Abstract
Evidence for the regulation of body energy is reviewed from the homeostatic perspective of Claude Bernard and Walter Cannon. Considered are the complementary roles of food intake and energy expenditure in the maintenance and defense of energy balance. Particular attention is paid to the roles adjustments in energy expenditure play in this process and to recent investigations identifying their metabolic underpinnings. This is followed by a consideration of the many newly-identified signals of body energy status and the pathways and feedback loops they utilize to inform the central regulating system. Finally, various naturally-occurring and experimentally-induced alterations in the regulated level of body energy are described and discussed. It is concluded that, though early investigators did not expressly consider energy a regulated feature of the milieu interieur, more recent research has provided a sound basis for judging the regulation of body energy to be another homeostatic process.
Keywords: body energy regulation, monitors of body energy, energy expenditure
Claude Bernard attributed the stability of the milieu interieur to the particular ability of the body both to monitor its internal condition and to make the adjustments necessary to sustain that stability. Stephen Cooper’s (2008) scholarly historical review of the complementary concepts of the stability of the internal milieu and homeostasis offers an excellent perspective from which to explore the question of whether body energy, or body weight which can serve as its index, displays the features necessary to conclude that it too is an aspect of our internal environment that is homeostatically regulated. The present paper critically examines several lines of evidence that address this question.
BODY ENERGY: EVIDENCE OF ITS DEFENSE
It is useful at the outset to note that, despite what are often wide disparities in body weight within a population, the individual typically displays impressive weight stability, matching or even surpassing that of many other regulated body conditions. Coefficients of weight variation over relatively short periods are on the order of only 0.5 to 0.6 % (Khosha & Billewicz, 1964) and cross-sectional observations indicate this pattern persists over more extended periods (Ten-State Nutrition Survey, 1968’70).
Evidently, such weight stability is achieved only by bringing into play adjustments in energy intake and/or energy expenditure appropriate to compensating for threats to that stability. Certainly, there is no question but that food intake is so controlled. Weight losses induced by caloric restriction produce marked increases in food intake (Levitsky, 1970), whereas intake is suppressed by over-nutrition and weight gain (Cohn and Joseph, 1962). An extensive literature exists documenting the important role that food intake adjustments play in the maintenance of energy balance, and the phenomena are sufficiently well known that they are not re-examined in the present review.
There is decidedly less agreement about whether energy expenditure adjustments play a corresponding role in the homeostatic maintenance of body energy. Unlike body fluid balance, where it has long been accepted that the control of both fluid intake and its rate of clearance are key to its maintenance, considerations of how energy balance is maintained have tended traditionally to focus upon food intake control.
That energy expenditure does play a rather significant role in the defense of body energy homeostasis is, however, indicated by more recent observations. The section which follows examines these findings and uses the unique relationship of energy expenditure to body mass to derive a useful perspective for viewing body energy regulation.
A. DAILY ENERGY EXPENDITURE AND BODY WEIGHT: A UNIVERSAL RULE
It was Max Kleiber (1947) who first demonstrated the remarkably consistent relationship, holding over a wide range of animal species, between daily energy expenditure and body weight. Initially studying 26 species ranging in size from small rodents to large mammals, Kleiber generated a function relating the daily energy expenditure of each, in kilocalories per day (kcal/d), to its body mass in kilograms (kg). A best fit of the resulting function yielded the equation kcal/d= 66.5BWkg.75. The body weight exponent of .75 indicates that, as species increase in size, their daily energy needs increase at a rate ¾ that of body mass. Raising an animal’s body mass to the ¾ power thus provided an index of what Kleiber characterized as its “metabolic body size” or BWkg.75. Simply by multiplying an animal’s metabolic body size by the derived constant (k) of 66.5, accurate estimates of its daily energy flux are obtained. Remarkably, subsequent investigators have demonstrated this formulation of Kleiber’s can be successfully applied across the entire animal kingdom, from unicellular organisms to the largest mammals (Hemmingsen, 1956–60).
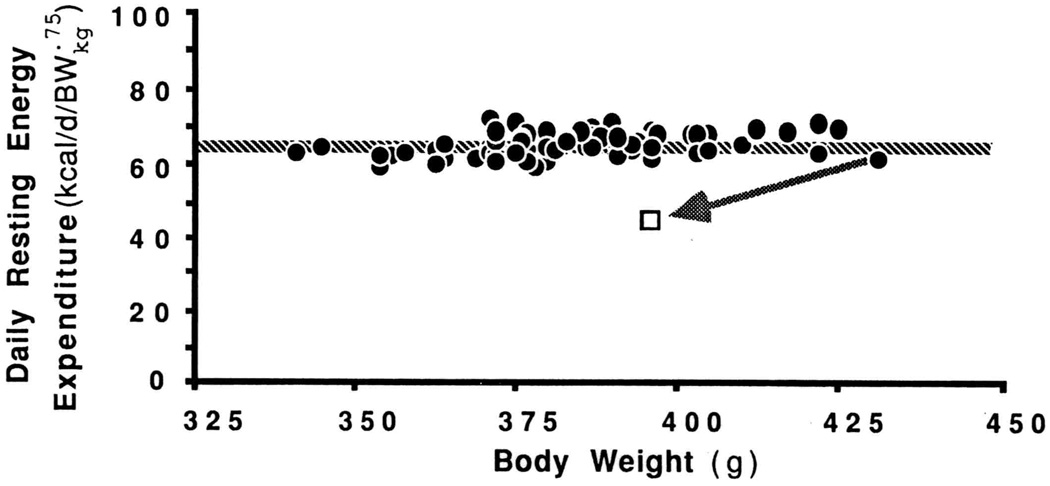
Though Kleiber did not directly address the issue of whether this interspecies relationship between daily energy expenditure and body mass would apply to different-sized member of the same species, subsequent study of a group of laboratory rats, of the same age but differing in body weight, appears to support such an extension. In Fig.1, the daily expenditures of each of these rats, each expressed relative to its metabolic body size, has been plotted against its maintained body weight. It can be seen that, when so expressed, the daily expenditures of all rats are essentially the same, indicating that all conform to the Kleiber rule. Note that even the value of the constant k, obtained here by extending the best-fit line in Fig. 1 to its Y-axis, very closely approximates the interspecies value of 66.5. Evidently, Kleiber’s interspecies formula for estimating daily energy from metabolic body size provides an equally good estimate of the daily energy needs of different-sized members of the same species.
Fig. 1.
Daily resting energy expenditure (kcal/d), expressed per body weight raised to the ¾ power (BW.75), of individual male rats of the same strain and age. The body weight of the largest rat was reduced by 8% by restricting its intake. The arrow indicates the resulting change in daily energy expenditure. (Unpublished observations of Hirvonen and Keesey)
B. ENERGY EXPENDITURE IN THE DEFENSE OF BODY ENERGY
While this orderly relationship between daily energy expenditure and body mass is suggestive of body energy regulation, it does not speak directly to the issue of energy expenditure’s role in the defense of body energy homeostasis. It does, however, provide a very useful perspective for doing so. Referring again to Fig. 1, note the effects of perturbing the body weight of one of the rats, actually the heaviest of the group. At the normally-maintained body weight, its daily energy expenditure, expressed relative to metabolic body size, matches that of all the other rats. However, restricting its intake so as to lower its body weight by 8% causes its daily energy expenditure to decline significantly below that of other rats of this weight. Clearly, the resulting hypometabolic condition will favor weight gain, even on a daily ration otherwise only sufficient for weight maintenance. Furthermore, this metabolic advantage can be shown to persist until this rat’s weight has been restored to its initial high level. Adjustments in daily expenditure would similarly serve to stabilize the weight of any animal in this group at its characteristic level.
Others (Leibel et al., 1995) have documented these adjustments in energy expenditure in dieting patients, emphasizing their significance in the context of the difficulties of their achieving and sustaining weight loss. Contributing further to our understanding of these expenditure adjustments are the results of a recent investigation in which the primary source of these energy savings in dieters has been identified as an enhanced efficiency of skeletal work efficiency (Rosenbaum et al., 2003). Apparently, it is the decline in leptin in dieters upon which this enhanced efficiency of muscle action depends since leptin replacement abolishes it (Rosenbaum et al., 2005).
C. A PERSPECTIVE ON ENERGY EXPENDITURE AND ENERGY BALANCE
The preceding observations show that individuals displaying stable weight maintenance can be expected to expend energy at a rate predictable from their metabolic body size (i.e., kcal/d= 66.5BWkg.75). Yet, perturbing energy balance so as either to elevate or lower body weight will cause energy expenditure, respectively, to increase (Rothwell and Stock, 1982) or, as seen in Fig. 1, to decline to a far greater extent that expected from the associated change in metabolic body size. It thus follows that, at any one time, there is for each individual but one body weight at which its daily energy expenditure will conform to that predicted by Kleiber’s rule. It is proposed that this particular body weight be viewed as representing the homeostatic null or set-point of that individual’s energy regulating system.
MONITORS OF BODY ENERGY STATUS
As Bernard and Cannon anticipated in their considerations of the general requirements for homeostasis, effective feedback to the regulating system would be essential. In the case of body energy, its stable maintenance and its defense when its balance has been perturbed implies—indeed requires--adequate feedback concerning energy status. In this regard, the last two decades have produced dramatic advances in our understanding of mechanisms that supply information on energy balance to the neural circuitry of energy intake and expenditure. These developments provide a detailed picture of multiple signaling pathways involved as feedback loops. And importantly, in the context of the present discussion, the new findings have established that the first of the two key criteria of a homeostatic system recognized by Bernard and Cannon (i.e. signals for regulating internal condition) unequivocally is met. The observations have also, of course, identified candidate targets for pharmacological and other interventions designed to modify the regulation of net body energy stores.
The gastrointestinal tract, including the pancreas and liver, is now known to provide extensive hormonal feedback to the neural circuits of ingestion and expenditure; this feedback reflects the fluxes of energy consumed and energy utilized. In fact, the GI tract is the largest endocrine organ of the body, measured either in terms of the number of endocrine cells it contains or in terms of the number of hormones those cells secrete (cf. Rehfeld, 1998). Genes for over 30 hormones and prohormones are expressed in the GI tract, and it secretes, including products of alternative splicing, some 100 or more active peptides. These hormones include, among others, the extensively investigated examples of ghrelin, gastric leptin, secretin, CCK, peptide, YY, glucagon-like peptide 1 from the stomach and intestines and insulin and glucagon from the pancreas.
Adipose tissue is also a major source of humoral signals, specifically signals that reflect lipogenesis, storage, and lipolysis and that supply this feedback to the neural circuits controlling energy balance. These last two decades have seen the traditional view of adipose tissue as a passive storage depot be replaced by the recognition that fat is a dynamic organ elaborating multiple hormones and adipokines. The revolution, which began with the discovery of leptin and its roles in energy balance, has expanded to include a list of well over a dozen other factors (e.g., adiponectin, resistin, adipsin) that are secreted in proportion to one or more of the functions of the adipocytes (cf. Badman & Flier, 2007).
Paralleling the change in perspective on the gut and adipose tissue as endocrine organs, in the last two decades it has also been recognized that neural afferent feedback from the peripheral organs that determine energy homeostasis is far more extensive than had been traditionally assumed. Modern advances in neural tract-tracing have established that the sensory or afferent innervation of the peripheral organs is both more extensive and more specialized than expected (cf. Powley & Phillips, 2002).
Both the neurons of the visceral sensory inflows and those of the rest of the neural circuitry determining energy intake and expenditure express receptors for the peptide hormones and humoral factors secreted by the GI tract and adipose tissue (cf. Havel, 2001; Moran, 2004). Elements of the central nervous systems circuits that control energy balance are also directly sensitive to circulating metabolites such as glucose and free fatty acids (e.g. Cota et al., 2006; Levin, 2001), establishing yet other feedback loops organized for monitoring energy status.
Thus, myriad signals generated in the processes of energy intake, digestion, metabolism and storage throughout the body converge on the central nervous system networks executing energy homeostasis. For some elements of the circuitry, such as the hypothalamic melanocortin system, and some of the peripheral hormones, such as leptin, insulin and CCK, the neuronal elements and cellular signaling pathways are now known in considerable detail (e.g., Schwartz et al., 2000). For other elements, fewer details are yet known (see Footnote 1). Nonetheless, there is extensive evidence that the feedback criterion, or the criterion that the system monitors the milieu interieur for body energy homeostasis, is satisfied.
ADJUSTMENTS IN REGULATED BODY ENERGY
There are times over an organism’s lifespan when body weight undergoes change. Were such a change to result from, for example, a scarcity of food, the significant accompanying reductions in daily energy expenditure would clearly identify the resulting weight loss as a displacement from its regulated level. In some instances, however, weight changes are not resisted but rather give the appearance of being homeostatically regulated. For example, the sizeable seasonable vatiation in body weight some species display have been shown be highly regular and, if challenged, effectively defended (Mrosovsky & Fisher, 1970). Likewise, the weight losses typically associated with injury or inflammatory processes (Lennie et al., 1995), or to various toxins (Seefield et al., 1984; Schwid et al., 1992) are accompanied by both by intake and expenditure adjustments appropriate to the maintenance of a lower body weight. Apparently, the mechanisms responsible for setting the level at which body energy is regulated can be adjusted so as to accommodate the special energy demands these conditions present.
EXPERIMENTALLY-INDUCED ALTERATIONS IN REGULATED BODY ENERY
Claude Bernard recognized the essential role the nervous system must play in coordinating and integrating the various components of a regulatory system. It is not unexpected, therefore, that vital insights into how the regulated level of body energy is set, and how this level might be adjusted, have emerged from particular experimental CNS interventions, particularly those involving hypothalamus.
It was Anand and Brobeck (1951) who first demonstrated that lesions of the lateral hypothalamus (LH) of rats produced a protracted aphagia and anorexia before feeding was resumed. While they and others viewed this as evidence for a hypothalamic ‘feeding center’, subsequent studies suggest that this lesion’s effects on food intake may actually be secondary to an altered level of body energy regulation. This possibility was first raised by the observation that, even after they had resumed feeding, LH animals maintained a chronically-reduced body weight which they appeared actively to defend (Powley & Keesey, 1970). If force-fed so as to restore their body weights to the level of nonlesioned animals, they again became aphagic or anorexic, remaining so until their body weight had again declined to this reduced level. If their weights were further reduced by caloric restriction, LH rats overate but again only until restoring body weight to this reduced level. (Mitchel & Keesey, 1977). Particularly compelling was the observation that animals whose body weight had been reduced prior to lesioning the LH would eat normally, or even be hyperphagic, immediately postlesion (Powley & Keesey, 1970). Evidently, the usual postlesion aphagia and anorexia did not reflect the loss of intake control but rather served as one means of lowering body weight to a reduced level of maintenance.
Daily energy expenditure observations provide further evidence of the effective defense of a reduced body weight by LH rats. First, their daily energy expenditure is appropriate to the smaller metabolic body mass they now maintain (Corbett et al., 1985). Lowering their body weight from its already-reduced level, causes a decline in energy expenditure significantly greater than expected from the change in metabolic body size, just as one sees when nonlesioned rats of normal weight are subjected to similar weight reductions. But, if overfed so as to restore their body weight to normal levels, their energy expenditure rises significantly above those of nonlesioned rats of this weight. 1 Effectively, LH-lesioned rats are metabolically normal only when at a body weight significantly below normal. Their capacity for the homeostatic regulation of body energy is apparently unimpaired but the body weight now defended has clearly been lowered.
Whether these naturally-occurring and hypothalamically-induced shifts in regulated body energy are the result of an altered feedback to the CNS regulatory centers or to a change in an internal standard or set-point to which that feedback is compared, remains to be determined. Nonetheless, it is reasonable to presume that a key role is played by this hypothalamic area in integrating the feedback and control functions of body energy regulation, and possibly in setting the level at which a balance is achieved.
BODY ENERGY HOMEOSTASIS: A SUMMARY
Bernard, Cannon, and others provided the framework for conceptualizing how the body’s internal environment is regulated. They identified the critical importance of systems for monitoring the body’s internal status and of systems for effectively compensating for perturbations of its stability.
While the contributions of these early workers led to the successful investigation of a host of regulated internal factors over past years, consideration of body energy from this perspective is a more recent development. This is probably not surprising when one considers how limited, until the last decade or so, our knowledge has been about systems for monitoring and transmitting information about the body’s energy status or about how metabolic processes are controlled in stabilizing body energy. The contributions of leptin and adiponectin, ghrelin, peptide YY, and other feedback signals to the careful monitoring of body energy status have been realized only rather recently. Likewise, the role of compensatory adjustments in whole body energy expenditure, complementing those in food intake, are only now being recognized and appreciated. The same is true for the means for experimentally intervening in the regulatory process so as to alter the level of maintained body energy. Thus, though the early workers failed to include body energy among the conditions of the body subject to homeostatic regulation, a sound foundation, based upon the work of the past several decades, appears now to be in place for its inclusion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Observations from the LH-lesioned animals induced to overeat and restore body weight to the level of nonlesioned controls provide a different perspective on energy expenditure adjustments in the service of body energy regulation. Traditionally, energy expenditure has been assumed to reflect the cost of maintaining the body’s lean tissue mass, its most metabolically-active component. Indeed, it is common practice to express the daily energy expenditure of individuals relative to an estimate of their lean body mass. However, as shown by the body composition and energy expenditure of the LH-lesioned rats restored to a normal body weight, under conditions calling for expenditure’s participation in the maintenance of energy balance, its relation to the lean tissue mass becomes more tenuous.
At the reduced body weight LH-lesioned rats ordinarily maintain, both their lean and adipose tissue masses are significantly below normal. But, when overfed so as to elevate their weight to the level of nonlesioned rats, nearly all the weight gain is in the form of adipose tissue. In effect, at a normal body weight LH rats are quite obese. Yet, as previously noted, their daily energy expenditure is markedly higher than that of nonlesioned rats having the same weight but significantly more lean tissue. Apparently responsible for this disconnect between lean tissue and daily energy expenditure is the participation of energy expenditure adjustments in the maintenance of body energy balance.
Certainly, the lean tissue mass is an important factor contributing to one’s daily energy needs. When in energy balance, it is likely the primary determinant. But, when body energy is perturbed and compensatory expenditure adjustments occur, the contribution of lean tissue to daily expenditure may be sharply reduced. In fact, in the example of the LH rats cited here, it seems probable that it is likely the large adipose mass that accounts for a significant part of the variance in daily expenditure.
Contributor Information
Richard E. Keesey, Department of Psychology, University of Wisconsin
Terry L. Powley, Department of Psychological Sciences, Purdue University
References
- Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus. Proceedings of the Society for Experimental Biology and Medicine. 1851;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132:2103–2115. doi: 10.1053/j.gastro.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Cohn C, Joseph D. Influence of body weight and body fat on appetite of “normal” lean and obese rats. Yale Journal of Biology and Medicine. 1962;34:598–607. [PMC free article] [PubMed] [Google Scholar]
- Corbett SW, Wilterdink EJ, Keesey RE. Resting oxygen consumption on over- and underfed rats with lateral hypothalamic lesions. Physiology and Behavior. 1985;35:971–977. doi: 10.1016/0031-9384(85)90268-9. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KAB, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Peripheral signals conveying metabolic information to the brain: Short-term and long-term regulation of food intake and energy homeostasis. Experimental Biology and Medicine. 2001;226:963–977. doi: 10.1177/153537020122601102. [DOI] [PubMed] [Google Scholar]
- Hemmingsen AM. Energy metabolism as related to body size and respiratory surfaces, and its evolution. Copenhagen Steno Memorial Hospital Reports. 1956–60:6–9. [Google Scholar]
- Hirvonen MD, Keesey RE. Chronically altered body protein levels following lateral hypothalamic lesions. American Journal of Physiology. 1996;39:R738–R743. doi: 10.1152/ajpregu.1996.270.4.R738. [DOI] [PubMed] [Google Scholar]
- Hirvonen MD, Keesey RE. Unpublished observations. [Google Scholar]
- Khosha T, Billewicz WZ. Measurement of changes in body weight. British Journal of Nutrition. 1964;18:225–235. doi: 10.1079/bjn19640022. [DOI] [PubMed] [Google Scholar]
- Kleiber M. Body size and metabolic rate. Physiological Reviews. 1947;15:511–541. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Lennie TA, McCarthy DO, Keesey RE. Body energy status and the metabolic response to acute inflammation. American Journal of Physiology. 1995;269:R1024–R1031. doi: 10.1152/ajpregu.1995.269.5.R1024. [DOI] [PubMed] [Google Scholar]
- Levin BE. Glucosensing neurons do more than just sense glucose. International Journal of Obesity. 2001;25 Suppl. 5:S68–S72. doi: 10.1038/sj.ijo.0801916. [DOI] [PubMed] [Google Scholar]
- Levitsky KA. Feeding patterns of rats in response to fasts and changes in environmental conditions. Physiology and Behavior. 1970;5:291–300. doi: 10.1016/0031-9384(70)90101-0. [DOI] [PubMed] [Google Scholar]
- Mitchel JS, Keesey RE. Defense of a lowered weight maintenance level in lateral hypothalamically lesioned rats: Evidence from a restriction-refeeding regimen. Physiology and Behavior. 1977;18:1121–1125. doi: 10.1016/0031-9384(77)90020-8. [DOI] [PubMed] [Google Scholar]
- Moran TL. Gut peptides in the control of food intake: 30 years of ideas. Physiology & Behavior. 2004;82:175–180. doi: 10.1016/j.physbeh.2004.04.048. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Fisher KC. Sliding set-points for body weight in ground squirrels during the hibernation season. Canadian Journal of Zoology. 1970;48:241–247. doi: 10.1139/z70-040. [DOI] [PubMed] [Google Scholar]
- Powley TL, Keesey RE. Relationship of body weight to the lateral hypothalamic feeding syndrome. Journal of Comparative and Physiological Psychology. 1970;70:25–36. doi: 10.1037/h0028390. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. American Journal of Physiology. 2002;283:G1217–G1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. The new biology of gastrointestinal hormones. Physiological Reviews. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau J-A, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of weight change on skeletal muscle work efficiency in human subjects. American Journal of Physiology. 2003;285:R183–R192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel R. Low dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. The Journal of Clinical Investigation. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Energy expenditure of “cafeteria”-fed rats determined from measurements of energy balance and indirect calorimetry. Journal of Physiology. 1982;328:371–377. doi: 10.1113/jphysiol.1982.sp014270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley JJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Schwid SS, Hirvonen MD, Keesey RE. Nicotine effects on body weight: A regulatory perspective. American Journal of Clinical Nutrition. 1992;55:878–884. doi: 10.1093/ajcn/55.4.878. [DOI] [PubMed] [Google Scholar]
- Seefeld MD, Keesey RE, Peterson RF. Body weight regulation in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology and Applied Pharmacology. 1984;76:526–536. doi: 10.1016/0041-008x(84)90357-0. [DOI] [PubMed] [Google Scholar]
- Ten-State Nutrition Survey (1968–1970) US DHEW Publication # (HSM) 72-8131.