Abstract
Organic anion transporting polypeptide 1B3 (OATP1B3, SLCO1B3) is normally expressed in hepatocytes. In this study, we demonstrated frequent overexpression of OATP1B3 in colorectal adenocarcinomas. Quantitative RT-PCR analysis of 17 colon tumors indicated tumoral overexpression of OATP1B3 by ~100 fold, compared to 20 normal colon samples (p<0.0001). Using immunohistochemistry on a tissue microarray containing 93 evaluable colon tumor specimens, we detected immunostaining of OATP1B3 in 75 colon adenocarcinomas (81%) and no immunostaining in normal samples. To determine the functional effects of OATP1B3 expression on drug-induced apoptosis, we used camptothecin and oxaliplatin on a panel of colorectal cancer cell lines stably overexpressing OATP1B3. The results indicated that OATP1B3 overexpression enhanced cell survival in RKO, HCT-8 and HCT116p53+/+ cells that harbor wildtype p53 but not in Caco-2 and HCT116p53-/- cells that lack p53, compared to the respective empty vector controls (p<0.01). The TUNEL assay confirmed that HCT116p53+/+ cells overexpressing OATP1B3 had significantly lower apoptotic levels compared to empty vector control (P<0.001). The overexpression of OATP1B3 reduced the transcriptional activity of p53, with subsequent reductions in transcript and protein levels of its downstream transcription targets (P21WAF1 and PUMA). Overexpression of a point mutation (G583E) variant of OATP1B3 lacking transport activity did not confer an antiapoptotic effect or affect p53 transcriptional activity, suggesting that the antiapoptotic effect of OATP1B3 may be associated with its transport activity. Taken together, our results suggest that OATP1B3 overexpression in colorectal cancer cells may provide a survival advantage by altering p53-dependent pathways.
Introduction
Colorectal cancer (CRC) represents a major public health problem accounting for over 1 million cases of new cancer and about half a million deaths worldwide annually (1, 2). The lifetime risk of developing CRC is 1 in 17, affecting men and women alike, with 90% of cases occurring after the age of 50 years (3). CRC is thought to develop from the progressive accumulation of genetic mutations, many of which affect the control of apoptosis (4-6). Abnormalities in apoptosis mechanisms may promote the selection of cells that are resistant to apoptosis and consequently have increased rates of mutations (7).
Organic anion transporting polypeptide 1B3 (OATP1B3, gene name: SLCO1B3) belongs to the organic anion transporting polypeptide (OATP/SLCO) superfamily and is expressed on the basolateral membrane of hepatocytes around the central veins (8). When expressed in the normal liver, OATP1B3 acts as an uptake transporter for a variety of endogenous compounds (e.g. bile acids, cholecystokinin, conjugated steroids, thyroid hormones) as well as xenobiotic compounds (e.g. pravastatin, paclitaxel) (9-12). OATP1B3 has been shown to be overexpressed in various human cancer tissues as well as in cancer cell lines derived from colon, pancreas, gall bladder, lung and breast cancers (9, 13, 14). Recent studies have reported that certain members of the OATP family are overexpressed in breast and brain tumors and may play a role as regulators of cellular processes such as proliferation and apoptosis (15-20). However, it is not known whether tumoral expression of OATP1B3 has any pathobiological significance. In this study, we investigated the expression of OATP1B3 in established colorectal adenocarcinomas and its functional impact on cancer cell survival following chemotherapy treatment using in vitro CRC cell line models.
Materials and Methods
Cells, plasmids and reagents
The human CRC lines, Caco-2, HCA-7, HCT-8, and RKO cells were obtained from the American Type Culture Collection (ATCC). The isogenic HCT116p53+/+ (p53-wildtype) and HCT116p53-/- (p53-null), were gifts from Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD) (21). All cells were maintained in DMEM supplemented with 10% fetal bovine serum and 2 mM L-glutamine. The expression plasmid for OATP1B3 (wildtype) was prepared by inserting the open reading frame of the OATP1B3 cDNA sequence (NM 019844) into the pcDNA3.1-Zeo+ vector (Invitrogen). The expression plasmid for the OATP1B3 G583E mutant was prepared using the QuikChange® site-directed mutagenesis kit (Stratagene) and the following primers; 583E.fw, GGTTATAAGAACACTAGAAGGAATTCTAGCTCCAATATATTTTG and 583E.rv, CAAAATATATTGGAGCTAGAATTCCTTCTAGTGTTCTTATAACC. The plasmids used for the reporter assay, pcDNA3-Fp53, PG13-luc (containing 13-tandem repeats of the p53 consensus DNA binding site), P21WAF1-luc and PUMA-Luc reporter plasmids have been described elsewhere (22). The polyclonal antibody against the C-terminal peptide sequence of OATP1B3 has been previously used and shown to be specific for OATP1B3 (12, 23). The antibodies against p53 (DO-1) and P21WAF1 (WA-1) were from Abcam. The antibodies against caspase 3, PUMA, PARP and ß-actin were from Cell Signaling. The antibody against NOXA was from Imgenex. The antibody against p53 (BP53.12) was obtained from Chemicon. The reagents for immunohistochemistry were from Biogenex. Fluorescently labeled deoxycholic acid (fluorescein isothiocyanate (FITC)-DCA) was a gift from Dr. Jesse Martinez (University of Arizona, Tucson, AZ) and previously reported to induce signaling changes in a similar manner to unlabeled deoxycholic acid (DCA) (24).
Quantitative RT-PCR and immunoblotting analyses on colon tumors
Total RNA was isolated from 20 normal colonic mucosa samples and 17 primary colon tumors (7 samples were from matched donors). Single-stranded cDNA was synthesized from a total RNA amount of 1 μg using the iScript cDNA synthesis Kit (Bio-Rad). Quantitative RT-PCR was performed using an iCycler with the iQ SYBR-green Supermix (Bio-Rad) and the following gene-specific primers reported previously (25, 26); OATP1B3.fw, 5′-GTCCAGTCATTGGCTTTGCA-3′; OATP1B3.rv,5′-CAACCCAACGAGAGTCCTTAGG-3′; OATP1B1.fw, 5′-TGAACACCGTTGGAATTGC-3′; OATP1B1.rv, 5′-TCTCTATGAGATGTCACTGGAT-3′; OATP1A2.fw, 5′-AAGACCAACGCAGGATCCAT-3′; OATP1A2.rv, 5′-GAGTTTCACCCATTCCACGTACA-3′; β-actin.fw, 5′-GCATCCTCACCCTGAAGTAC-3′; β-actin.rv, 5′-GATAGCACAGCCTGGATAGC-3′. Reactions were performed in duplicate and mRNA copy numbers were quantified using purified PCR products as calibration samples. The results were normalized to 106 copies of β-actin mRNA.
The overexpression of OATP1B3 protein in colon tumors was confirmed by immunoblotting using protein lysates prepared from normal and cancerous colonic tissues from the same donors (n=5 pairs). Tissue homogenate containing 25 μg of total protein was subjected to immunoblotting and probed for OATP1B3 and β-actin. Immunoactive bands were visualized by enhanced chemiluminescence (Amersham Pharmacia).
Immunohistochemistry
A tissue microarray contained 93 de-identified, archival cases of colon adenocarcinomas of all American Joint Committee on Cancer criteria (AJCC) stages and 12 normal colonic mucosa sections. An avidin-biotin immunoperoxidase assay was performed following the antigen retrieval procedure using citrate buffer and a polyclonal antibody against OATP1B3 (1:200 dilution) was used (23). The immune reaction was visualized using 3,3’-diaminobenzidine (DAB) and nuclei were counter-stained with hematoxylin. The specificity of immunoreactive signals was verified by omitting either primary or secondary antibody as well as by incubating with polyclonal OATP1B3 antiserum that has been neutralized by pre-incubation with the antigenic peptide.
OATP1B3 expression was evaluated by an experienced pathologist (E.H.). The intensity of immunostaining was assigned as negative (0), weakly positive (1), moderately positive (2), or strongly positive (3). In normal colonic mucosa, the staining for OATP1B3 was negative. We therefore designated staining of intensity ≥ 1 as “positive” staining for OATP1B3.
Similarly, the immunohistochemical staining for p53 was performed on the same colorectal tumor microarray using a monoclonal p53 antibody (BP53.12, 1:100 dilution). The accumulation of p53 protein was evaluated by an experienced pathologist (E.M.B.). The staining for p53 was designated to be positive if ≥ 10% of tumor cells exhibit p53 immunostaining (27). In normal colonic mucosa, there was no accumulation of p53 protein.
Development of in vitro model cell lines overexpressing OATP1B3
CRC cell lines Caco-2, HCT-8, RKO and isogenic HCT-116 cells with wildtype or null p53 status were used to generate stable cell line models overexpressing OATP1B3 or the G583E variant of OATP1B3. Cells were transfected with the pcDNA3-based mammalian expression plasmid containing the open reading frame of OATP1B3 or the empty vector (control) using Lipofectamine 2000 (Invitrogen) and selected with the optimal concentrations of zeocin (800 μg/ml or 520 μM for HCT-8 and Caco-2, 400 μg/ml or 260 μM for RKO and isogenic HCT-116) for 3 weeks. OATP1B3 protein expression levels in the resulting resistant clones were examined by immunoblotting.
Cell viability assay
The functional impact of OATP1B3 overexpression on tumor cell survival following the incubation with chemotherapeutic drugs, camptothecin (CPT) or oxaliplatin was examined using the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) assay or ATP-based assay (Cell Titer Glo, Promega). Cells were plated at a density of 5,000 – 20,000 cells/well in a 96-well plate and treated with CPT (10 μM), oxaliplatin (10 μM) or vehicle (DMSO) and cell viability was assessed. The relative cell viability in the drug-treated group was calculated in comparison to the vehicle-treated group.
Apoptosis assay: TUNEL (terminal deoxynucleotidyl transferase–mediated nick-end labeling) assay
HCT-116p53+/+ cells stably expressing OATP1B3 or empty vector were seeded into 8-well chamber slides. Cells were treated with CPT (10 μM) or vehicle (DMSO) for 24 hrs and stained by TUNEL (In situ cell death detection kit, Roche Diagnostics). Twenty random fields at 200x magnification, >1000 cells were examined and photographed using fluorescence microscopy. The percentage of apoptosis was calculated by counting TUNEL-positive (red fluorescence) and healthy non-apoptotic cells.
Luciferase assay
To investigate whether the antiapoptotic effects of OATP1B3 involve the suppression of exogenous or endogenous p53 transcriptional activity, luciferase activity assays were performed in HCT-116p53-/- and HCT-116p53+/+ cells using the PG13-luc, P21WAF1-luc or PUMA-luc reporter plasmid, as previously described (22). Briefly, PG13-luc is a reporter plasmid which has a firefly luciferase under the control of 13 tandem repeats of the p53 response elements and P21WAF1-luc and PUMA-luc reporter plasmids contain a firefly luciferase under the P21WAF1 and PUMA gene promoter sequences with p53 response elements (28, 29). HCT-116p53-/- cells were transiently transfected with the expression plasmids for OATP1B3, p53 or empty vector as well as PG13-luc or P21WAF1-luc and pRL-TK using Fugene 6 (Roche Diagnostics). After 48 hours, luciferase activity normalized for renilla luciferase activity was obtained using a Dual Luciferase assay kit (Promega). In separate experiments, HCT-116p53+/+ cells stably overexpressing OATP1B3 or empty vector were transfected with the PG13-luc or PUMA-luc and pRL-TK. After 24 hours, cells were treated with CPT (10 μM) or vehicle (DMSO) for 4 hours and further incubated in fresh medium for 24 hours. Luciferase activity was measured as previously described.
Chemotherapy treatment and immunoblotting analyses
HCT-116p53+/+ cells stably expressing OATP1B3 or empty vector were treated with CPT (10 μM), oxaliplatin (10 μM) or vehicle (DMSO). After incubation, cells were harvested in RIPA buffer and subjected to immunoblotting. Following the incubation with respective primary and secondary antibodies, immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Pharmacia). β-actin was used as a gel loading control.
Effects of the OATP1B3 G583E variant
A point mutation variant (G583E) of OATP1B3 lacking transport activity was used to assess the relationship between OATP1B3 transport activity and its antiapoptotic effect. Luciferase activity assays were performed using the PG13-luc plasmid following transient transfection of OATP1B3 (wildtype), OATP1B3 G583E variant or empty vector in HCT-116p53-/- cells. HCT-116p53+/+ cells stably overexpressing OATP1B3 (wildtype), OATP1B3 G583E variant or empty vector were developed and used to assess the cellular uptake of a fluorescently labeled substrate (FITC-DCA) by measuring the green fluorescent signal associated with cell lysates following incubation with FITC-DCA (10 μM, 10 min) and subsequent PBS washes. In addition, HCT-116p53+/+ cells stably overexpressing OATP1B3 (wildtype), OATP1B3 G583E variant or empty vector were treated with CPT (10 μM, 24 hours) to measure cell survival and protein levels of p53 and its target genes, P21WAF1 and NOXA.
Statistical analysis
The results were expressed as the mean with standard deviation. Statistical significance between groups was determined using the Mann Whitney test, the unpaired Student’s t-test or ANOVA. P-values less than 0.05 were considered to indicate statistical significance.
Results
OATP1B3 mRNA and protein is frequently overexpressed in colorectal adenocarcinomas
The results from the quantitative RT-PCR analysis indicated that OATP1B3 mRNA is markedly overexpressed (96-fold differences in the median values) in the colorectal adenocarcinoma samples tested in comparison to normal colonic mucosa (Fig. 1A, p<0.0001 by Mann Whitney test). In seven cases, the tumoral and normal colon tissue samples analyzed were from the same donors and OATP1B3 mRNA was upregulated in all seven tumors compared to the matched normal colonic tissue samples (average fold differences in seven matching pairs was 76, ranging from 3 to 176). The expression levels of closely related members of the OATP family (namely OATP1B1 and OATP1A2) in these samples were also measured using quantitative RT-PCR. The results indicated minimal expression of OATP1B1 and OATP1A2 mRNA in both tumor and normal colonic mucosa, suggesting that marked overexpression is specific to OATP1B3, not accompanied by other tested OATP family members (data not shown). The results from OATP1B3 immunoblotting analysis confirm that OATP1B3 protein is overexpressed in colon tumors (n=3 out of 5), but not in the adjacent normal colonic tissues from the same donor (Fig. 1B). OATP1B3 expression in colon tissue appears to be tumor-specific and not related to the characteristics of an individual patient.
Fig. 1.
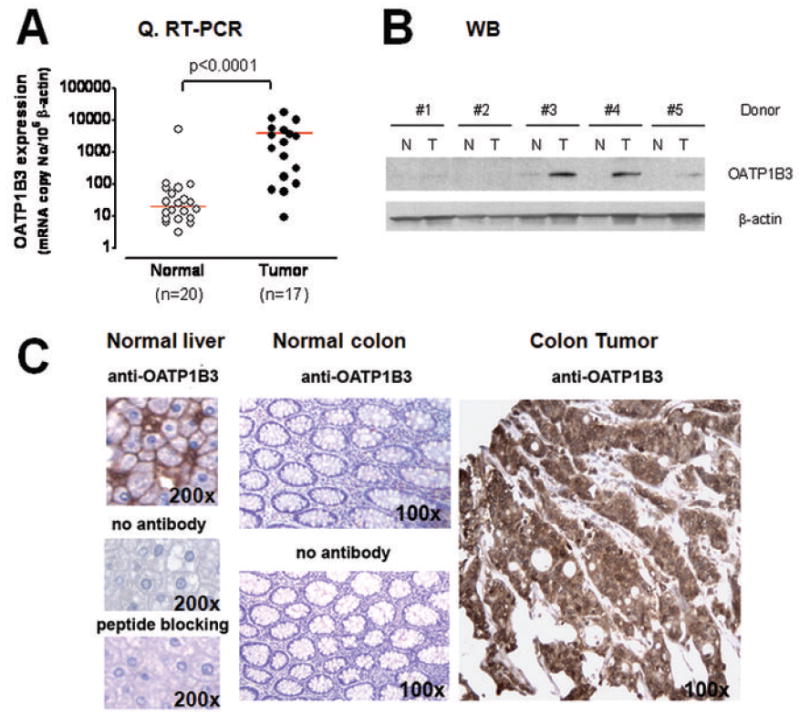
A: Log10[OATP1B3 mRNA expression normalized to β-actin] in normal and tumor colonic tissue samples measured by quantitative RT-PCR analysis. (horizontal bars = median values, p<0.0001 by Mann Whitney test). B: Immunoblotting analysis for OATP1B3 using protein lysates prepared from normal and cancerous colonic tissues from the same donors (n=5 pairs). Colon tumor tissue (3 out 5 pairs) showed OATP1B3 overexpression while normal colon tissue did not. C: Immunohistochemical staining for OATP1B3 using the avidin-biotin complex method. The positive signal was visualized by diaminobenzidine (DAB) staining (dark brown color). Normal human liver was used as a positive control to confirm the specificity of polyclonal OATP1B3 antiserum and showed membranous immunostaining for OATP1B3. Colon tumor tissue showed intense cytoplasmic OATP1B3 immunostaining while normal colon tissue showed no detectable immunostaining.
The OATP1B3 protein expression was assessed by immunohistochemistry. Because of the known expression of OATP1B3 in human hepatocytes (10), liver sections served as positive controls and were used to confirm the specificity of OATP1B3 antiserum by antigenic peptide blocking (Fig. 1C). In normal colon tissue, OATP1B3 protein expression was not detectable, consistent with its minimal mRNA expression in normal colon (Fig. 1C). In contrast, colon tumor sections showed intense OATP1B3 staining localized in tumor cells with a primarily cytoplasmic staining pattern, which was different from the exclusive membrane staining pattern in the liver (Fig. 1C).
We assessed the extent and frequency of OATP1B3 protein expression in established colorectal adenocarcinomas using a colon tumor tissue array containing 93 evaluable tumor and 12 normal colon tissue specimens. Normal colonic mucosa did not demonstrate OATP1B3 staining (intensity=0), however tumor tissue sections showed OATP1B3 staining primarily in the cytoplasm. We therefore designated staining of intensity ≥1 as “positive” staining for OATP1B3. The results indicate that ~81% of colon tumor sections evaluated (n=75 out of 93 specimens evaluated) were positive for OATP1B3 staining (Table 1). OATP1B3 expression was prevalent in all clinical stages with no statistically significant association with tumor stage (Fisher’s exact test, p>0.05). In addition, there was no statistically significant association between OATP1B3 immunostaining and any known clinico-pathologic factors (e.g. gender, age, tumor location, tumor size, Fisher’s exact test p>0.05).
Table 1.
Summary of OATP1B3 immunostaining from colon tumor tissue microarray
| Normal Colon | Colon Tumor
|
|||||
|---|---|---|---|---|---|---|
| Tumor stage | ||||||
| OATP1B3 positive staining (intensity of ≥1) | 1 | 2 | 3 | 4 | total | |
| Number stained/Number of total specimens | 0/12 | 8/8 | 35/41 | 26/35 | 6/9 | 75/93 |
| (Percentage) | (0%) | (100%) | (85%) | (74%) | (67%) | (81%) |
OATP1B3 overexpression provides a survival advantage to colon cancer cells
We assessed the expression of OATP1B3 in a number of established CRC cell lines, RKO, HCT-8, HCA-7 and Caco-2 cells. The quantitative RT-PCR results indicated that OATP1B3 mRNA is expressed in all the cell lines tested except Caco-2 cells. The OATP1B3 mRNA levels per 106 copies of β-actin were as follows; 22707 (HCA-7), 1473 (RKO), 250 (HCT-8) and not detectable (Caco-2). Considering a possibility that the functional impact of OATP1B3 overexpression may be affected by cell line-dependent alterations, we developed cell line models stably overexpressing OATP1B3 or empty vector using Caco-2, RKO and HCT-8 and examined whether OATP1B3 expression alters cell viability upon camptothecin (CPT, 10 μM, 24 hrs) treatment. The results indicated that OATP1B3 overexpression led to a significant cell survival advantage following CPT treatment in RKO and HCT-8 cells, but not in Caco-2 cells (Fig. 2A). Noting that the cellular p53 status differs between RKO and HCT-8 (p53 wildtype) and Caco-2 (harboring a p53 mutation causing a premature stop codon) (30), we examined whether cellular p53 status is an important factor determining the effect of OATP1B3 on cancer cell survival using isogenic HCT-116 cells with wildtype and null p53 status. OATP1B3 overexpression enhanced cell survival upon CPT (10 μM, 24 hrs) or oxaliplatin treatment (10 μM, 72 hrs) in HCT-116p53+/+ cells, but not in HCT-116p53-/- cells sharing the same genetic background (Fig. 2B and 2C, upper panels). These findings indicate that p53-dependent pathways may be potentially important for the OATP1B3 effect to enhance cell survival after chemotherapy treatment.
Fig. 2.
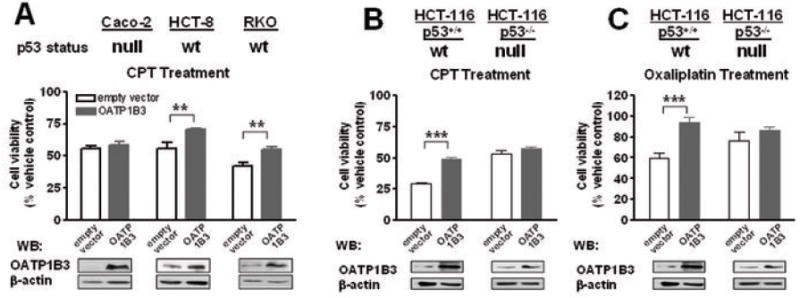
The overexpression of OATP1B3 conferred a cell survival advantage following Camptothecin (CPT) treatment to cells harboring wildtype p53, but not to cells lacking p53. Cell viability following CPT treatment (10 μM, 24 hrs) was determined using the MTT assay and expressed as the relative percentage compared to the vehicle (DMSO)-treated groups. A: Caco-2, RKO and HCT-8 cells. B: Isogenic HCT-116 cells with wildtype or null p53 status. Increased OATP1B3 protein expression in cells stably transfected with OATP1B3 was confirmed by western blotting using a polyclonal OATP1B3 antiserum (Lower panels). (**, p<0.01; ***, p<0.001, ANOVA with post-hoc Tukey test)
To examine whether OATP1B3 confers a survival advantage through apoptotic pathways, we measured the apoptosis outcome in HCT-116p53+/+ cells stably overexpressing OATP1B3 using the TUNEL assay after CPT treatment (10 μM, 24 hrs). A representative image shown in Fig. 3A demonstrates that HCT-116p53+/+ cells overexpressing OATP1B3 are protected against apoptosis induced by CPT (10 μM) compared to the empty vector control cells. The quantitative analyses of apoptotic (TUNEL-positive) and healthy non-apoptotic cells indicated that stable overexpression of OATP1B3 in HCT-116p53+/+ cells leads to a significant decrease (by 2.7-fold) in apoptosis (Fig. 3A, p<0.001). Consistent with these results, the activation/cleavage of caspase-3 and poly(ADP-ribose) polymerase (PARP) was substantially reduced in CPT-treated HCT-116p53+/+ cells overexpressing OATP1B3 compared to CPT-treated empty vector controls (Fig. 3B, lanes 2 vs 4).
Fig. 3.
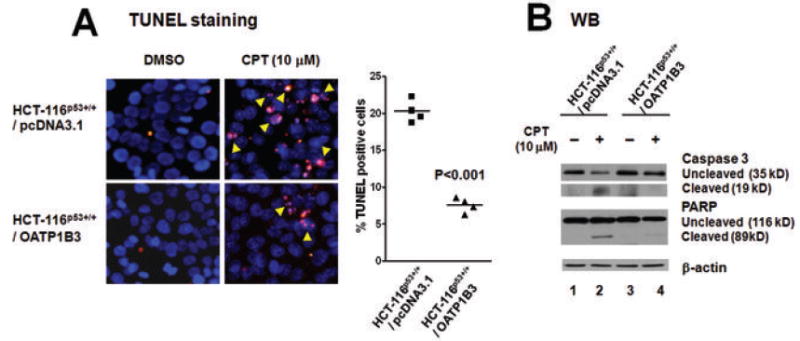
The overexpression of OATP1B3 in HCT-116p53+/+ cells provided apoptotic resistance following Camptothecin (CPT) treatment. A: HCT-116p53+/+ cells stably transfected with OATP1B3 or the empty vector were treated with CPT (10 μM, 24 hrs). Apoptotic cells were visualized by TUNEL staining (red fluorescence, indicated by arrows). Representative images and the results from quantitative analyses indicate that OATP1B3 overexpression is associated with a significantly decreased rate in apoptosis (p<0.001, unpaired t-test). B: Following treatment with CPT (10 μM, 24 hrs), the cleavage/activation of caspase-3 and PARP was substantially less in HCT-116p53+/+ cells stably overexpressing OATP1B3 compared to their empty vector controls.
OATP1B3 overexpression interferes with p53 transcriptional activity
Using p53-responsive reporter assays, we examined whether OATP1B3 expression interferes with the transcriptional activity of p53. We first examined the effect of OATP1B3 expression on the transcriptional activity of exogenous p53 by transiently transfecting the expression plasmids for p53 and OATP1B3 as well as p53-responsive reporter plasmids (PG13-luc and P21WAF1-luc). Relative luciferase activity following co-transfection of p53 and OATP1B3 in HCT-116p53-/- cells was substantially lower than that following transfection of p53 alone (Fig. 4A). To ascertain the inhibitory effect of OATP1B3 on p53 transcriptional activity, we examined the protein levels of p53 and its downstream targets using HCT-116p53-/- or HCT-116p53+/+ cells following transient expression of p53 and/or OATP1B3. The results indicate that co-transfection of OATP1B3 and p53 results in a substantial decrease in the protein levels of a p53 downstream target, P21WAF1 compared to transfection of p53 alone (Fig. 4B).
Fig. 4.
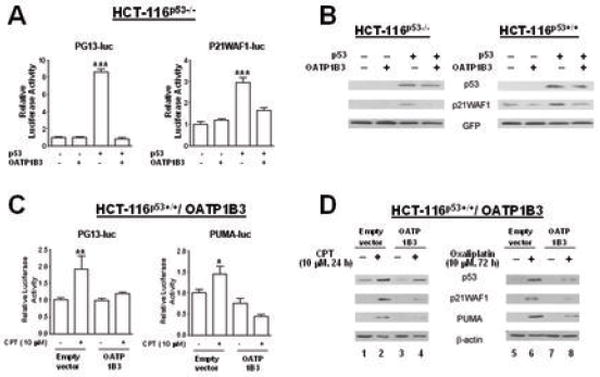
Co-expression of OATP1B3 affects p53 transcriptional activity. A: The results from the p53-responsive PG13-luc and P21WAF1-luc reporter assays in HCT-116p53-/- cells demonstrate that OATP1B3 expression causes a decrease in the transcriptional activity of exogenous p53. B: The results from immunoblotting analysis indicate that co-transfection of OATP1B3 with p53 results in decreases in the protein level of P21WAF1, a p53 downstream target in HCT-116p53-/- and HCT-116p53+/+ cells. C: The results from the p53-responsive PG13-luc or PUMA-luc reporter assay in HCT-116p53+/+ cells stably overexpressing OATP1B3 or empty vector show that CPT treatment increased reporter activity in the empty vector controls, but not in cells overexpressing OATP1B3. D: HCT-116p53+/+ cells stably overexpressing OATP1B3 or the empty vector showed induction of p53 protein upon chemotherapy treatment. However, the levels of the p53 downstream targets, P21WAF1 and PUMA were substantially lower or undetectable in chemotherapy-treated OATP1B3 overexpressing cells compared to chemotherapy-treated empty vector control cells (lanes 2 vs 4 for CPT treatment, 6 vs 8 for oxaliplatin treatment). (*, p<0.05; **, p<0.01; ***, p<0.001, different from the rest, ANOVA followed by post hoc Tukey test)
In addition, we investigated the effect of OATP1B3 overexpression on the transcriptional activity of endogenous p53 by comparing the effects of CPT treatment in HCT-116p53+/+ cells stably overexpressing OATP1B3 or empty vector. The results indicated that CPT treatment increased PG13-luc or PUMA-luc reporter activity in the empty vector controls, but not in cells overexpressing OATP1B3 (Fig. 4C). These results suggested that OATP1B3 expression causes a decrease in p53 transcriptional activity. The inhibitory effect of OATP1B3 on endogenous p53 transcriptional activity was further verified by examining the protein levels of p53 and its downstream targets, P21WAF1 and PUMA. HCT-116p53+/+ cells stably overexpressing OATP1B3 or the empty vector showed induction of p53 protein upon CPT (10 μM, 24 hrs) or oxaliplatin (10 μM, 72 hrs) treatment. However, the levels of the p53 downstream targets, P21WAF1 and PUMA were substantially lower or undetectable in chemotherapy-treated OATP1B3 overexpressing cells compared to chemotherapy-treated empty vector control cells (Fig. 4D, lanes 2 vs 4 for CPT treatment and lanes 6 vs 8 for oxaliplatin treatment). These results from the p53-responsive reporter assays and immunoblotting analysis suggest that OATP1B3 overexpression interferes with the transcriptional activity of p53 and its downstream signaling.
Antiapoptotic effect of OATP1B3 appears to be associated with its transporter function
To investigate a potential association between the antiapoptotic effect of OATP1B3 and its transport function, we utilized a point mutation (G583E) variant of OATP1B3 which lacks transport activity (31). In contrast to OATP1B3 (wildtype), the OATP1B3 variant (G583E) did not affect the p53 transcriptional activity after transient transfection with the PG13-luc plasmid in HCT-116p53-/- cells (Fig. 5A). The cellular uptake of the fluorescently labeled bile acid (FITC-DCA, 10 μM, 10 min) was substantially lower in HCT-116p53+/+ cells stably overexpressing the OATP1B3 variant (G583E) or empty vector than in cells overexpressing OATP1B3 (wildtype) (Fig. 5B). In addition, the OATP1B3 variant (G583E) did not confer a survival advantage following CPT treatment (Fig. 5C). Unlike the results obtained from OATP1B3 (wildtype), immunoblotting analyses indicated that CPT treatment leads to increases in p53 downstream targets, P21WAF1 and NOXA to a comparable level between cells overexpressing the OATP1B3 variant (G583E) or empty vector (Fig. 5D).
Fig. 5.
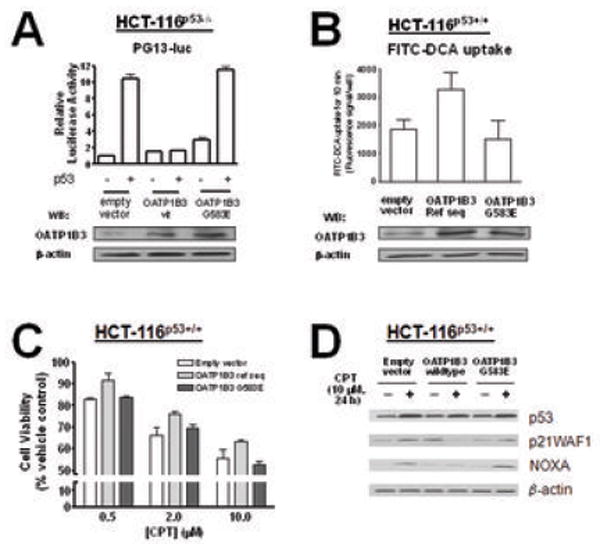
The OATP1B3 variant (G583E) lacking the transport activity confers neither an inhibitory effect on p53 transcriptional activity nor a survival advantage following CPT treatment. A: The results from the p53-responsive PG13-luc reporter assays in HCT-116p53-/- cells demonstrate that the OATP1B3 G583E variant does not affect the transcriptional activity of p53 in contrast to OATP1B3 (wildtype). B: The results from a cellular uptake assay using a fluorescently labeled deoxycholic acid (FITC-DCA, 10 μM) indicate that HCT-116p53+/+ cells stably overexpressing OATP1B3 (wildtype) show an increased cellular uptake of FITC-DCA compared to the cells stably overexpressing the OATP1B3 G583E variant or empty vector controls. C: Overexpression of the OATP1B3 G583E variant does not confer a cell survival advantage following camptothecin (CPT) treatment to HCT-116p53+/+ cells, in contrast to OATP1B3 (wildtype). D: The levels of the p53 downstream targets, P21WAF1 and NOXA are increased following CPT treatment in HCT-116p53+/+ cells stably overexpressing OATP1B3 (wildtype) or empty vector, but not in those stably overexpressing the OATP1B3 G583E variant.
Discussion
Colorectal cancer remains one of the leading causes of cancer-related death worldwide and resistance to chemotherapy is a major issue in the management of recurrent and metastatic colorectal cancer. In this study, we demonstrate for the first time that OATP1B3 is aberrantly overexpressed at both mRNA and protein levels in the majority of colorectal adenocarcinomas and that OATP1B3 overexpression confers an antiapoptotic effect against chemotherapy treatment in colon cancer cells by altering the p53-dependent pathways. These findings may explain one of the potential mechanisms contributing to chemotherapeutic resistance in tumors harboring wildtype p53.
The expression of OATP1B3 in colon tumors and in the colon tumor cell lines (data not shown) was mainly cytoplasmic and clearly different from the membranous expression pattern in the normal liver. Cytoplasmic localization of OATP1B3 in tumor cells has also been found in primary breast cancer tissues (14). The aberrant expression of OATP1B3 in the cytoplasm of colon tumors was the impetus to investigate whether OATP1B3 functions differently in the setting of malignancy. The results from the current study suggest that OATP1B3 overexpression in the cytoplasm of colon tumor cells confers apoptotic resistance. Previous studies have reported cytoplasmic localization of other membrane proteins in cancer cell line models due to a defect in plasma membrane protein recycling or tumoral changes in signaling (e.g. PI3K activation) (32, 33). However, the mechanism responsible for the cytoplasmic expression of OATP1B3 in colon tumors remains to be determined. In further studies we have found that OATP1B3 expression is increased in premalignant adenomatous polyps (data not shown). However, in polyps with low malignant potential (hyperplastic) OATP1B3 was not detected, comparable to our observations in normal colonic mucosa. These results suggest that OATP1B3 upregulation may be an early event associated with colorectal tumorigenesis and maintained throughout colon cancer progression and not just a marker of cellular proliferation. We aim to further investigate the pathobiological significance of OATP1B3 overexpression in colon epithelium by assessing the levels and variability of OATP1B3 expression in larger groups of colon polyps with differing malignant potential and histology.
In the present study, the pro-survival/anti-apoptotic effect of OATP1B3 appears to involve the interference with the p53-dependent apoptosis pathways. OATP1B3 expression substantially decreased the levels of p53 downstream targets despite elevated p53 protein levels after chemotherapy treatment. The p53 protein, often referred to as “a guardian of the genome,” is the master regulator of apoptosis following exposure to DNA damage, hypoxia, and cytotoxic drugs and it is the most commonly mutated gene in human cancers (34). While p53 mutations have been associated with resistance to chemotherapy (35, 36), the predictive value of p53 mutations in determining clinical outcomes in CRC patients has not been clearly established (37-40). This may be in part due to methodological problems assessing p53 mutation status in clinical samples (41, 42). Resistance to colorectal cancer treatment is observed in patients whose tumors harbor wildtype p53. Therefore other mechanisms may interfere with p53 function, altering response to chemotherapy. Our results suggest that OATP1B3 overexpression may be one of the mechanisms underlying chemotherapy resistance in tumors harboring wildtype p53. These findings may provide additional insights into understanding the complexity in determining chemotherapy response. In an attempt to identify the p53 wildtype/mutant status in colon tumors overexpressing OATP1B3 and to discover possible correlations between these two proteins in clinical samples, we performed immunohistochemical staining for p53 using a monoclonal p53 antibody (BP53.12) on the same tumor microarray that was used for OATP1B3 staining. On analysis, approximately one third of the samples showed an accumulation of p53 protein (26 out of the total 89 tumor samples evaluable for p53 staining and 20 out of 75 OATP1B3 overexpressing tumors). The distribution of p53 positive tumors was comparable among the OATP1B3 staining designations indicating no apparent correlation between p53 detection and OATP1B3 expression in these clinical samples (p=0.325, Fisher’s exact test). Further evaluation of a possible relationship between p53 accumulation and OATP1B3 expression is warranted given our in vitro results and the inherent limitations of immunohistochemistry as a means of assessing p53 mutational status. It is possible that the apparent lack of association between p53 staining and OATP1B3 overexpression may be due to other regulatory factors that affect the complex p53 pathway and mask the molecular relationships between p53 and OATP1B3 (43).
The results from our current study indicated that OATP1B3 overexpression in colon tumors is associated with the lower transcriptional activation of well-known p53 downstream target genes, P21WAF1, NOXA, and PUMA. These results are consistent with previously published reports documenting that P21WAF1, NOXA, and PUMA are transcriptionally regulated by p53 and play important roles in apoptosis (44-46). Our results also suggest that OATP1B3 overexpression in colon cancer cells harboring wildtype p53 reduces the activation of the DNA damage response protein PARP, an important marker of apoptosis (47). These findings are consistent with decreased apoptosis levels and attenuation of caspase activation in the presence of OATP1B3 overexpression.
OATP1B3 is known to mediate the transport of various endogenous and exogenous substrates including steroid and thyroid hormones, prostaglandins and glutathione (8). Our results indicate that OATP1B3 overexpression in p53 wildtype HCT-116 cells confers a pro-survival/antiapoptotic effect and also maintains its transporter function in this setting. The overexpression of the OATP1B3 variant (G583E) lacking the transport activity resulted in no pro-survival/antiapoptotic effect, suggesting that the antiapoptotic effect of OATP1B3 may be associated with its transport activity. However it is not a certainty that the antiapoptotic effect of OATP1B3 is due to alterations in the cellular transport of chemotherapy drugs, since the antiapoptotic effect of OATP1B3 was observed with two drugs (CPT, oxaliplatin) that have differing OATP1B3 transport properties. There are differing reports regarding the transport of CPT and its structurally related analog, CPT-11, by OATP1B3 (48-50). Oxaliplatin and other platinum drugs have been shown not to interact with organic anion transporters including OATP1B3 (50). While our current results with OATP1B3 G583E variant suggest an association between the antiapoptotic effect of OATP1B3 and its transport activity, we cannot rule out the possibility that a point mutation of G583E in OATP1B3 affects both the antiapoptotic function and the transport activity of OATP1B3 simultaneously, and that the two functions are not necessarily linked. The results from our current study suggest potential complexity in the molecular mechanisms underlying the antiapoptotic effect of OATP1B3 overexpression. Further investigation using additional OATP1B3 variants or specific inhibitors of the transporter activity would be necessary to confirm the association between the antiapoptotic function and transport activity of OATP1B3 and to determine the involvement of additional mechanisms. Certain transporters such as MDR1 have been shown to be localized in cytoplasmic organelles/vesicles such as mitochondria and golgi apparatus and to be functionally active as transporters (51, 52). Interestingly, MDR1 has been also reported to provide protection against apoptosis independently of its transport function (53, 54). Additional studies are required to clarify the molecular mechanisms and/or transport substrates contributing to the functional changes associated with OATP1B3 overexpression.
In conclusion, OATP1B3 is frequently overexpressed in a majority of colon tumor samples, but not in normal colonic mucosa. Overexpression of OATP1B3 in colon cancer cells conferred an antiapoptotic advantage against chemotherapy treatment, by interfering with p53 transcriptional activity. These findings provide further justifications to investigate the molecular interactions between OATP1B3 and p53 as well as the clinical significance of OATP1B3 overexpression as a potential factor in determining chemotherapy resistance.
Acknowledgments
A.C. Lockhart’s effort in this research was supported by NCI Grant 5K23CA098011. H. Glaeser is supported by ‘Deutsche Forschungsgemeinschaft’ DFG GL 588/2-1.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Gill S, Blackstock AW, Goldberg RM. Colorectal cancer. Mayo Clin Proc. 2007;82:114–29. doi: 10.4065/82.1.114. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 5.Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet. 2002;3:101–28. doi: 10.1146/annurev.genom.3.022502.103043. [DOI] [PubMed] [Google Scholar]
- 6.Ilyas M, Straub J, Tomlinson IP, Bodmer WF. Genetic pathways in colorectal and other cancers. Eur J Cancer. 1999;35:1986–2002. doi: 10.1016/s0959-8049(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 7.Watson AJ. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol. 2006;57:107–21. doi: 10.1016/j.critrevonc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 9.Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120:1689–99. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- 10.Konig J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000;278:G156–64. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi H, Okada M, Akitaya S, et al. Transport of fluorescent chenodeoxycholic acid via the human organic anion transporters OATP1B1 and OATP1B3. J Lipid Res. 2006 doi: 10.1194/jlr.M500532-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Seithel A, Eberl S, Singer K, et al. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos. 2007;35:779–86. doi: 10.1124/dmd.106.014407. [DOI] [PubMed] [Google Scholar]
- 13.Monks NR, Liu S, Xu Y, Yu H, Bendelow AS, Moscow JA. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1- and OATP1B3-expressing HeLa cells. Mol Cancer Ther. 2007;6:587–98. doi: 10.1158/1535-7163.MCT-06-0500. [DOI] [PubMed] [Google Scholar]
- 14.Muto M, Onogawa T, Suzuki T, et al. Human liver-specific organic anion transporter-2 is a potent prognostic factor for human breast carcinoma. Cancer Sci. 2007;98:1570–6. doi: 10.1111/j.1349-7006.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronger H, Konig J, Kopplow K, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65:11419–28. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 16.Al Sarakbi W, Mokbel R, Salhab M, Jiang WG, Reed MJ, Mokbel K. The role of STS and OATP-B mRNA expression in predicting the clinical outcome in human breast cancer. Anticancer Res. 2006;26:4985–90. [PubMed] [Google Scholar]
- 17.Nozawa T, Suzuki M, Takahashi K, et al. Involvement of estrone-3-sulfate transporters in proliferation of hormone-dependent breast cancer cells. J Pharmacol Exp Ther. 2004;311:1032–7. doi: 10.1124/jpet.104.071522. [DOI] [PubMed] [Google Scholar]
- 18.Nozawa T, Suzuki M, Yabuuchi H, Irokawa M, Tsuji A, Tamai I. Suppression of cell proliferation by inhibition of estrone-3-sulfate transporter in estrogen-dependent breast cancer cells. Pharm Res. 2005;22:1634–41. doi: 10.1007/s11095-005-7096-0. [DOI] [PubMed] [Google Scholar]
- 19.Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem. 2006;281:29542–57. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- 20.Miki Y, Suzuki T, Kitada K, et al. Expression of the steroid and xenobiotic receptor and its possible target gene, organic anion transporting polypeptide-A, in human breast carcinoma. Cancer Res. 2006;66:535–42. doi: 10.1158/0008-5472.CAN-05-1070. [DOI] [PubMed] [Google Scholar]
- 21.Bunz F, Dutriaux A, Lengauer C, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 22.Belkhiri A, Zaika A, Pidkovka N, Knuutila S, Moskaluk C, El-Rifai W. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer Res. 2005;65:6583–92. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 23.Ho RH, Tirona RG, Leake BF, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Jean-Louis S, Akare S, Ali MA, Mash EA, Jr, Meuillet E, Martinez JD. Deoxycholic acid induces intracellular signaling through membrane perturbations. J Biol Chem. 2006 doi: 10.1074/jbc.M506710200. [DOI] [PubMed] [Google Scholar]
- 25.Briz O, Serrano MA, MacIas RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003;371:897–905. doi: 10.1042/BJ20030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glaeser H, Bailey DG, Dresser GK, et al. Intestinal Drug Transporter Expression and the Impact of Grapefruit Juice in Humans. Clin Pharmacol Ther. 2007 doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 27.Resnick MB, Routhier J, Konkin T, Sabo E, Pricolo VE. Epidermal growth factor receptor, c-MET, beta-catenin, and p53 expression as prognostic indicators in stage II colon cancer: a tissue microarray study. Clin Cancer Res. 2004;10:3069–75. doi: 10.1158/1078-0432.ccr-03-0462. [DOI] [PubMed] [Google Scholar]
- 28.Tomkova K, Belkhiri A, El-Rifai W, Zaika AI. p73 isoforms can induce T-cell factor-dependent transcription in gastrointestinal cells. Cancer Res. 2004;64:6390–3. doi: 10.1158/0008-5472.CAN-04-2176. [DOI] [PubMed] [Google Scholar]
- 29.Tomkova K, El-Rifai W, Vilgelm A, Kelly MC, Wang TC, Zaika AI. The gastrin gene promoter is regulated by p73 isoforms in tumor cells. Oncogene. 2006;25:6032–6. doi: 10.1038/sj.onc.1209610. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Bodmer WF. Analysis of P53 mutations and their expression in 56 colorectal cancer cell lines. Proc Natl Acad Sci U S A. 2006;103:976–81. doi: 10.1073/pnas.0510146103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letschert K, Keppler D, Konig J. Mutations in the SLCO1B3 gene affecting the substrate specificity of the hepatocellular uptake transporter OATP1B3 (OATP8) Pharmacogenetics. 2004;14:441–52. doi: 10.1097/01.fpc.0000114744.08559.92. [DOI] [PubMed] [Google Scholar]
- 32.Liang XJ, Shen DW, Garfield S, Gottesman MM. Mislocalization of membrane proteins associated with multidrug resistance in cisplatin-resistant cancer cell lines. Cancer Res. 2003;63:5909–16. [PubMed] [Google Scholar]
- 33.Knostman KA, McCubrey JA, Morrison CD, Zhang Z, Capen CC, Jhiang SM. PI3K activation is associated with intracellular sodium/iodide symporter protein expression in breast cancer. BMC Cancer. 2007;7:137. doi: 10.1186/1471-2407-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–23. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 35.Boyer J, McLean EG, Aroori S, et al. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res. 2004;10:2158–67. doi: 10.1158/1078-0432.ccr-03-0362. [DOI] [PubMed] [Google Scholar]
- 36.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–67. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 37.Popat S, Chen Z, Zhao D, et al. A prospective, blinded analysis of thymidylate synthase and p53 expression as prognostic markers in the adjuvant treatment of colorectal cancer. Ann Oncol. 2006;17:1810–7. doi: 10.1093/annonc/mdl301. [DOI] [PubMed] [Google Scholar]
- 38.Iacopetta B, Russo A, Bazan V, et al. Functional categories of TP53 mutation in colorectal cancer: results of an International Collaborative Study. Ann Oncol. 2006;17:842–7. doi: 10.1093/annonc/mdl035. [DOI] [PubMed] [Google Scholar]
- 39.Russo A, Bazan V, Iacopetta B, Kerr D, Soussi T, Gebbia N. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: influence of tumor site, type of mutation, and adjuvant treatment. J Clin Oncol. 2005;23:7518–28. doi: 10.1200/JCO.2005.00.471. [DOI] [PubMed] [Google Scholar]
- 40.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–27. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 41.Munro AJ, Lain S, Lane DP. P53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer. 2005;92:434–44. doi: 10.1038/sj.bjc.6602358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soussi T, Ishioka C, Claustres M, Beroud C. Locus-specific mutation databases: pitfalls and good practice based on the p53 experience. Nat Rev Cancer. 2006;6:83–90. doi: 10.1038/nrc1783. [DOI] [PubMed] [Google Scholar]
- 43.Hall PA, McCluggage WG. Assessing p53 in clinical contexts: unlearned lessons and new perspectives. J Pathol. 2006;208:1–6. doi: 10.1002/path.1913. [DOI] [PubMed] [Google Scholar]
- 44.Yu J, Zhang L. No PUMA, no death: implications for p53-dependent apoptosis. Cancer Cell. 2003;4:248–9. doi: 10.1016/s1535-6108(03)00249-6. [DOI] [PubMed] [Google Scholar]
- 45.Oda E, Ohki R, Murasawa H, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 46.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–8. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 47.Scovassi AI, Poirier GG. Poly(ADP-ribosylation) and apoptosis. Mol Cell Biochem. 1999;199:125–37. doi: 10.1023/a:1006962716377. [DOI] [PubMed] [Google Scholar]
- 48.Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos. 2005;33:434–9. doi: 10.1124/dmd.104.001909. [DOI] [PubMed] [Google Scholar]
- 49.Anderson BD, Horn J, Monks N. Camptothecin analogue carboxylates are OATP1B1 and OATP1B3 substrates. AAPS Annual Meeting and Exposition; 2006; San Antonio, TX. 2006. [Google Scholar]
- 50.Yamaguchi H, Kobayashi M, Okada M, et al. Rapid screening of antineoplastic candidates for the human organic anion transporter OATP1B3 substrates using fluorescent probes. Cancer Lett. 2008;260:163–9. doi: 10.1016/j.canlet.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 51.Solazzo M, Fantappie O, Lasagna N, Sassoli C, Nosi D, Mazzanti R. P-gp localization in mitochondria and its functional characterization in multiple drug-resistant cell lines. Exp Cell Res. 2006;312:4070–8. doi: 10.1016/j.yexcr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Munteanu E, Verdier M, Grandjean-Forestier F, et al. Mitochondrial localization and activity of P-glycoprotein in doxorubicin-resistant K562 cells. Biochem Pharmacol. 2006;71:1162–74. doi: 10.1016/j.bcp.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Johnstone RW, Cretney E, Smyth MJ. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood. 1999;93:1075–85. [PubMed] [Google Scholar]
- 54.Smyth MJ, Krasovskis E, Sutton VR, Johnstone RW. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc Natl Acad Sci U S A. 1998;95:7024–9. doi: 10.1073/pnas.95.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]


