Abstract
Objective
The role of sphingosine-1-phosphate (S1P) receptors in acute vascular injury and smooth muscle cell (SMC) phenotypic modulation is not completely resolved.
Methods and Results
S1P receptor antagonists were used to test the hypothesis that specific S1P receptor subtypes differentially regulate SMC phenotypic modulation. In response to acute balloon injury of the rat carotid artery, S1P1/S1P3 receptor mRNA levels were transiently increased at 48 hours whereas S1P2 receptor expression was decreased. S1P2 expression was reinduced and increased at 7 to 10 days postinjury. Daily intraperitoneal injection of the S1P1/S1P3 antagonist VPC44116 decreased neointimal hyperplasia by ≈50%. In vitro, pharmacological inhibition of S1P1/S1P3 receptors with VPC25239 attenuated S1P-induced proliferation of rat aortic SMCs. Conversely, inhibition of S1P2 with JTE013 potentiated S1P-induced proliferation. Inhibition of S1P1/S1P3 resulted in S1P-induced activation of the SMC differentiation marker genes SMα-actin and SMMHC, whereas inhibition of S1P2 attenuated this response. S1P2-dependent activation of SMα-actin and SMMHC was shown to be mediated by L-type voltage-gated Ca2+ channels and subsequent RhoA/Rho kinase– dependent SRF enrichment of CArG box promoter regions.
Conclusion
Results provide evidence that S1P1/S1P3 receptors promote, whereas S1P2 receptors antagonize, SMC proliferation and phenotypic modulation in vitro in response to S1P, or in vivo after vascular injury.
Keywords: lipids, pharmacology, restenosis, calcium, phenotypic modulation
The phenotypic state of the vascular smooth muscle cell (SMC) plays a critical role in blood vessel development and several major human diseases, including the pathogenesis of atherosclerosis and restenosis after vascular injury.1 What makes the SMC unique compared to other muscle cell types is its ability to rapidly undergo phenotypic modulation from a contractile quiescent phenotype to a highly synthetic or migratory phenotype. For example, in response to vascular injury, differentiated SMCs downregulate expression of SMC-specific contractile genes, proliferate, migrate into the vessel intima (neointimal hyperplasia), and then reestablish the contractile phenotype once the injury is repaired. A hallmark of this SMC phenotypic plasticity is transcriptional repression of SMC-specific differentiation genes, such as SMα-actin, smooth muscle myosin heavy chain (SMMHC), and SM22α.2 Evidence from our laboratory and others has firmly established that CArG box [CC(A/T)6GG] DNA sequences present within the promoters of SMC genes play a pivotal role in controlling their transcription.1,3 CArG boxes bind serum response factor (SRF), which requires myocardin or myocardin-related transcription factors (MRTFs).4–7 Many growth factors and cytokines implicated in SMC phenotypic modulation have been shown to regulate this process, including platelet-derived growth factor (PDGF), angiotensin-II, transforming growth factor beta (TGF-β), and recently oxidized phospholipids.1,3,8 Moreover, recent studies have also shown that signaling through ion channels can positively and negatively regulate SMC differentiation marker genes in response to growth factor stimulation and vascular injury.9 For example, activation L-type voltage-gated Ca2+ channels (VGCC) increases SRF enrichment of CArG boxes through RhoA/rho kinase, whereas activation of intermediate conductance Ca2+-activated K+ channels (IKCa) suppress SMC differentiation marker genes.10,11
Sphingosine-1-phosphate (S1P) is a bioactive phospholipid with diverse cellular functions mediated by 5 G protein–coupled receptors (S1P1–5).12,13 Treatment of SMCs with S1P has functions that range from Ca2+-dependent contraction to proliferation and migration. S1P has also been shown to increase expression of the SMC differentiation marker genes SMα-actin, SMMHC, and SM22α through a RhoA/MRTF-dependent pathway.14 These diverse functional responses to S1P might be explained by the differences in relative S1P receptor isoform expression patterns in various cultured SMC lines compared to intact blood vessels and SMCs from various vascular beds. For example, studies by Kluk and Hla showed that rat pup intimal SMCs expressed greater mRNA levels of S1P1 than adult medial SMCs and had a greater proliferative response to S1P.15 This distinction is important because S1P1, S1P2, and S1P3 are coupled to different and opposing signaling cascades. For example, S1P1 is coupled to Gi and has been shown to activate extracellular signal regulated kinase (ERK), PI3K, and Rac whereas S1P2 is coupled to Gi/o, Gq, and G12/13 and signals in part through JNK and Rho (reviewed in13). Activation of S1P2 is thought to directly oppose S1P1-induced proliferation and migration through a Rho-dependent pathway and decreased Rac.16–18
Our understanding of true S1P receptor isoform expression levels, though, is challenged by the fact that there are few commercially available antibodies to these receptors and we must rely heavily on mRNA expression profiles. That being said, mice genetically null for specific S1P receptor isoforms have yielded valuable insight into the functional role of these receptors in SMCs in vivo. For example, S1P1 null mice die E12.5 to 14.5 and have impaired homing of SMCs to developing blood vessels, suggesting that S1P1 is critical for vascular SMC migration in vivo.19 In contrast, exciting recent studies by Reidy and colleagues revealed that carotid neointimal hyperplasia is increased in response to ligation in mice that are null for the S1P2 receptor and that mouse lines with higher levels of S1P1 mRNA, ie, FVB mice, show increased neointimal hyperplasia compared to C57/Bl6 mice.20,21
Taken together, the general paradigm that has emerged is that S1P1 regulates SMC proliferation and migration whereas S1P2 has opposing actions. However, given observations by Proia et al19,22 that S1P receptors play a critical role in vascular development, one must also consider the possibility that enhanced neointimal formation in S1P2 null mice may also be attributable to secondary adaptive changes in gene expression. In addition, as yet no studies have been reported to address whether S1P receptor subtypes are differentially expressed in response to vascular injury, nor have studies defined cellular and molecular mechanisms whereby different S1P subtypes mediate their effects on SMC differentiation or phenotypic switching. The present studies use a series of novel selective S1P analogues23–25 that function as selective S1P receptor agonists or antagonists to address each of these key questions.
Methods
For detailed methods, see online supplement (available online at http://atvb.ahajournals.org).
Rat aortic SMCs were isolated and cultured (passage 11 to 15) as previously described.11 For all experiments, SMCs were grown to full confluence and growth arrested in serum-free media before stimulation. Sphingosine-1-phosphate (Avanti Polar Lipids); JTE013 (Tocris); VPC44116, VPC01091, VPC25239 were synthesized at the University of Virginia23–25; Y27632 gift from Mitsubishi Pharma Corp, Koyata, Japan; (s)FTY720 a gift from Novartis. SMCs were transfected with plasmids using FuGENE6 (Roche) when the cells were ≈80% confluent. For vascular injury studies, male Sprague-Dawley rats (350 to 400 g) were anesthetized, and acute injury to the left common carotid artery was made with a 2F Fogarty balloon catheter as described previously.26 Total RNA was prepared from cultured rat aortic SMCs and tissues using TRIzol reagent (Invitrogen), cDNA synthesized using the iScript cDNA Synthesis Kit (BioRad) and real-time polymerase chain reaction (PCR) analysis (iCycler, BioRad) was performed on cDNA using SybrGreen, as previously described.11,27 Chromatin immunoprecipitation (ChIP) was performed as previously described.11,27 Quantification of protein:DNA interaction/enrichment was determined by the following 2Ct(Ref)-Ct(IP)-2Ct(Ref)-Ct(No antibody control). Statistical significance among treatment groups was confirmed with a 1-way ANOVA when appropriate. Statistical significance between specific groups was determined by a posthoc multiple comparison Student-Newman-Keuls test (P<0.05).
Results
S1P Receptors Are Differentially Expressed After Acute Balloon Injury of the Rat Carotid Artery
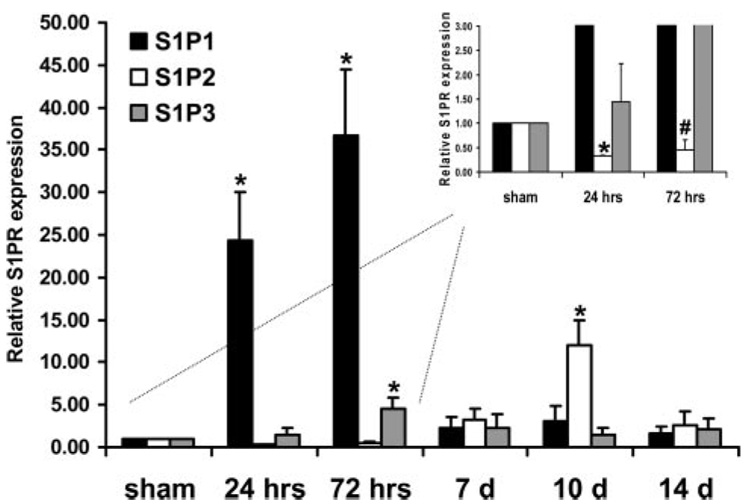
Previous studies have shown that expression profiles of multiple receptors can be rapidly altered after acute vascular injury. For example, studies by Majesky et al showed that the platelet-derived growth factor (PDGF) receptor subunit β is rapidly decreased during the first 24 hours of the initial response to acute balloon injury but reinduced 48 hours to 1 week later.28 We used the rat carotid artery balloon injury model26 to test the hypothesis that S1P receptors are differentially regulated after injury at 24 hours, 72 hours, and 7, 10, and 14 days. There were no significant differences in basal S1P1, S1P2, and S1P3 mRNA levels after sham operation (supplemental Figure I), and S1P4 and S1P5 mRNA levels were indistinguishable from no RT controls (data not shown). S1P1 and S1P3 receptor mRNA levels were significantly and transiently increased 36.68±7.86- and 4.57±1.25-fold, respectively, 24 to 72 hours postinjury and restored to control levels by 7 days (Figure 1). S1P2 receptor mRNA levels were reduced by ≈55% (P<0.05; Figure 1, inset) after acute injury at 24 hours but restored to normal levels by 72 hours and increased 12.07±2.86-fold 10 days after injury (P<0.05).
Figure 1.
S1P receptor mRNA levels are differentially expressed after acute balloon injury of the rat carotid artery. Temporal expression analysis by real-time RT-PCR of endogenous S1P1, S1P2, and S1P3 in balloon-injured carotid arteries. S1PR expression was normalized to 18S rRNA expression in the injured and uninjured contralateral control vessel. Each time point represents the mean±SE of the injured (S1PR:18S) vessel normalized to the uninjured (S1PR:18S) vessel (n=5 to 6 animals per time point). Inset, Higher magnification of 0, 24, and 72 hour time points to empha-size reduction in S1P2 mRNA after acute injury. *P<0.05 when compared to time zero. #P=0.09.
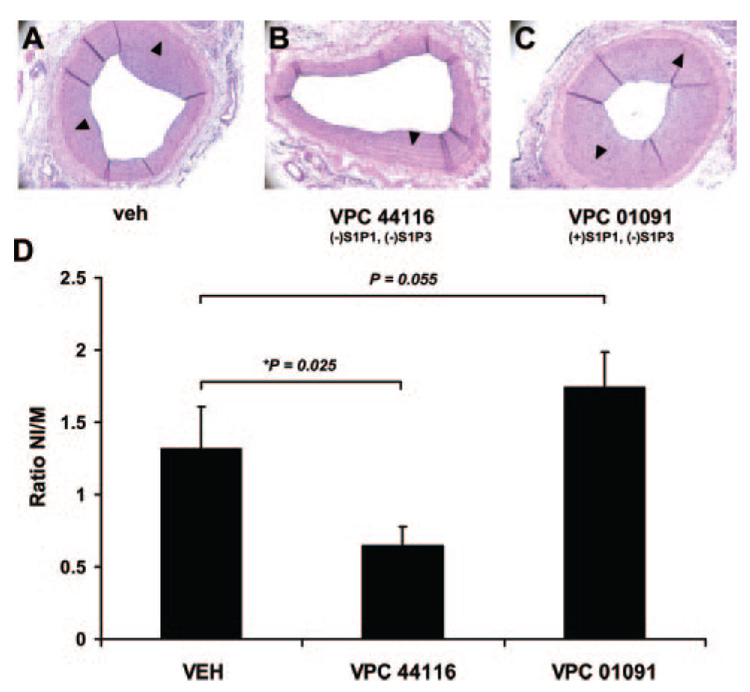
Treatment With VPC44116, an S1P1 Antagonist, Attenuates Neointimal Hyperplasia After Acute Balloon Injury of the Rat Carotid Artery
The S1P receptor mRNA expression profiles presented in Figure 1 are consistent with the concept that the S1P1 and S1P3 receptor might be critical for the onset of SMC proliferation, which occurs primarily during the first 48 to 72 hours in this model.26 Therefore, animals were treated daily I.P. with VPC44116 (10 mg/kg) 1 day before and 14 days after balloon injury. VPC44116 is a meta-substituted arylamide phosphonate discovered to be an antagonist at S1P1 and S1P3.23 In vivo, VPC44116 has been shown to block S1P receptor agonist-induced lymphonpenia,23 and this compound has a half life of approximately 3.1 hours in rats (unpublished data, Kevin Lynch, 2006) VPC44116 treatment reduced neointimal hyperplasia by ≈50% (P<.025; Figure 2). Medial areas in vehicle (0.1±0.05 mm2) and VPC44116 (0.12±0.06) were not different after vascular injury; neointimal areas in vehicle and VPC44116 were 0.132±0.04 mm2 and 0.065±0.03 mm2, respectively. Of interest, treatment with VPC01091 1 day before and 7 days after injury I.P. (10 mg/kg) showed a trend for increased neointimal hyperplasia at 14 days postinjury, although not significant (P=0.055). VPC01091 is a compound with S1P1 agonist activity and S1P3 antagonist activity and has been shown to cause lymphopenia in vivo.23
Figure 2.
Treatment with VPC44116, an S1P1 antagonist, attenuates neointimal hyperplasia after acute balloon injury of the rat carotid artery. Representative cross sectional histological images of carotid artery 14 days after balloon injury in vehicle-treated (A, veh), VPC44116-treated (B, S1P1/S1P3 antagonist), and VPC01091 (S1P1 agonist/S1P3 antagonist). C, Quantitative analysis of neointimal hyperplasia plotted as the ratio neointima (NI) area to medial (M) area. n=6 for all treatment groups. Arrow heads point to the IEL.
Pharmacological Inhibition of Select S1P Receptors Differentially Regulates S1P-Induced SMC Proliferation
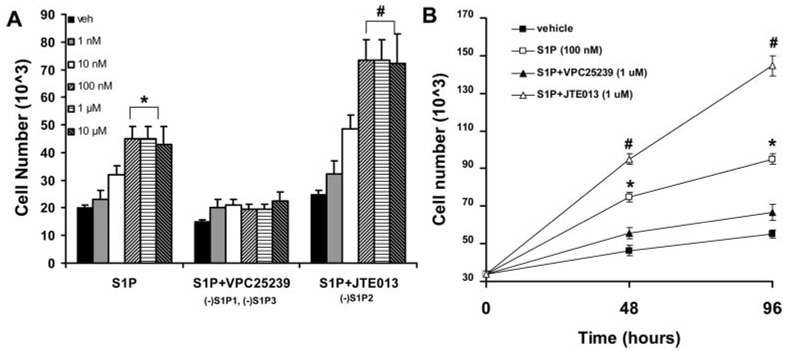
To further understand the mechanism by which S1P receptors regulate SMC phenotypic modulation, we used several selective S1P receptor antagonists in in vitro experiments. We first assessed whether these receptor antagonists could alter the S1P-induced SMC proliferation. Rat aortic SMCs were grown to 100% confluence and growth arrested for 72 hours before treatment with S1P. S1P treatment induced SMC proliferation in a dose-dependent manner (Figure 3). SMCs from various vascular beds and differing mouse strains express different mRNA levels of S1P receptors. Under these specific cell culture conditions, quantitative real-time PCR revealed that rat aortic SMCs expressed equivalent mRNA levels of S1P1, S1P2, and S1P3 but no expression of S1P4 and S1P5 (supplemental Figure I). This does not rule out that endogenous S1P receptor proteins levels are different; however, there are no commercially available antibodies that meet our criteria for S1P receptor antibody selectivity. Pretreatment of SMCs with VPC25239 (1.0 µmol/L), an S1P1 and S1P3 antagonist with similar affinity for both receptors and no activity at S1P2,24 attenuated S1P-induced proliferation in a dose-dependent manner (Figure 3A). Conversely, pretreatment of cells with JTE013 (1.0 µmol/L), an S1P2 receptor antagonist with IC50 = ≈ 20 nmol/L (IC50 values >10 µmol/L at S1P1/S1P3),29 potentiated S1P-induced SMC proliferation in a dose-dependent manner. The enhanced proliferation response in the presence of VPC25239 and blunted proliferation response in the presence of JTE013 were sustained out to 96 hours (Figure 3B). Taken together, pharmacological inhibition of S1P1 and S1P3, not S1P2, prevents S1P-induced SMC proliferation.
Figure 3.
Pharmacological inhibition of S1P1 and S1P3, not S1P2, prevents S1P-induced SMC proliferation. Rat aortic SMCs were serum-starved for 72 hours and pretreated with vehicle (veh), VPC25239 (1.0 µmol/L, S1P1/S1P3 antagonist), or JTE013 (1.0 µmol/L, S1P2 antagonist) for 30 minutes followed by S1P treatment. Cell proliferation was assessed by changes in cell number. A, Dose response to S1P in the presence of veh, VPC25239, or JTE013 after 48 hours of stimulation with S1P. B, Proliferation response over 96 hours, media/reagents were changed after 48 hours. *S1P treatment > veh or S1P+VPC25239 (P<0.05). #S1P+JTE013 > veh, S1P, or S1P+VPC25239 (P<0.05).
Differential Regulation of SMC Differentiation Marker Genes by S1P Receptors
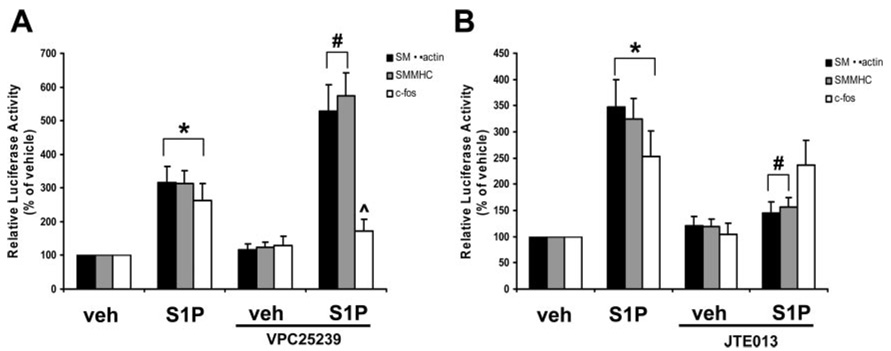
A hallmark of SMC phenotypic modulation associated with acute vascular injury is transient suppression of SMC differentiation marker genes that define the contractile phenotype; these genes include SM α-actin, SMMHC, and SM22α.1,2 Recent studies by Lockman et al showed that treatment of rat aortic SMCs with S1P increased expression of the SMC differentiation marker genes SM α-actin, SM22α, and SMMHC.14 We repeated these studies by testing the effects of S1P on the activity of a series of promoter-luciferase constructs: SM α-actin (−2.6/+2.8 kb), −125 SM α-actin (−125 bp/+2.8 kb), SMMHC (−4.2/≈ + 11.6 kb), SM22α (−447/+89 bp), c-fos (−356/+109 bp), and ACLP (−2502/+176 bp). In supplemental Figure IIA, treatment of SMCs with S1P resulted in a dose-dependent increase in promoter activity of SM α-actin, SM22α, and SMMHC, as well as promoter activity of c-fos. The non–CArG-mediated ACLP promoter construct showed no changes in promoter activity in response to S1P, suggesting that CArG cis-regulatory elements, which bind SRF, may play a critical role in the response to S1P (addressed below). Treatment of SMCs with S1P also increased SMα-actin and SMMHC mRNA but had no effect on the SRF cofactor myocardin (supplemental Figure IIB).Pretreatment of SMCs with the S1P1/S1P3 antagonist, VPC25239 (1.0 µmol/L), potentiated S1P-induced SMα-actin and SMMHC promoter activity, not c-fos (Figure 4A). Conversely, blocking S1P2 with JTE013 (1.0 µmol/L) pretreatment resulted in S1P-induced suppression of SMα-actin promoter activity, with little effect on c-fos promoter activity (Figure 4B). We next performed cotransfection experiments overexpressing S1P1 or S1P2 with the SMα-actin promoter reporter construct. Overexpression of S1P1 attenuated whereas overexpression of S1P2 potentiated S1P-induced activation of SMα-actin luciferase (supplemental Figure III). Of interest, treatment of SMCs FTY720 (fingolimid), a drug that, after phosphorylation, is an agonist at S1P1, S1P3, S1P4, and S1P5, not S1P2, resulted in suppression of SMC differentiation marker gene promoter activity (supplemental Figure IV). Taken together, these pharmacological results and overexpression studies show that activation of the S1P1 and S1P3 receptors suppress genes that define the contractile SMC phenotype whereas the S1P2 receptor promotes SMC differentiation. Moreover, c-fos is differentially regulated by S1P receptors.
Figure 4.
S1P differentially regulates SMC differentiation marker gene expression and c-fos by select S1P receptors. A and B, Transiently transfected SMCs were pretreated with VPC25239 (1.0 µmol/L, S1P1/S1P3 antagonist) or JTE013 (1.0 µmol/L, S1P2 antagonist) and stimulated with S1P (1.0 µmol/L) for 24 hours. Pharmacological inhibition of the S1P1 and S1P3 receptors potentiated S1P-induced activation of the SMα-actin and SMMHC promoter-luciferase construct whereas inhibition of the S1P2 receptor prevents S1P-induced activation of SMα-actin and SMMHC promoter-luciferase activity. *P<0.05 compared to veh; #P<0.05 compared to S1P treatment.
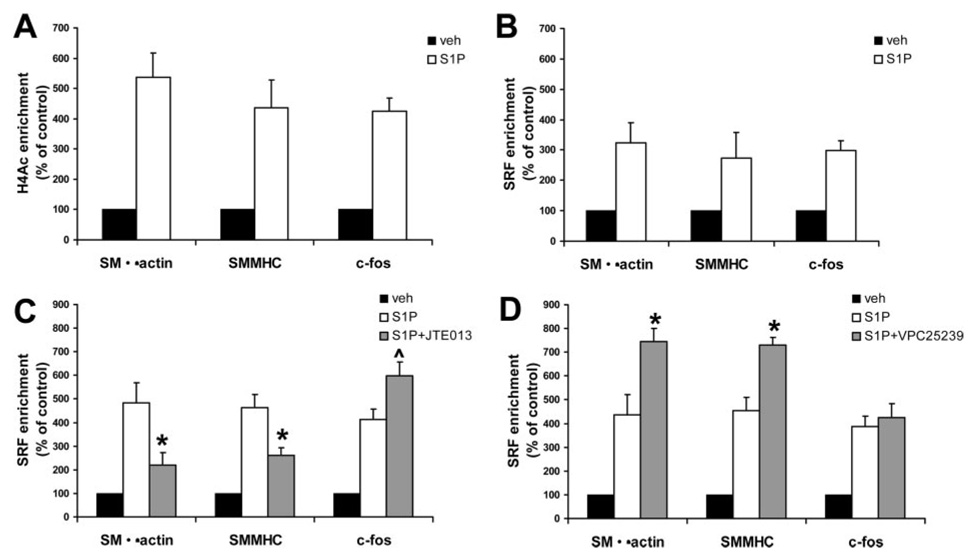
S1P2 Mediates Increased H4Ac and SRF Binding to CArG Promoter Regions Within Intact Chromatin
Multiple SMC differentiation marker gene promoters contain 2 or more CArG boxes which bind SRF (serum response factor) that are required for promoter activity in vitro and in vivo, whereas c-fos contains 1 CArG in its promoter.1,3 We recently showed that histone H4 acetylation (H4Ac) of these CArG promoter regions is required for SRF binding both in vitro and in vivo.27 Although work by Lockman et al showed that S1P did not induce SRF enrichment of the SMα-actin CArG B element by EMSA,14 it is not clear whether S1P alters SMC differentiation marker chromatin structure and SRF binding at the endogenous chromatin level in an intact SMC. Moreover, there is no evidence linking S1P receptors to alterations in histone modifications and SRF interactions within CArG regions of SMC differentiation marker gene promoters in intact chromatin. Therefore, quantitative ChIP assays were used to directly test whether S1P treatment altered SRF enrichment of CArG-containing regions in the endogenous SMα-actin, SMMHC, or c-fos. S1P treatment increased H4Ac and SRF enrichment of the CArG-containing regions in the SMα-actin, SMMHC, and c-fos endogenous promoters (Figure 5A and 5B, respectively). S1P-induced SRF enrichment was significantly reduced by pretreating cells with the S1P2 antagonist JTE013 (Figure 5C). However, blocking S1P2 had no effect on SRF enrichment of the c-fos CArG promoter region. Subsequent studies tested whether blocking S1P1 and S1P3 with VPC25239 effected S1P-induced SRF enrichment of these promoters. Indeed, blocking S1P1 and S1P3 increased S1P-induced SRF enrichment of the SMα-actin and SMMHC promoter but did not increase the c-fos promoter over control levels (Figure 5D). Treatment of cells with either VPC25239 or JTE013 did not effect basal H4Ac or SRF enrichment of these promoters (data not shown).
Figure 5.
Inhibition of the S1P1 and S1P3 receptors increases S1P-induced H4Ac and SRF enrichment of the SMα-actin and SMMHC CArG promoter region. Quantitative chromatin immunoprecipitation (ChIP) assays were performed to determine the effects of S1P histone H4Ac (A) and SRF (B) enrichment of endogenous promoter regions containing CArG cis-regulatory elements. SRF enrichment of CArG regions is expressed as percent of control (vehicle-treated cells) ±SEM. C and D, ChIP assays on SMCs pretreated with JTE013 (S1P2 antagonist) or VPC25239 (S1P1/S1P3 antagonist). *Significant difference from S1P alone, P<0.05. P̂=0.11 when compared to S1P alone.
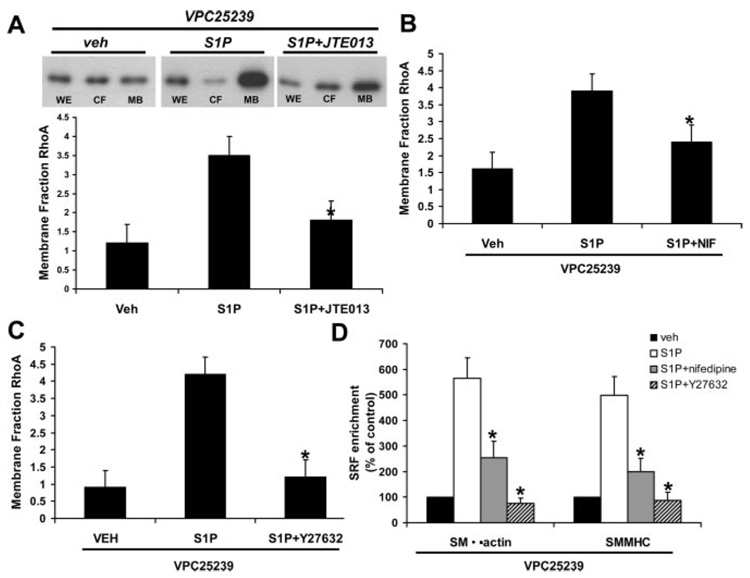
S1P2 Signals Through L-Type VGCCs and ROK to Regulate SRF Binding to Intact Chromatin
Activation of the RhoA pathway in smooth muscle by agonists leads to RhoA dissociation from Rho-GDI, nucleotide exchange of GTP for GDP, and translocation of RhoA and Rho kinase (ROK) from the cytosol to the membrane fraction to initiate signaling cascades. We previously showed that depolarization-induced activation of L-type VGCCs resulted in a ROK-dependent enrichment of SRF to CArG promoter regions,11 and S1P has been shown to modulate human airway SMC contraction by activating L-type VGCCs.30 Moreover, several studies have shown S1P can activate ROK.13 In Figure 6, cells were pretreated with VPC25239 to block S1P1/S1P3 and then stimulated with (1) S1P ± JTE013 (1 µmol/L), S1P2 antagonist, (2) S1P ± nifedipine (1.0 µmol/L), an L-type voltage-gated Ca2+ channel (VGCC) blocker, or (3) S1P ± Y27632 (10 µmol/L), a ROK inhibitor. Pretreatment with VPC25239 did not alter RhoA translocation compared to vehicle alone (data not shown). In the presence of VPC25239, S1P induced a robust induction of RhoA translocation to the membrane that was blocked by JTE103 (Figure 6A). Blocking either L-type VGCCs (Figure 6B) or ROK (Figure 6C) prevented S1P-induced translocation of RhoA from the cytosolic fraction (CY) to the MB fraction (detergent-soluble particulate fraction) compared with the vehicle-treated MB. In Figure 6D, we show that blocking S1P1 and S1P3 with VPC25239 in the presence of nifedipine or the ROK inhibitor Y27632 prevented S1P-induced SRF enrichment of the SMα-actin and SMMHC CArG promoter regions. Taken together, these results suggest that activation of the S1P2 receptor mediates SRF enrichment of SMα-actin and SMMHC CArG promoter regions via L-type VGCC activation and RhoA/ROK signaling.
Figure 6.
S1P2 signals through L-type VGCCs and ROK to regulate SRF binding to intact chromatin. SMCs were pretreated VPC25239 to block S1P1/S1P3 for 60 minutes before treatment with S1P. Western blot for RhoA was performed on whole cell extract (WE, loading control), cytosolic (CY), and membrane detergent-soluble particulate fractions (MB) and densitometry analysis performed. A, SMCs were stimulated with S1P for 12 hours in the presence or absence of the S1P2 antagonist JTE013 (30 minutes pretreatment, n=4). B, SMCs were stimulated with S1P for 12 hours in the presence or absence of the L-type voltage-gated Ca2+ channel blocker nifedipine (1 µmol/L, 30 minutes pretreatment, n=3). C, SMCs were stimulated with S1P for 12 hours in the presence or absence of the Rho kinase inhibitor Y27632 (10 µmol/L, pretreatment 30 minutes, n=3). *Significant difference in RhoA translocation to MB compared with vehicle and P<0.05. D, ChIP assays for SRF enrichment of SMα-actin and SMMHC CArG promoter regions. SMCs were pretreated with VPC25239 and nifedipine (1 µmol/L) or Y27632 (10 µmol/L) for 30 minutes and then stimulated with S1P (N=3). *Significant difference compared to S1P treatment, P<0.05.
Discussion
The major focus of the studies presented herein was to determine whether S1P receptors are differentially regulated after acute vascular injury and whether we could use this information to target select S1P receptors to inhibit neointimal hyperplasia in response to vascular injury. Recent studies by Reidy and colleagues20,21 provided evidence showing that S1P2 null mice have enhanced neointimal hyperplasia in response to vascular injury whereas mouse strains with higher expression levels of S1P2 show diminished neointima formation. Their results were the first to suggest that different S1P receptor subtypes mediate opposing effects after vascular injury in vivo. Results of the present studies confirm and extend these findings in several key aspects. First, our results provide evidence that S1P1 and S1P3 are transiently induced after acute vascular injury which is concomitant with a decrease in S1P2. Second, we were able to attenuate neointimal hyperplasia by treating animals with an S1P1/S1P3 selective antagonist before and after acute injury. These results are important in that they indicate that the major effects observed by Reidy and colleagues were not secondary to adaptive changes in gene expression. Moreover, results clearly demonstrate the feasibility of using selective S1P receptor antagonists or agonists for treating neointimal hyperplasia. Finally, given that transcriptional repression of SMC cell differentiation marker genes is a hallmark response in SMC phenotypic modulation associated with vascular injury, we also wanted to define cellular and molecular mechanisms whereby different S1P subtypes mediate their effects on SMC differentiation or phenotypic switching. We show that signaling through the S1P2 receptor increases expression of the SMC differentiation marker genes whereas S1P1/S1P3 suppress these genes. Furthermore, molecular regulation of these genes by S1P2 is dependent on VGCCs and RhoA/ROK signaling which results in increased binding of SRF to CArG cis regulatory elements in the endogenous SMα-actin and SMMHC promoters.
Over the past ≈8 years, studies detailing the role of S1P in vascular function suggest that the variety of effects mediated by S1P through multiple receptor isoforms in SMCs could provide an abundance of therapeutic targets for angiogenesis, atherosclerosis, and vascular injury.12,13,31 Results of the present studies and findings by Reidy and colleagues suggest that an S1P2 receptor agonist alone or in combination with S1P1/3 antagonist may be effective in inhibiting intimal hyperplasia. However, no selective S1P2 agonist has yet been developed to our knowledge. In addition, treatment with an S1P2 agonist alone may not be effective given our observations that S1P2 receptor mRNA levels are decreased after vascular injury (Figure 1, inset). Interestingly, Kuel et al recently showed that the investigational drug FTY720 (fingolimid), a potent immunosuppressant which is an S1P1, S1P3, S1P4, S1P5 agonist, attenuates experimental athero-sclerosis development in ApoE−/− mice placed on a Western-type diet for 20 weeks.32 At first, this result might seem difficult to reconcile with observations in our studies showing that S1P1/S1P3 antagonists inhibit rather than promote injury-induced neointimal hyperplasia. However, there are fundamental differences in lesion formation after acute vascular injury versus during atherosclerosis that may account for these differences. For example, neointima lesions that form after acute vascular injury are predominantly composed of SMCs whereas lesions that develop in ApoE−/− mice are composed of macrophages, other inflammatory cells, and SMCs. Indeed, FTY720 treatment inhibited monocyte recruitment and decreased macrophage content in lesions,32 and others have shown that treatment of endothelial cells with SEW2871, a selective S1P1 agonist, decreases tumor necrosis factor (TNF)-alpha induced monocyte recruitment.33 Taken together, results suggest that S1P receptor therapeutic strategies for inhibiting development of atherosclerosis or development of instent restenosis are likely to work by targeting different cell types but paradoxically target the same S1P1 receptor subtype. Moreover, understanding the temporal expression profiles of S1P receptors after vascular injury versus the progression of atherosclerosis and in multiple cell types will be critical in regulating these disease processes. For example, gene analysis of human blood vessels showed that S1P1 was increased in the neointima of human lesions identified with instent restenosis compared normal/nondiseased blood vessels.34 The cell type(s) that contributed to this change in expression were not identified.
An unresolved but very important question raised by the present studies is to define the source of S1P which plays a role in mediating neointimal hyperplasia after acute vascular injury. The most logical source is the blood or platelets which are activated at the site of vascular injury.35,36 Also, in humans, the severity of atherosclerosis is positively correlated with serum S1P levels.37 Thus, one possibility is that S1P is readily available for SMCs once the endothelium has been denuded by balloon injury and platelets home to the injury site. However, an alternative possibility is that SMCs may also be a source of S1P. There are several indications that this may be the case. Sphingosine arises when sphingo-myelin is broken down to ceramide and subsequent ceramide hydrolysis by ceramidases. Sphingosine is then phosphorylated to S1P by sphingosine kinase (Sphk1 or Sphk2).38 Forced expression of a dominant-negative isoform of SphK1 in SMCs of resistance arteries inhibited the development of tone and myogenic responses, suggesting that there is basal vessel production and release of S1P that maintains vessel homeostasis.39 Furthermore, in cultured human coronary artery SMCs, Sphk1 is required for growth and PDGF-BB increases Sphk1 mRNA and protein levels. Indeed, preliminary results from our laboratory (Wamhoff, unpublished data, 2006) suggest that Sphk1 mRNA is increased and sustained after acute vascular injury. Thus, future studies are required to test the hypothesis that changes in endogenous S1P generation is necessary for S1P-mediated SMC phenotypic modulation. A challenge to testing this hypothesis will be the difficult task of measuring S1P levels in intact blood vessel after acute vascular injury and distinguishing S1P stored in platelets from S1P being produced by SMCs.
A number of distinct SMC phenotypes have been described based on gene expression profiles or cellular function. These include a contractile phenotype, a proliferative phenotype, an inflammatory phenotype, and the migratory phenotype.1 We have presented data herein and discussed results from others clearly implicating S1P1 in SMC proliferation and migration, with S1P2 having opposing effects. That being said, it is still unclear which S1P receptor isoforms regulate SMC differentiation marker genes that define the contractile phenotype. Previous studies by Lockman et al have shown that treating SMCs with S1P increased SMC proliferation and paradoxically increased expression of SMα-actin, SMMHC, and SM22α.14 Results of the present studies have confirmed these findings (Figure 3 and Figure 4). Using a pharmacological approach, we further deduced that S1P1/S1P3 negatively regulate these genes and positively regulate proliferation whereas S1P2 opposes this action. Of interest, the role of ion channels have recently been shown to play a critical role in regulating SMC differentiation marker gene expression in vitro and in vivo; a concept referred to as excitation transcription coupling (ETC).9–11 S1P-induced contraction of smooth muscle involves S1P2 signaling which can couple Ca-influx via VGCCs and RhoA/ROK to elicit contraction.13 We previously reported that VGCC-dependent increases in [Ca2+]i are associated with activation of expression of SMC differentiation marker genes through mechanisms that are dependent on ROK and increased binding of SRF to CArG cis regulatory promoter elements.11 Herein we show that S1P treatment increases histone H4Ac of CArG-containing promoter regions, which is indicative of an open chromatin state,27 and increased SRF enrichment to these CArG-containing regions. We further deduced that blocking S1P1/S1P3 increased SRF enrichment and that this could be attenuated by blocking VGCCs with nifedipine and prevented by inhibiting ROK with Y27632. Moreover, FTY720 treatment suppressed SMα-actin and SMMHC promoter-reporter activity. We speculate that suppression of these genes is mediated by ERK and Elk-1 via S1P1/S1P3, although this mechanism needs to be further explored. Taken together, S1P-induced activation of SMα-actin and SMMHC appears to be mediated by S1P2 signaling through L-type VGCCs, RhoA/ROK, and subsequent enrichment of SRF to CArG-containing promoter regions. These findings, although not proven, might link the differential regulation of S1P receptors after vascular injury with changes in SMC differentiation marker gene expression. That is (as shown in Figure 1), induction of S1P1/S1P3, coupled to reduction of S1P2, early in the injury response might drive SMC proliferation, migration, and suppression of SMC differentiation marker genes whereas reinduction of S1P2 late in the injury response begins to reverse this response, assisting in arresting neointimal growth and reinduction of the contractile phenotype. This S1P2 expression profile parallels transcriptional reactivation of several SMC differentiation marker genes in vivo after acute vascular injury, including SMα-actin, SMMHC, and SM22α.2
In summary, data presented herein support a model whereby S1P receptors are differentially regulated by vascular injury and pharmacological treatment with an S1P1/S1P3 antagonist can attenuate neointimal hyperplasia. Furthermore, the S1P2 receptor plays a critical role in regulating SMC differentiation marker gene expression. There are several outstanding issues that need to be addressed. First, what is the source of S1P in atherosclerosis and in-stent restenosis? Second, how are S1P receptors regulated at the transcriptional level? Our understanding of the latter is in its infancy but will provide insight into the differential regulation of these disparate receptor subtypes in multiple diseases.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the Pfizer Atorvastatin Research Award and NIH HL081682 to B.R.W., NIH P01 HL19242 to G.K.O., and NIH R01 GM067958 to K.R.L. and T.L.M.
Footnotes
Disclosures
None.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003;92:856–864. doi: 10.1161/01.RES.0000068405.49081.09. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 7.Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14:558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 9.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–878. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 10.Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2006;291:H2493–H2503. doi: 10.1152/ajpheart.01254.2005. [DOI] [PubMed] [Google Scholar]
- 11.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a Rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 12.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 13.Watterson KR, Ratz PH, Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine-1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 15.Kluk MJ, Hla T. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ Res. 2001;89:496–502. doi: 10.1161/hh1801.096338. [DOI] [PubMed] [Google Scholar]
- 16.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophos-pholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single g protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and pro-liferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue S, Nakazawa T, Cho A, Davastan F, Shilling D, Daum G, Reidy M. Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: A role for sphingosine-1-phosphate receptors. J Vasc Surg. 2007;46:756–763. doi: 10.1016/j.jvs.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy MA. Sphingosine 1-phosphate receptor 2 negatively regulates neointimal formation in mouse arteries. Circ Res. 2007;101:995–1000. doi: 10.1161/CIRCRESAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 22.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 23.Foss FW, Jr, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, Macdonald TL. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 25.Zhu R, Snyder AH, Kharel Y, Schaffter L, Sun Q, Kennedy PC, Lynch KR, Macdonald TL. Asymmetric synthesis of conformationally constrained fingolimod analogues-discovery of an orally active sphingosine 1-phosphate receptor type-1 agonist and receptor type-3 antagonist. J Med Chem. 2007;50:6428–6435. doi: 10.1021/jm7010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 27.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majesky MW, Reidy MA, Bowen-Pope DF, Hart CE, Wilcox JN, Schwartz SM. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990;111:2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Comm. 2002;299:483–487. doi: 10.1016/s0006-291x(02)02671-2. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA, Jr, Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J. 2003;17:1789–1799. doi: 10.1096/fj.02-0836com. [DOI] [PubMed] [Google Scholar]
- 31.Waeber C, Blondeau N, Salomone S. Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors. Drug News Perspect. 2004;17:365–382. doi: 10.1358/dnp.2004.17.6.829028. [DOI] [PubMed] [Google Scholar]
- 32.Keul P, Tolle M, Lucke S, Wnuck Lipinski K, Heusch G, Schuchardt M, van der Giet M, Levkau B. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 33.Bolick DT, Srinivasan S, Kim KW, Hatley ME, Clemens JJ, Whetzel A, Ferger N, Macdonald TL, Davis MD, Tsao PS, Lynch KR, Hedrick CC. Sphingosine-1-phosphate prevents tumor necrosis factor-{alpha}-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler Thromb Vasc Biol. 2005;25:976–981. doi: 10.1161/01.ATV.0000162171.30089.f6. [DOI] [PubMed] [Google Scholar]
- 34.Zohlnhofer D, Richter T, Neumann FJ, Nuhrenberg T, Wessely R, Brandl R, Murr A, Klein CA, Baeuerle PA. Transcriptome analysis reveals a role of interferon-γ in human neointima formation. Molecular Cell. 2001;7:1059–1069. doi: 10.1016/s1097-2765(01)00239-8. [DOI] [PubMed] [Google Scholar]
- 35.Yatomi Y, Yamamura S, Ruan F, Kume S, Ozaki Y, Igarashi Y. N,N-Dimethylsphingosine 1-phosphate activates human platelets. FEBS Lett. 1997;417:341–344. doi: 10.1016/s0014-5793(97)01321-5. [DOI] [PubMed] [Google Scholar]
- 36.Siess W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim Biophys Acta. 2002;1582:204–215. doi: 10.1016/s1388-1981(02)00173-7. [DOI] [PubMed] [Google Scholar]
- 37.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metabol. doi: 10.1016/j.tem.2007.07.005. In press. [DOI] [PubMed] [Google Scholar]
- 39.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.