Abstract
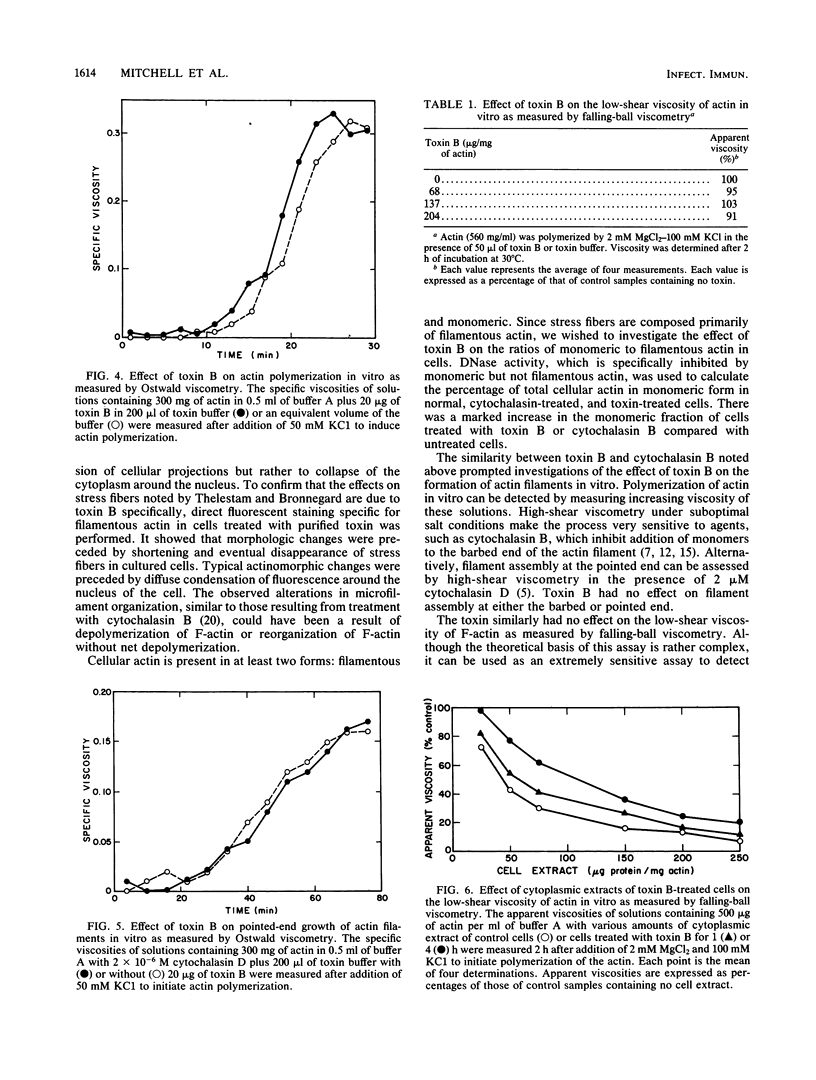
We describe a simplified procedure for purification of Clostridium difficile toxin B. In this procedure, cytotoxicity is associated with a single protein band with a molecular mass of 230 kilodaltons. We used direct fluorescent staining of actin filaments to study the effect of this toxin on cultured cells. Morphologic changes were preceded by a decrease in the number and length of stress fibers followed by their disappearance with condensation of cellular actin around the nucleus. We then showed that cells treated with either cytochalasin B or toxin B had a significant increase in the monomeric actin pool as quantitated by DNase I inhibition. In contrast to the cytochalasins, toxin B had no direct effect on the rate or extent of actin polymerization or network formation in vitro. Cytoplasmic extracts of toxin B-treated cells had a significantly lower level of modulating activity on actin assembly and interactions in vitro compared with extracts of untreated cells. These results suggest that the action of toxin B on cells is due to direct or indirect effects on cellular proteins involved in controlling the state of actin assembly in the cells.
Full text
PDF

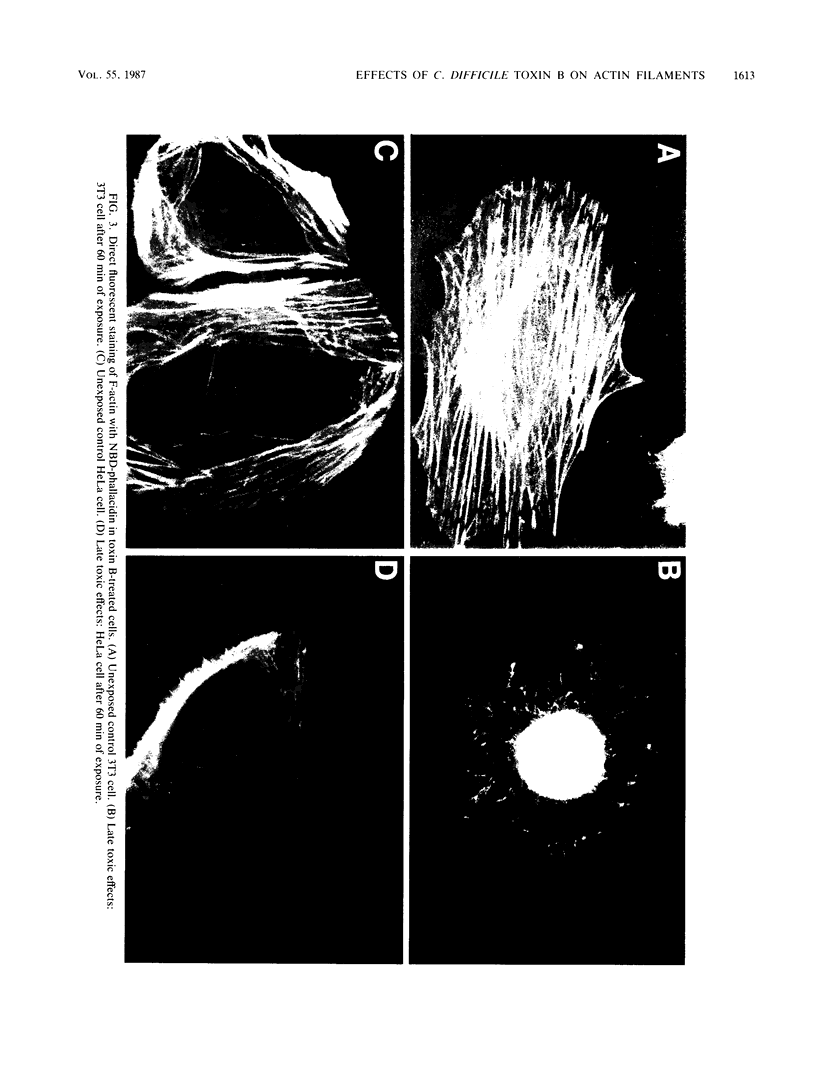

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Moon N., Chang T. W., Taylor N., Onderdonk A. B. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978 Nov;75(5):778–782. [PubMed] [Google Scholar]
- Blikstad I., Carlsson L. On the dynamics of the microfilament system in HeLa cells. J Cell Biol. 1982 Apr;93(1):122–128. doi: 10.1083/jcb.93.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Flanagan M. D., Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980 Feb 10;255(3):835–838. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libby J. M., Jortner B. S., Wilkins T. D. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982 May;36(2):822–829. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby J. M., Wilkins T. D. Production of antitoxins to two toxins of Clostridium difficile and immunological comparison of the toxins by cross-neutralization studies. Infect Immun. 1982 Jan;35(1):374–376. doi: 10.1128/iai.35.1.374-376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. C., Lin S. Actin polymerization induced by a motility-related high-affinity cytochalasin binding complex from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 May;76(5):2345–2349. doi: 10.1073/pnas.76.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. C., Tobin K. D., Grumet M., Lin S. Cytochalasins inhibit nuclei-induced actin polymerization by blocking filament elongation. J Cell Biol. 1980 Feb;84(2):455–460. doi: 10.1083/jcb.84.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Wilkins J. A., Cribbs D. H., Grumet M., Lin D. C. Proteins and complexes that affect actin-filament assembly and interactions. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):625–632. doi: 10.1101/sqb.1982.046.01.058. [DOI] [PubMed] [Google Scholar]
- MacLean-Fletcher S. D., Pollard T. D. Viscometric analysis of the gelation of Acanthamoeba extracts and purification of two gelation factors. J Cell Biol. 1980 May;85(2):414–428. doi: 10.1083/jcb.85.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Magargal W. W., Lin S. Transformation-dependent increases in endogenous cytochalasin-like activity in chicken embryo fibroblasts infected by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8201–8205. doi: 10.1073/pnas.83.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C., Barone L. M., Ely R., Faris B., Clark M. E., Franzblau C., LaMont J. T. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986 Jan 25;261(3):1316–1321. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Brönnegård M. Interaction of cytopathogenic toxin from Clostridium difficile with cells in tissue culture. Scand J Infect Dis Suppl. 1980;(Suppl 22):16–29. [PubMed] [Google Scholar]
- Wedel N., Toselli P., Pothoulakis C., Faris B., Oliver P., Franzblau C., LaMont T. Ultrastructural effects of Clostridium difficile toxin B on smooth muscle cells and fibroblasts. Exp Cell Res. 1983 Oct 15;148(2):413–422. doi: 10.1016/0014-4827(83)90163-5. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary J. M., Cleveland G., Kwock L., Lawrence T., Weissman R. M., Nabell L., Fried F. A., Staab E. V., Risinger M. A., Lin S. Actin filament organization of the Dunning R3327 rat prostatic adenocarcinoma system: correlation with metastatic potential. Cancer Res. 1986 Feb;46(2):926–932. [PubMed] [Google Scholar]