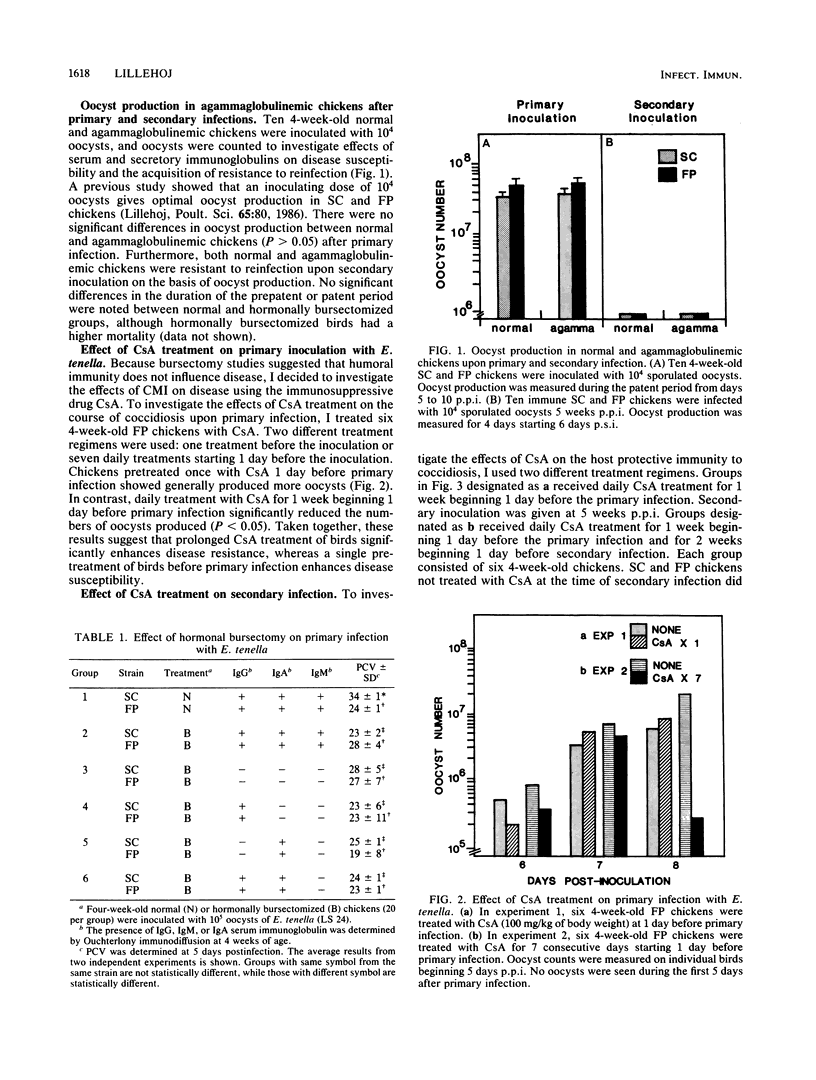
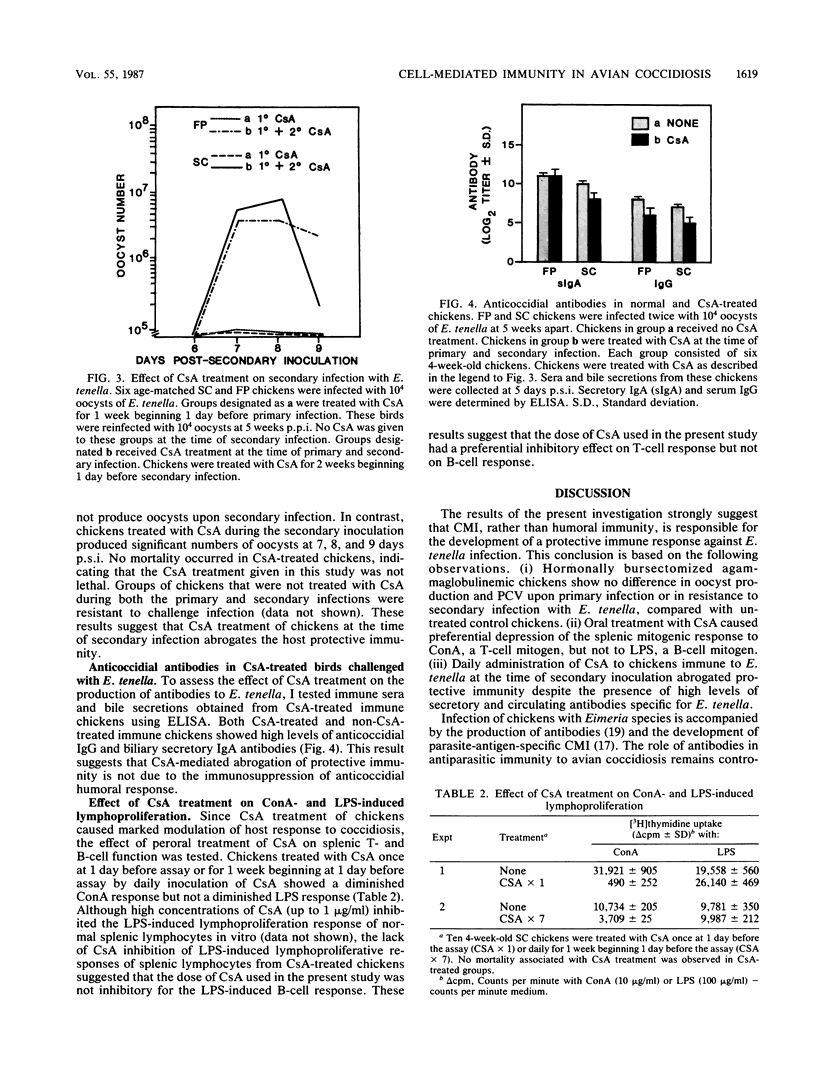
Abstract
The effects of cyclosporin A (CsA) treatment and hormonal bursectomy on Eimeria tenella infection of chickens were investigated to evaluate the role of humoral antibody and cell-mediated immunity (CMI) in the host protective immunity to an intestinal protozoan disease, coccidiosis. Hormonal bursectomy had no significant effect on the host response to E. tenella. CsA treatment had a differential effect on the course of disease depending on how CsA was given relative to infection. Daily administration of CsA for 7 days beginning 1 day before primary infection with E. tenella enhanced disease resistance, whereas a single dose of CsA given before primary infection enhanced disease susceptibility compared with that of untreated controls. Chickens treated with CsA during the primary infection were resistant to reinfection at 5 weeks post-primary infection. Treatment of chickens immune to E. tenella with CsA at the time of secondary infection abrogated their resistance to reinfection despite the presence of high levels of coccidia-specific secretory immunoglobulin A and serum immunoglobulin G. Splenic lymphocytes obtained after CsA treatment demonstrated a substantially depressed concanavalin A response, but not a depressed lipopolysaccharide response. Because CsA was not directly toxic to parasites in vivo when administered during the secondary infection, these results suggest that CsA interacts with the immune system to allow priming during the primary infection, while interfering with the effector function of CMI during the secondary infection. Taken together, present findings indicate that CMI plays a major role in host protective immunity to E. tenella.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behforouz N. C., Wenger C. D., Mathison B. A. Prophylactic treatment of BALB/c mice with cyclosporine A and its analog B-5-49 enhances resistance to Leishmania major. J Immunol. 1986 Apr 15;136(8):3067–3075. [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Bout D. T., Deslee D., Capron A. R. Protection against schistosomiasis produced by cyclosporin A. Am J Trop Med Hyg. 1984 Jan;33(1):185–186. doi: 10.4269/ajtmh.1984.33.185. [DOI] [PubMed] [Google Scholar]
- Bout D., Deslèe D., Capron A. Antischistosomal effect of cyclosporin A: cure and prevention of mouse and rat schistosomiasis mansoni. Infect Immun. 1986 Jun;52(3):823–827. doi: 10.1128/iai.52.3.823-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne R. Y. Immunosuppression for organ grafting -- observations on cyclosporin A. Immunol Rev. 1979;46:113–124. doi: 10.1111/j.1600-065x.1979.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- Giambrone J. J., Klesius P. H., Eckamn M. K., Edgar S. A. Influence of hormonal and chemical bursectomy on the development of acquired immunity to coccidia in broiler chickens. Poult Sci. 1981 Dec;60(12):2612–2618. doi: 10.3382/ps.0602612. [DOI] [PubMed] [Google Scholar]
- Grebenau M. D., Lerman S. P., Chi D. S., Thorbecke G. J. Transfer of agammaglobulinemia in the chicken. I. Generation of suppressor activity by injection of bursa cells. Cell Immunol. 1980 Apr;51(1):92–108. doi: 10.1016/0008-8749(80)90241-5. [DOI] [PubMed] [Google Scholar]
- Hughes H. P., Speer C. A., Kyle J. E., Dubey J. P. Activation of murine macrophages and a bovine monocyte cell line by bovine lymphokines to kill the intracellular pathogens Eimeria bovis and Toxoplasma gondii. Infect Immun. 1987 Mar;55(3):784–791. doi: 10.1128/iai.55.3.784-791.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Reid W. M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970 Aug;28(1):30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kaibara N., Hotokebuchi T., Takagishi K., Katsuki I. Paradoxical effects of cyclosporin A on collagen arthritis in rats. J Exp Med. 1983 Dec 1;158(6):2007–2015. doi: 10.1084/jem.158.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Gottlieb C. A., Budzko D. B. Exacerbation of Trypanosoma cruzi infection in mice treated with the immunoregulatory agent cyclosporin A. Tropenmed Parasitol. 1983 Mar;34(1):4–6. [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkl A., Klaus G. G. Selective effects of cyclosporin A on functional B cell subsets in the mouse. J Immunol. 1980 Dec;125(6):2526–2531. [PubMed] [Google Scholar]
- Larsson E. L. Cyclosporin A and dexamethasone suppress T cell responses by selectively acting at distinct sites of the triggering process. J Immunol. 1980 Jun;124(6):2828–2833. [PubMed] [Google Scholar]
- Lillehoj H. S. Immune response during coccidiosis in SC and FP chickens. I. In vitro assessment of T cell proliferation response to stage-specific parasite antigens. Vet Immunol Immunopathol. 1986 Dec;13(4):321–330. doi: 10.1016/0165-2427(86)90025-5. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S., Malek T. R., Shevach E. M. Differential effect of cyclosporin A on the expression of T and B lymphocyte activation antigens. J Immunol. 1984 Jul;133(1):244–250. [PubMed] [Google Scholar]
- Lillehoj H. S., Ruff M. D. Comparison of disease susceptibility and subclass-specific antibody response in SC and FP chickens experimentally inoculated with Eimeria tenella, E. acervulina, or E. maxima. Avian Dis. 1987 Jan-Mar;31(1):112–119. [PubMed] [Google Scholar]
- Mesfin G. M., Bellamy J. E. Thymic dependence of immunity to Eimeria falciformis var. pragensis in mice. Infect Immun. 1979 Feb;23(2):460–464. doi: 10.1128/iai.23.2.460-464.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell S. P., Scheibel L. W., Cole G. A. Inhibition by cyclosporin A of rodent malaria in vivo and human malaria in vitro. Infect Immun. 1982 Sep;37(3):1093–1100. doi: 10.1128/iai.37.3.1093-1100.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Long P. L. Immunity to coccidiosis: protective effects of transferred serum and cells investigated in chick embryos infected with Eimeria tenella. Parasitology. 1971 Oct;63(2):299–313. doi: 10.1017/s0031182000079610. [DOI] [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Solbach W., Forberg K., Kammerer E., Bogdan C., Röllinghoff M. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J Immunol. 1986 Jul 15;137(2):702–707. [PubMed] [Google Scholar]
- Thomson A. W., Moon D. K., Inoue Y., Geczy C. L., Nelson D. S. Modification of delayed-type hypersensitivity reactions to ovalbumin in cyclosporin A-treated guinea-pigs. Immunology. 1983 Feb;48(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Tutschka P. J., Beschorner W. E., Allison A. C., Burns W. H., Santos G. W. Use of cyclosporin A in allogeneic bone marrow transplantation in the rat. Nature. 1979 Jul 12;280(5718):148–151. doi: 10.1038/280148a0. [DOI] [PubMed] [Google Scholar]
- Wick G., Müller P. U., Schwarz S. Effect of cyclosporin A on spontaneous autoimmune thyroiditis of Obese strain (OS) chickens. Eur J Immunol. 1982 Oct;12(10):877–881. doi: 10.1002/eji.1830121014. [DOI] [PubMed] [Google Scholar]


