Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bix G, Iozzo RV. Matrix revolutions: "tails" of basement-membrane components with angiostatic functions. Trends Cell Biol. 2005;15:52–60. doi: 10.1016/j.tcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Bix G, Iozzo RV. Novel interactions of perlecan: Unraveling perlecan's role in angiogenesis. Microsc. Res. 2008;71:339–348. doi: 10.1002/jemt.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoeller JJ, Iozzo RV. Proteomic profiling of endorepellin angiostatic activity on human endothelial cells. Proteome Sci. 2008;6:7. doi: 10.1186/1477-5956-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA, Cardi C, Thakur MT, Barker CA, Camphausen KC, Iozzo RV. Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism. J. Natl. Cancer Inst. 2006;98:1634–1646. doi: 10.1093/jnci/djj441. [DOI] [PubMed] [Google Scholar]
- 47.Woodall BP, Nyström A, Iozzo RA, Eble JA, Niland S, Krieg T, Eckes B, Pozzi A, Iozzo RV. Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem. 2008;283:2335–2343. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, Greenspan DS, Iozzo RV. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 49.Oda O, Shinzato T, Ohbayashi K, Takai I, Kunimatsu M, Maeda K, Yamanaka N. Purification and characterization of perlecan fragment in urine of end-stage renal failure patients. Clin. Chim. Acta. 1996;255:119–132. doi: 10.1016/0009-8981(96)06395-4. [DOI] [PubMed] [Google Scholar]
- 50.O'Riordan E, Orlova TN, Mendelev N, Patschan D, Kemp R, Chander PN, Hu R, Hao G, Gross SS, Iozzo RV, Delaney V, Goligorsky MS. Urinary proteomic analysis of chronic renal allograft nephropathy. Proteomics Clin. Appl. 2008;2:1025–1035. doi: 10.1002/prca.200780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thadikkaran L, Crettaz D, Siegenthaler MA, Gallot D, Sapin V, Iozzo RV, Queloz PA, Schneider P, Tissot JD. The role of proteomics in the assessment of premature rupture of fetal membranes. Clin. Chim. Acta. 2005;360:27–36. doi: 10.1016/j.cccn.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 52.Tsangaris GT, Karamessinis P, Kolialexi A, Garbis SD, Antsaklis A, Mavrou A, Fountoulakis M. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics. 2006;6:4410–4419. doi: 10.1002/pmic.200600085. [DOI] [PubMed] [Google Scholar]
- 53.Laplante P, Raymond M-A, Labelle A, Abe J-I, Iozzo RV, Hebért M-J. Perlecan proteolysis induces α2β1 integrin and src-family kinases dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation. J. Biol. Chem. 2006;281:30383–30392. doi: 10.1074/jbc.M606412200. [DOI] [PubMed] [Google Scholar]
- 54.Chang JW, Kang U-B, Kim DH, Yi JK, Lee JW, Noh D-Y, Lee C, Yu M-H. Identification of circulating endorepellin LG3 fragment: Potential use as a serological biomarker for breast cancer. Proteomics Clin. Appl. 2008;2:23–32. doi: 10.1002/prca.200780049. [DOI] [PubMed] [Google Scholar]
- 55.Cailhier J-F, Sirois I, Raymond M-A, Lepage S, Laplante P, Brassard N, Prat A, Iozzo RV, Pshezhetskyz AV, Hebért M-J. Caspase-3 activation triggers extracellular release of cathepsin L and endorepellin proteolysis. J. Biol. Chem. 2008 doi: 10.1074/jbc.M801164200. In press. [DOI] [PubMed] [Google Scholar]
- 56.Abboud-Jarrous G, Atzmon R, Peretz T, Palermo C, Gadea BB, Joyce JA, Vlodavsky I. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J. Biol. Chem. 2008;283:18167–18176. doi: 10.1074/jbc.M801327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephan A, Mateos JM, Kozlov SV, Cinelli P, Kistler AD, Hettwer S, Rülicke T, Streit P, Kunz B, Sonderegger P. Neurotrypsin cleaves agrin locally at the synapse. FASEB J. 2008;22:1861–1873. doi: 10.1096/fj.07-100008. [DOI] [PubMed] [Google Scholar]
- 58.Hilgenberg LGW, Su H, Gu H, O'Dowd DK, Smith MA. α3Na+/K+-ATPase is a neuronal receptor for agrin. Cell. 2006;125:359–369. doi: 10.1016/j.cell.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 59.Meinen S, Barzaghi P, Lin S, Lochmüller H, Rüegg MA. Linker molecules between laminins and dystroglycan ameliorate laminin-α2-deficient muscular dystrophy at all disease stages. J. Cell Biol. 2007;176:979–993. doi: 10.1083/jcb.200611152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grønborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins MG, Maitra A, Pandey A. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell. Proteom. 2006;5:157–171. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]