Abstract
Objective
To develop and evaluate the feasibility of a cue reactivity paradigm for young marijuana smokers.
Method
A laboratory procedure was developed involving neutral- and marijuana-related imagery, video, and in vivo cues. Fifteen adolescents and young adults with cannabis use disorders completed the procedure. Continuous Skin Conductance (SC) and Heart Rate (HR) were measured throughout the procedure. Participants completed questionnaires regarding marijuana craving before, during and after cue presentations.
Results
Higher levels of craving and SC were observed during marijuana cue presentations.
Conclusions
The procedure appears to elicit cue reactivity among adolescents and young adults with cannabis use disorders and should be further evaluated and refined with a larger sample. Implications for future studies are discussed.
Keywords: Marijuana, Cannabis, Craving, Cue reactivity, Adolescent, Young Adult
The initiation of marijuana use typically occurs in adolescence, and young marijuana smokers are more likely than their older counterparts to exhibit dependence symptoms and difficulty cutting down (Chen & Anthony, 2003). Among high school seniors, 5.1% use marijuana daily and 18.8% have used marijuana in the past 30 days (Johnston et al., 2007). Cannabis use disorders (abuse or dependence) are present in 3.6% of adolescents and 5.9% of young adults, compared with only 0.7% of adults over the age of 25 (SAMHSA, 2007).
Factors contributing to continued use and relapse are not well understood in young marijuana smokers (Latimer et al., 2000; Ramo et al., 2005). Craving, which is common in adults and adolescents with cannabis use disorders, may be one of these factors (Budney et al., 1999; Heishman & Singleton, 2006; Milin et al., 2008; Vandrey et al., 2005). The laboratory-based cue reactivity paradigm is one means for assessing craving and the factors that influence it. In this paradigm, the researcher attempts to induce craving in substance-using participants by presenting them with cues associated with their respective substance of use. In general, this approach leads to robust increases in craving, along with modest increases in objective physiological measures such as heart rate and skin conductance (for review, see Carter & Tiffany, 1999).
Despite the fact that marijuana is the most commonly used illicit substance in the United States (SAMHSA, 2007), there has been minimal investigation of marijuana cue reactivity in adults or adolescents. In one study, adult participants reported increased craving in response to auditory imagery scripts (Singleton et al., 2002). In another, regular marijuana users spent more time viewing marijuana-related pictorial cues than neutral cues and were more likely to rate the marijuana cues as pleasant than non-marijuana users (Field et al., 2006).
The present study was conducted to address significant gaps in marijuana cue reactivity literature by including adolescent and young adult marijuana smokers and by exploring potential physiological correlates of marijuana craving. The goal was to assess the feasibility of conducting a laboratory-based study involving young marijuana smokers and to develop a set of procedures that could reliably evoke craving for marijuana within this understudied sample of individuals. It was predicted that participants would show greater craving and physiological reactivity in response to marijuana cues relative to matched neutral cues.
Method
Participants
Fifteen participants (mean age [± SD]: 19.0 ± 1.6 years; range 16 to 21 years) enrolled and completed the study. Six were age 16 to 18 and nine were age 19 to 21. Five were female (one Black, one Native American, three White) and ten were male (one Hispanic, one Native American, eight White). Nine met DSM-IV-TR criteria for Cannabis Abuse and six met criteria for Cannabis Dependence. In the 30 days prior to study entry, participants reported smoking marijuana an average of 2.1 ± 1.9 “joints”/day (range 0.4 to 7.4). They were required to abstain from marijuana for 24 hours prior to the cue reactivity session, confirmed using the creatinine-normalized tetrahydrocannabinol (THC) method (Budney et al., 2003; Huestis & Cone, 1998). Participants completing all study procedures were compensated with $75 in department store gift cards. The Medical University of South Carolina’s Institutional Review Board approved this study. All participants 18 to 21 years old provided informed consent. For participants under 18 years old, parents or legal guardians provided informed consent and subjects provided assent.
Cue Stimuli
In the cue reactivity procedure, there were two cue types, neutral and marijuana. For each cue type, there were three presentation types, presented successively, including an auditory imagery script, a video cue, and an in vivo cue-handling procedure. We reasoned that the successive use of three differing modalities of cue presentation would maximize our ability to evoke cue-related reactivity in our participants.
Imagery script
Scripts were based on those previously used and described in detail by Singleton and colleagues (Singleton et al., 2002). Participants were instructed to listen to the script and imagine themselves in the scene. The neutral script described a pleasant day at the beach. The marijuana script described sitting in a room surrounded by friends smoking marijuana. Each script lasted for 75 seconds, followed by 15 more seconds during which time participants were instructed to continue imagining themselves in the scene, at the end of which participants were told to stop (90 seconds total).
Video cue
The video cue consisted of brief video clips. Neutral clips depicted adolescents drinking water. Marijuana clips depicted adolescents preparing and smoking marijuana. Participants viewed the videos for 90 seconds each.
In vivo cue
The in vivo cue condition involved the presentation of a pre-recorded standardized set of instructions that instructed participants to handle neutral- or marijuana-related objects. Neutral objects included a pencil and eraser. Participants were asked to hold both objects and to smell the pencil. Marijuana objects included a marijuana cigarette and a lighter. Participants were asked to hold both objects, smell the marijuana cigarette, and briefly “flick” the lighter. The total duration of the in vivo cues was 90 seconds. Participants were instructed to handle both neutral and marijuana in vivo cues via standard, matched, strictly-timed instructions in order to minimize differences in movement between neutral and marijuana conditions.
Cue presentation order
Neutral and marijuana cues were structured as three-part serial presentations, spanning from imagery to video to in vivo cues, in order to progress from more abstract forms of cues to more concrete forms of cues. Neutral stimuli were presented first and all participants received the same fixed order (cf., Rohsenow et al., 2000; Rohsenow et al., 2001) in order to prevent carryover effects that can occur when drug-related stimuli are presented first (Monti et al., 1987). In order to further minimize carryover effects, there was a twenty-minute rest period consisting of a nature slide show between the presentation of neutral cues and the presentation of marijuana cues. Instructions were recorded digitally using Adobe Audition 1.5, and presented using DMDX stimulus presentation software (Forster & Forster, 2003). Participants received instructions through a pair of BOSE noise-canceling headphones.
Measures
Craving
Craving for marijuana was measured using the Marijuana Craving Questionnaire (MCQ, Heishman et al., 2001, Singleton et al., 2002, Heishman & Singleton, 2006), and using a single-item rating (described below). The MCQ is a likert-based self-assessment instrument for marijuana craving that has been validated in adults. In the present study, the 12-item version of the MCQ was used, which has the three items from each factor of the full 47-item MCQ that exhibited the most within-factor reliability. Each item is rated on a scale from 1 to 7. The four factors of the MCQ represent four constructs characterizing marijuana craving (Singleton et al., 2002). The Compulsivity factor reflects an inability to control marijuana use. The Emotionality factor reflects the use of marijuana in anticipation of relief from withdrawal or negative mood. The Expectancy factor reflects anticipation of positive outcomes from smoking marijuana. The Purposefulness factor reflects intention and planning to use marijuana for positive outcomes. Of note, Heishman, Singleton, and colleagues do not recommend combining scores on the four MCQ factors into a single (“grand total”) score. Instead, the mean of the three items in each factor is calculated. The MCQ ratings were collected four times: once before and once after the entire three-part series of neutral cues, as well as once before and once after the entire three-part series of marijuana cues. The single-item craving rating was a visual analog rating that involved the presentation of the following statement: “I have a desire to smoke marijuana.” The item was rated on a 21-point (0 to 20) scale. It was collected eight times: once immediately before presentation of the entire three-part series of neutral cues, and after each individual neutral imagery, video and in vivo cue presentation, as well as once before the entire three-part series of marijuana cues and after each individual marijuana imagery, video and in vivo cue presentation.
Skin Conductance (SC)
Continuous SC data were collected via two sensors placed on the hypothenar eminence of the non-dominant hand. A Coulbourn Lab Linc V Series V71-23 Isolated Skin Conductance Coupler was used. Data were sampled at 20 Hz stored in arbitrary A/D units calibrated to 10 microsiemens per 100 A/D units. For each participant, data were inspected to find the maximum and minimum SC values and then range corrected using the approach proposed by Lykken and Venables (1971). SC data were collected for 30 seconds before and 90 seconds during each individual presentation of imagery, video, and in vivo cues for both neutral and marijuana conditions. Within each data segment, means were taken for every 10 seconds of data. Thus, each 30-second data segment produced 3 mean values representing 10-second averages. Similarly, each 90-second data segment produced 9 mean values representing 10-second averages. Note: due to equipment error, SC data were not available for one participant.
Heart Rate (HR)
Heart rate sensors were placed on the right mid clavicle bone, left lower rib cage, and non-dominant forearm. Continuous heart rate data were sampled at 1000 Hz using a Coulbourn Lab Linc V series V71-01 Bioamplifier with a low pass filter of 40Hz and a high pass filter of 8 Hz. Heart beats were detected with a Coulbourn V21-10 Dual Comparator calibrated to detect the peak of the R-wave within the HR waveform. HR data were stored as inter-beat intervals and converted offline into beats per minute (BPM). Heart rate data were collected concurrently with SC data (as described for SC above). As with SC, mean values were calculated for every 10-seconds of data within each segment.
Statistical Analysis
For all four MCQ factors, ratings collected prior to cue presentation were subtracted from ratings collected after cue presentation for both neutral and marijuana conditions. The resulting change scores were examined using paired t-test comparisons, with a Bonferroni-corrected p-level of 0.0125 (0.05/4) to protect against inflation of Type I errors produced by multiple comparisons.
Change scores for single-item craving ratings in response to both neutral and marijuana cues were calculated by subtracting the rating made immediately before each respective three-part series of cues from the single-item ratings collected immediately after each individual cue presentation type (imagery, video, and in vivo). Similarly, physiological change scores were calculated by subtracting the minimum SC and HR values collected immediately prior to each cue presentation type (imagery, video, and in vivo) from the maximum 10-second mean values collected during the respective cue presentation. Change scores for the single-item craving rating and physiological measures were entered into repeated-measures ANOVA with two repeated factors: Cue Type (Neutral, Marijuana) and Presentation Type (imagery, video, and in vivo). Post-hoc ANOVA and t-test comparisons were used to further investigate main and interactive effects.
Results
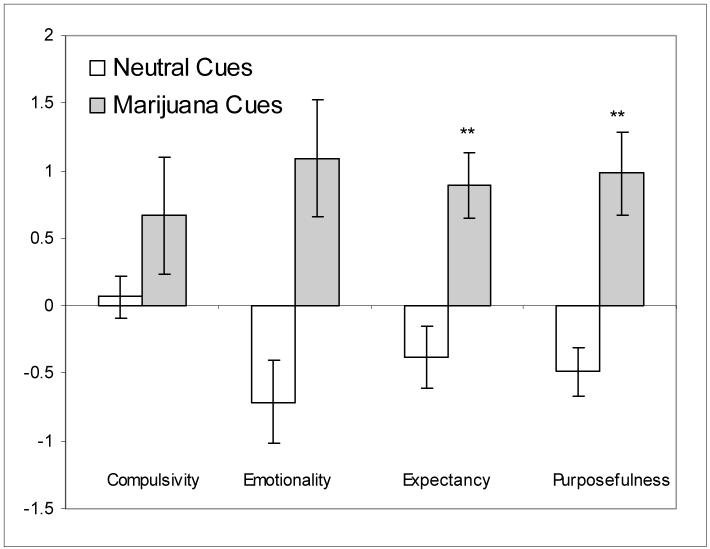
MCQ rating results revealed a significant effect for the Expectancy factor (t= 3.01, p < 0.01), with the Marijuana change score, M = 0.89, SD = 0.93 exceeding the Neutral change score, M = -0.38, SD = 0.88. A significant effect was also observed for the Purposefulness factor (t = 3.91, p < 0.01), with the mean Marijuana change score, M = 0.98, SD = 1.16, exceeding the Neutral change score, M = -0.49, SD = 0.70. The effects for the Compulsivity factor did not reach significance (p = 0.14), while the Emotionality factor did not exceed the Bonferroni-corrected level of significance (p = 0.02) (Figure 1).
Figure 1. Change scores for MCQ Factors (mean ± SEM).
Note: MCQ change scores reflect change from baseline to immediately after completion of the cue reactivity procedure. MCQ ratings range from 1 to 7.
** p < 0.01, paired t-test comparisons (marijuana v. neutral)
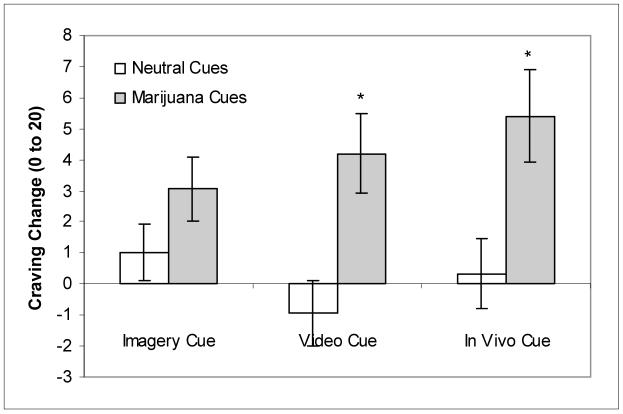
The single-item craving rating yielded significant effects for Cue Type, F(1,14) = 5.06, p < 0.05, Presentation Type, F(2, 28) = 4.54, p < 0.05, and Presentation Type x Cue Type interaction, F(2, 28) = 7.64, p < 0.01. Post-hoc t-tests revealed that craving ratings in response to the video (t = 2.54, df = 14, p < 0.05) and in vivo (t = 2.48, df = 14, p < 0.05) cue presentations were higher within the marijuana condition than the neutral condition (Figure 2).
Figure 2. Change scores for single-item craving rating for each cue type (mean ± SEM).
Note: In contrast to MCQ scores, ratings were collected immediately after each respective cue presentation. Craving ratings range from 0 to 20.
* p < 0.05, paired t-test comparisons (marijuana v. neutral)
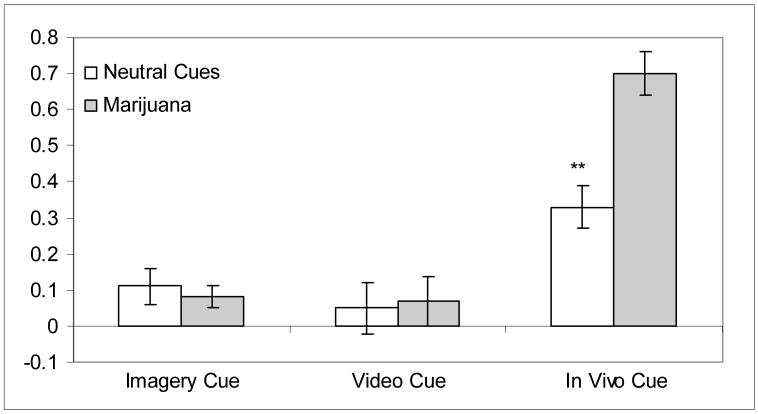
Repeated measures ANOVA for skin conductance revealed significant effects for Cue Type, F(1, 13) = 5.79, p < 0.05, Presentation Type, F(2, 26) = 64.46, p < 0.001, and the Cue Type x Presentation Type interaction, F(2, 26) = 12.98, p < 0.01. Examination of the data suggested that SC increased over the course of the cue presentation during presentations of both neutral and marijuana cues. Specifically, the third (in vivo) cue presentation, M = 0.52, SD = 0.13, in which participants moved to handle the cues, evoked greater SC than the first (imagery) and second (video) cue presentations, M = 0.09, SD = 0.15 and M = 0.06, SD = 0.22, respectively. Notably, SC during the third (in vivo) cue presentation was significantly higher during presentation of the marijuana cues relative to the neutral cues, t = -3.70, df = 13, p < 0.01 (Figure 3).
Figure 3. Change scores for corrected skin conductance values by cue type (mean ± SEM).
Note: Means reflect maximum change in SC during each respective cue presentation
** p < 0.01, paired t-test comparisons (marijuana v. neutral)
For heart rate, there was also evidence of an increase during both the neutral and marijuana presentations. The effect for Presentation Type was significant, F(2, 28) = 50.00, p < 0.001, with the third (in vivo) presentation type (when participants were moving) evoking greater HR change, M = 18.51, SD = 8.41, relative to the first (imagery), M = 4.44, SD = 3.14, t = 7.57, and second (video) presentations, M = 3.98, SD = 1.88, t = 7.26 (p < 0.001 for both). However, in contrast to the SC results, no significant differences in HR were observed between marijuana and neutral cue conditions.
Discussion
To our knowledge, this is the first published marijuana cue reactivity study to incorporate adolescent and young adult participants and to explore potential physiological correlates of marijuana craving. Within the present paradigm, greater craving and skin conductance were observed during the presentation of marijuana cues relative to neutral cues. Coupled with previous findings in young alcohol (Tapert et al., 2003; Thomas et al., 2005) and nicotine (Upadhyaya et al., 2004; Upadhyaya et al., 2006) users, these findings add to the emerging evidence that young substance users may exhibit subjective and physiological cue reactivity.
The present study involved a small, predominantly White and male sample, which limits the generalizability of the findings. Additionally, the study presented cues in a fixed order in which marijuana cues were always presented last, with a nature show preceding marijuana but not neutral cues. While prior publications (Monti et al., 1987; Rohsenow et al., 2000; Rohsenow et al., 2001) support the use of a fixed order of cue presentation (neutral first, followed by active) to avoid carryover effects, this limits the ability to rule out potential time-dependent confounding factors. Additionally, the fixed order of individual presentation types (imagery, video, in vivo) limits the ability to determine which of these evokes the most robust and reliable cravings. Still, the finding that in vivo cues coincided with the most robust reactivity is consistent with prior studies of cigarette smokers (Niaura et al., 1998, Shadel et al., 2001) and alcohol abusers (Staiger & White 1991).
In sum, despite the limitations indicated above, these results suggest that the present paradigm may indeed evoke craving and skin conductance reactivity to marijuana cues in young marijuana smokers. The present study also demonstrates that it is feasible to recruit young marijuana smokers to investigate cue-induced craving and physiological reactivity. Thus, future studies evaluating this paradigm are justified. Such investigations should strive to incorporate younger adolescent participants, a larger sample size, a counterbalanced order, and a method to examine the relationship between cue reactivity and subsequent marijuana use or relapse.
Acknowledgments
This work was supported by the American Academy of Child and Adolescent Psychiatry (AACAP)/National Institute on Drug Abuse Physician Scientist in Substance Abuse K12 Award (5K12 DA 000357), the AACAP Pilot Research Award, and the General Clinical Research Center (GCRC) USPHS M01 RR 01070 Grant. The authors would like to thank Doug Christie, Erin EuDaly, Gina Frattaroli, Christine Horne, Ashley Leinbach, Laura Grace Rollins, and the MUSC GCRC staff for their efforts in this study. The authors gratefully acknowledge the editorial contributions of Michael E. Saladin and Stephen J. Heishman.
References
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. Journal of Abnormal Psychology. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1321. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chen CY, Anthony JC. Possible age-associated bias in reporting of clinical features of drug dependence: epidemiological evidence on adolescent-onset marijuana use. Addiction. 2003;98:71–82. doi: 10.1046/j.1360-0443.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug and Alcohol Dependence. 2006;85:75–82. doi: 10.1016/j.drugalcdep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Forster KI, Forster JC. DMDX: a windows display program with millisecond accuracy. Behavior Research Methods, Instruments, & Computers. 2003;35:116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana craving questionnaire: Development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG. Assessment of cannabis craving using the Marijuana Craving Questionnaire. Methods in Molecular Medicine. 2006;123:209–216. doi: 10.1385/1-59259-999-0:209. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal of Analytical Toxicology. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Overall, illicit drug use by American teens continues gradual decline in 2007. University of Michigan News and Information Services; Ann Arbor, MI: 2007. Retrieved February 11, 2007, from http://www.monitoringthefuture.org. [Google Scholar]
- Latimer WW, Winters KC, Stinchfield R, Traver RE. Demographic, individual, and interpersonal predictors of adolescent alcohol and marijuana use following treatment. Psychology of Addictive Behaviors. 2000;14:162–73. doi: 10.1037//0893-164x.14.2.162. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Milin R, Manion I, Dare G, Walker S. Prospective assessment of cannabis withdrawal in adolescents with cannabis dependence: a pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:174–179. doi: 10.1097/chi.0b013e31815cdd73. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg T D, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. Journal of Abnormal Psychology. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addictive Behaviors. 1998;23:209–224. doi: 10.1016/s0306-4603(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Ramo DE, Anderson KG, Tate SR, Brown SA. Characteristics of relapse to substance use in comorbid adolescents. Addictive Behaviors. 2005;30:1811–23. doi: 10.1016/j.addbeh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. Naltrexone’s effects on reactivity to alcohol cues among alcoholic men. Journal of Abnormal Psychology. 2000;109:738–742. [PubMed] [Google Scholar]
- Rohsenow D, Monti P, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, et al. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96:1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Niaura R, Abrams DB. Effect of different cue stimulus delivery channels on craving reactivity: comparing in vivo and video cues in regular cigarette smokers. Journal of Behavior Therapy and Experimental Psychiatry. 2001;32:203–209. doi: 10.1016/s0005-7916(01)00035-0. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Trotman AJM, Zavahir M, Taylor RC, Heishman SJ. Determination of the reliability and validity of the marijuana craving questionnaire using imagery scripts. Experimental and Clinical Psychopharmacology. 2002;10:47–53. doi: 10.1037//1064-1297.10.1.47. [DOI] [PubMed] [Google Scholar]
- Staiger PK, White JM. Cue reactivity in alcohol abusers: stimulus specificity and extinction of the responses. Addictive Behaviors. 1991;16:211–221. doi: 10.1016/0306-4603(91)90014-9. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2007. Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293. [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, et al. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Deas D. Alcohol cue reactivity in alcohol-dependent adolescents. Journal of Studies on Alcohol. 2005;66:354–360. doi: 10.15288/jsa.2005.66.354. [DOI] [PubMed] [Google Scholar]
- Upadhyaya HP, Drobes DJ, Thomas SE. Reactivity to smoking cues in adolescent cigarette smokers. Addictive Behaviors. 2004;29:849–856. doi: 10.1016/j.addbeh.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Upadhyaya HP, Drobes DJ, Wang W. Reactivity to in vivo smoking cues in older adolescent cigarette smokers. Nicotine & Tobacco Research. 2006;8:135–140. doi: 10.1080/14622200500432112. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug and Alcohol Dependence. 2005;78:205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]