Abstract
The protein alpha-synuclein is implicated in the development of Parkinson’s disease. The molecule forms Lewy body aggregates that are hallmarks of the disease, has been associated with the spread of neuropathology from the peripheral to the central nervous system, and appears to be involved with the autonomic disorders responsible for the gastrointestinal (GI) symptoms of individuals afflicted with Parkinson’s. To characterize the normative expression of alpha-synuclein in the innervation of the GI tract, we examined both the postganglionic neurons and the preganglionic projections by which the disease is postulated to retrogradely invade the CNS. Specifically, in Fischer 344 and Sprague Dawley rats, immunohistochemistry in conjunction with injections of the tracer Dextran-Texas Red was used to determine, respectively, the expression of alpha-synuclein in the myenteric plexus and in the vagal terminals. Alpha-synuclein is expressed in a subpopulation of myenteric neurons, with the proportion of positive somata increasing from the stomach (~3%) through duodenum (proximal, ~6%; distal, ~13%) to jejunum (~22%). Alpha-synuclein is co-expressed with the nitrergic enzyme NOS or the cholinergic markers calbindin and calretinin in regionally specific patterns: ~90% of forestomach neurons positive for alpha-synuclein express NOS, whereas ~92% of corpus-antrum neurons positive for alpha-synuclein express cholinergic markers. Vagal afferent endings in the myenteric plexus and the GI smooth muscle do not express alpha-synuclein, whereas, virtually all vagal preganglionic projections to the gut express alpha-synuclein, both in axons and in terminal varicosites in apposition with myenteric neurons. Vagotomy eliminates most, but not all, alpha-synuclein-positive neurites in the plexus. Some vagal preganglionic efferents expressing alpha-synuclein form varicose terminal rings around myenteric plexus neurons that are also positive for the protein, thus providing a candidate alpha-synuclein-expressing pathway for the retrograde transport of putative Parkinson’s pathogens or toxins from the ENS to the CNS.
Keywords: Calbindin, Calretinin, Enteric Nervous System, Fischer 344, Lewy Neurites/Bodies, Nitric Oxide Synthase
Introduction
Lewy bodies and neurites are neuropathological markers of Parkinson’s disease that are composed of the protein alpha-synuclein (George, 2002), and antibodies to the molecule are commonly used diagnostically to identify aggregations of Lewy material in the nervous system. The normal function(s) of alpha-synuclein is not entirely known, but its presence in high concentrations in presynaptic terminals suggests a role in the maintenance of synaptic vesicle pools (Cabin et al., 2002) and more specifically a possible modulatory role in vesicle priming (Larsen et al., 2006; Gitler and Shorter, 2007). Abnormal processing of alpha-synuclein leads to pathological changes in its binding properties with over-expression and excess accumulation of alpha-synuclein resulting in compromised function (e.g., impaired axonal transport and interference in normal membrane trafficking) and reduced viability of neurons (Galvin et al., 1999; Chua and Tang, 2006; Gitler and Shorter, 2007).
Alpha-synuclein is widely, but not universally, expressed in the CNS (Li et al., 2002; Brenz Verca et al., 2003; Adamczyk et al., 2005; Yu et al., 2007). Furthermore, though the patterns are less thoroughly characterized for the periphery, the protein is also expressed in some elements of the peripheral autonomic nervous system (Bloch et al., 2006; Braak et al., 2007). Recently, from analyses of the temporal and spatial patterns of the spread of Lewy aggregates throughout the nervous system over the progression of Parkinson’s disease, Braak and colleagues have suggested that alpha-synuclein may be causally involved in the development of idiopathic Parkinson’s disease. Braak and co-workers (Del Tredici et al., 2002; Braak et al., 2003, 2006) determined that the appearance of alpha-synuclein-positive Lewy pathology initially occurs, during the earliest stage of Parkinson’s disease, in both the enteric nervous system (ENS) and the dorsal motor nucleus of the vagus (dmnX). These observations, combined with the fact that within the CNS, Lewy inclusion bodies first appear in the brainstem and then spread through susceptible brain regions in an ascending course to the cerebral cortex, led Braak and his colleagues to put forth the novel explanation that Parkinson’s disease starts in the ENS and spreads, as has been demonstrated for other “infective” agents such as prion proteins and viruses (van Keulen et al., 2000; McBride et al., 2001) and for neural tracers such as Fluorogold (Powley et al., 1987; Powley and Berthoud, 1991), to the CNS via vagal preganglionic innervation of the gut. Moreover, they suggest that the occurrence of alpha-synuclein aggregations in specific enteric neurons and projections to these peripheral targets involved in the pathological process advocates for the expression of alpha-synuclein as a prerequisite for development of later stages of Parkinson’s disease. Thus the Braak model suggests the general proposal that Parkinson’s disease may be produced by an environmental pathogen that breaches the mucosal barrier of the gastrointestinal (GI) tract (and presumably the olfactory system as well; Hawkes et al., 2007) in susceptible individuals. Further, the model suggests that the stomach is the most likely starting point for any pathological insult to gain access to axons from the dorsal vagal complex, because of the heavy innervation of the GI tract by vagal preganglionics (Berthoud et al., 1991; Holst et al., 1997).
While offering a general mechanism, the model does not adequately define a particular pathway (i.e., a specific subset of enteric neurons or vagal efferents) from mucosa to brainstem and does not provide sufficient specifics to fully formulate detailed and testable hypotheses about the involvement of particular intrinsic and extrinsic pathways in the GI tract in the pathology. A fuller analysis of the expression of alpha-synuclein in the intrinsic innervation of the gut and the extrinsic projections to the stomach would elucidate the putative mechanisms involved in transporting a pathogenic signal that initiates alpha-synuclein misfolding and aggregation from the GI tract to the CNS.
Such an analysis of alpha-synuclein expression would also be particularly instructive, independent of the validity or details of the Braak model. The health of both the myenteric plexus, located within the smooth muscle wall of the GI tract, and the vagal preganglionic motor innervation of the myenteric plexus are crucial to the maintenance of daily GI function and health, and disruption of normal GI function is a common complaint of Parkinson’s patients, with signs of GI dysfunction predating the onset of motor movement symptoms by several years (Abbott et al., 2002; Ueki and Otsuka, 2004; Abbott et al., 2007). The neural basis of the functional GI disturbances afflicting Parkinson’s patients is unknown, but the symptoms suggest that deterioration of the neural mechanisms that regulate GI physiology might be occurring. The occurrence of Lewy material aggregates in the innervation of the GI tract are consistent with such an explanation, and a fuller characterization of the alpha-synuclein expressing elements of the autonomic innervation of the GI tract should thus help understand and ultimately treat the GI symptoms of Parkinson’s patients, regardless of any causal role in spreading the disease throughout the nervous system. Finally, since there are a variety of synucleinopathies other than Parkinson’s disease, a better characterization of the normative patterns of expression of alpha-synuclein in the autonomic nervous system may elucidate other disorders that can afflict the GI tract.
The goals of the present study, therefore, were threefold: First, to provide a thorough description of the normative innervation of the myenteric plexus of the stomach and duodenum by neural elements immunoreactive for alpha-synuclein. Second, to establish the distribution and neurochemical coding of alpha-synuclein neurons within the myenteric plexus. And, finally, to determine the relationship of vagal preganglionic terminals to these alpha-synuclein neurons.
Experimental procedures
Subjects
Three to ten-month-old, virgin male Fischer 344 (F344; n = 42) and Sprague-Dawley (SD; n = 12) rats were purchased from Harlan (Indianapolis, IN). Rats were housed individually and maintained on a 12:12 hour light:dark schedule at 22–24°C. Solid chow (laboratory diet No. 5001; PMI Feeds, Inc., Brentwood, MO) and tap water were available ad libitum. All procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996, and approved by the Purdue University Animal Care and Use Committee. Every effort was made to minimize the number of rats used and their suffering.
Selective Labeling of Vagal Preganglionic Efferents or Vagal Afferents
Overnight food-deprived rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). To label selectively the vagal efferent innervation of the myenteric plexus, the red fluorescent dye Dextran-Texas Red (5%; 10,000 MW, lysine fixable; D-1863; Molecular Probes, Eugene, OR) was pressure injected (PicoSpritzer II; General Valve Corporation, Fairfield, NJ) bilaterally into the dmnX using a glass micropipette. The rats (F344, n = 18; SD, n = 8) were maintained for 19 days post-dmnX-injection to allow for transport of the dye (Powley and Phillips, 2005). To label selectively vagal afferent innervation of the myenteric plexus and longitudinal smooth muscle, Dextran-Texas Red was pressure injected bilaterally into the right and the left nodose ganglia. The rats (SD, n = 4) were maintained for 14 days post-nodose-injection to allow for transport of the dye (Powley and Phillips, 2005).
Fluorescent Immunohistochemistry
Rats were euthanized with a lethal dose of sodium pentobarbital (180 mg/kg, i.p.) and perfused through the left ventricle of the heart with 200 ml of 0.01 M sodium phosphate buffer saline (PBS; pH 7.4; 38°C), followed by 500 ml of 4% paraformaldehyde (PF) in 0.1 M PBS (pH 7.4; 4°C). The stomach and duodenum (i.e., the first 4 cm of the small intestine distal to the pyloric sphincter) were removed. The stomach was divided into dorsal and ventral whole mounts by cutting along the greater and lesser curvatures. The duodenum was opened with a longitudinal cut along the mesenteric attachment. Whole mounts were fixed for an additional 2 h in 4% PF. The mucosa, submucosa, and circular muscle were then removed by fine dissection to facilitate penetration of the primary antibodies to the myenteric plexus.
Whole mounts were rinsed in PBS and then soaked for 5 to 6 days at room temperature in PBS containing 5% normal goat serum, 2% bovine serum albumin, 0.5% Triton X-100, and 0.08% Na Azide, followed by incubation for 24 h at room temperature in a cocktail consisting of a mouse primary and a rabbit primary diluted with PBS containing 2% normal goat serum, 2% bovine serum albumin, 0.3% Triton X-100, and 0.08% Na Azide. The primary antibodies used were mouse alpha-synuclein (1:2,500; 610787; BD Biosciences, San Jose, CA) and one of the following three rabbit antibodies: NOS (1:2,500; SC-648; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), calbindin (1:8,000; CB-38; SWant, Bellinzona, Switzerland), or calretinin (1:16,000; 7699/4; SWant). Whole mounts were then rinsed in PBS and 0.3% Triton X-100 (PBST), and incubated for 2 h at room temperature in a cocktail consisting of goat anti-mouse ALEXA Fluor 488 (1:500; A11029; Molecular Probes) and biotinylated anti-rabbit raised in goat (1:500; 111-065-144; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted with PBST. Next, whole mounts were further rinsed in PBS and then incubated for 1 h with streptavidin ALEXA Fluor 350 (1:500; S11249; Molecular Probes). Finally, labeled whole mounts were rinsed in PBS, mounted on gelatin-coated slides, air-dried overnight, dehydrated in alcohol, cleared in xylene, cover-slipped with DePeX (Electron Microscopy Sciences, Hatfield, PA), and allowed at least one month to cure (Espada et al., 2005). Specificity of the staining was assessed for every rat by omitting one of the primary antibodies on randomly sampled jejunal whole mounts. In some cases (n = 4), Dextran-Texas Red was injected and the primary antibodies were omitted from the processing steps to evaluate possible bleed-through of Texas Red from the red to the green and blue channels.
Complete Subdiaphragmatic Vagotomy
Twenty F344 rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and received either a complete subdiaphragmatic vagotomy (n = 15) or sham (n = 5) surgery. A complete subdiaphragmatic vagotomy consisted of cutting and cauterizing a) the posterior trunk above the point where it bifurcates into the gastric and celiac branches, and b) the anterior trunk above the hepatic branch (Phillips et al., 2000, 2003). For sham surgeries both vagal trunks were exposed and isolated but not cut. Rats were given ad libitum access to a nutritionally complete liquid diet (Isocal; 1.05 kcal/ml; Mead Johnson, Evansville, IN) to minimize any post-surgical problems resulting from vagotomy.
FluoroGold Verification of Vagotomy
Five days prior to perfusion, sham and vagotomized rats were injected intraperitoneally with 1 to 2 mg of FluoroGold (Fluorochrome, Inc., Englewood, CO) in 1 ml of saline to label the cell bodies of intact vagal efferent fibers innervating the GI tract (Powley et al., 1987). Following perfusion for permanent labeling (see below), the brainstems were removed and cryoprotected overnight in 15% sucrose in 4% PF. The medullas were then blocked, sectioned coronally at 30 µm in a cryostat, and mounted on gelatin coated slides. Slides were air-dried overnight, dehydrated in alcohol, cleared in xylene, and cover-slipped with DePeX.
Permanent Immunohistochemistry
A minimum of 30 days post-vagotomy, rats were killed with a lethal dose of sodium pentobarbital (180 mg/kg, i.p.) and perfused through the left ventricle of the heart with 0.01 M PBS followed by 500 ml of 4% PF. The stomach, duodenum, and jejunum (two 3 cm whole mounts taken approximately 52 cm distal to the pyloric sphincter) were removed, separated into whole mounts, post-fixed for 2 h in 4% PF, and then dissected, leaving whole mounts of the longitudinal muscle with the myenteric plexus attached.
Whole mounts of the GI tract were rinsed in PBS and then incubated for 30 min in a hydrogen peroxide:methanol (1:4) solution to quench endogenous peroxidase. Following several more PBS rinses, whole mounts were soaked for 72 h at room temperature in PBS containing 5% normal horse serum, 2% bovine serum albumin, 0.5% Triton X-100, and 0.08% Na Azide. Free-floating whole mounts were then incubated for 24 h at room temperature with a mouse antiserum raised against alpha-synuclein (1:5,000; BD Biosciences) diluted with PBS containing 2% normal horse serum, 2% bovine serum albumin, 0.3% Triton X-100, and 0.08% Na Azide. Next, whole mounts were rinsed in PBS and then incubated for 1 h at room temperature with biotinylated anti-mouse IgG, rat absorbed, raised in horse (diluted as per the recommendations of the supplier; BA-2001; Vector Laboratories, Inc., Burlingame, CA), followed by incubation for 1 h with an avidin-biotin-horseradish peroxidase complex (Vectastain Elite ABC Kit, Standard; Vector Laboratories, Inc.). Horseradish peroxidase was reacted with diaminobenzidine (DAB) and H2O2 for 5 min to yield a permanent deposit. Finally, stained whole mounts were rinsed in distilled water, mounted on gelatin-coated slides, air-dried overnight, dehydrated in alcohol, cleared in xylene, and cover-slipped with cytoseal (Richard-Allan Scientific, Kalamazoo, MI).
Whole mounts from an additional four unoperated control rats were permanently labeled with DAB to visualize the alpha-synuclein innervation of the GI tract, with the GI tissue from one of the four controls counterstained with 0.5% Cuprolinic Blue (quinolinic phthalocyanine; Polysciences, Inc., Warrington, PA) to label the entire population of myenteric neurons (Phillips et al., 2004a). Specificity of the staining was assessed for each rat by omitting the primary antibody in randomly chosen jejunal whole mounts.
Brightfield Image Capture, Confocal Microscopy, and Image Processing
Brightfield photomicrographs were acquired using a LEICA DM microscope with a SPOT RT Slider camera controlled using SPOT Software (V4.6; Diagnostic Instruments, Sterling Heights, MI). Confocal images were obtained using an Olympus DSU (Disk Scanning Unit) spinning disk confocal attached to a BX61 motorized microscope (Olympus, Center Valley, PA). The system was controlled using SlideBook digital microscopy software (V4.0; Intelligent Imaging Innovations, Denver, CO). Double- and triple-labeled sections were imaged by using filter sets appropriate for the specific visualization of Texas Red (DSU-MRFPHQ), ALEXA Fluor 488 (U-MNIBA2), and ALEXA Fluor 350 (U-MNUA2). A X60 water-immersion objective lens (n.a. = 1.2) was used. Extended-focus images or z-series of up to ten optical sections at z-increments of 1.0 µm were created. Images were captured, analyzed, and post-processed using the SlideBook software. In some cases, z-stacks were compressed into one focus plane (i.e., maximum value projection). To test for co-localization, single sections at the same focus plane were taken out of the z-stacks, and the two or three channels, respectively, were merged.
Final post-production was done using Photoshop CS3 (Adobe Systems, San Jose, CA). Adobe Photoshop CS3 was used to: (1) apply text and scale bars; (2) make minor adjustments to the color, brightness, contrast, and sharpness of the images to match as closely as possible the appearance of the original material viewed under the microscope; (3) remove artifact (i.e., dust and lint); and (4) to organize the final layout of the figures.
Quantitative Analysis
The density of alpha-synuclein-positive neurons in the myenteric plexus of the stomach, duodenum, and jejunum was determined using whole mounts from control rats permanently labeled with DAB. Our quantification and sampling protocols have been described previously (Phillips and Powley, 2001, 2007). Briefly, a grid that defined the location of the sampling sites was adjusted to fit each whole mount, and then a counting grid (area = 0.25 mm2) was positioned at each of the sites. The neurons within the counting grid and those overlapping the upper and left edges were counted; those neurons overlapping the lower and right edges were excluded. For the stomach, the counting grid was positioned at 140 equidistant sampling sites and the number of neurons positive for alpha-synuclein was determined for each site that fell within a defined region (i.e., forestomach, corpus, and antrum). The small intestine was sampled in transverse strips (i.e., columns) that were divided into seven sampling sites equidistant around the circumference of the organ. In this way, the mesenteric attachment and anti-mesenteric aspect of the tissue were both sampled. The duodenal columns were spaced every 0.5 cm, with the pyloric (P) boundary of the tissue serving as the zero point. Whole mounts of the jejunum were sampled in three equidistant transverse strips.
The percentage of alpha-synuclein-positive neurons co-reactive for either NOS, calbindin, or calretinin (n = 6/antibody) was determined using the quantitative sampling strategy described and validated by Burnstock and colleagues (Van Nassauw et al., 2002, Xiang and Burnstock, 2004) for fluorescently labeled myenteric neurons. Briefly, the entire whole mount of the ventral stomach wall was systematically scanned, the location of all alpha-synuclein-positive neurons was recorded, and co-localization with a second marker was determined. For whole mounts of the duodenum, 12 randomly chosen visual fields that contained myenteric ganglia with alpha-synuclein-positive neurons were captured using the Olympus DSU, and the percentage of co-localization with the second antibody was determined. Co-localization of the various antibodies could not be determined for jejunal whole mounts because they were only labeled for one antibody and served as deletion controls for the immunohistochemical protocol.
Results
Pattern of Innervation by Axons expressing Alpha-Synuclein
Throughout the stomach and proximal duodenum, axons immunoreactive for alpha-synuclein were so densely distributed that the myenteric plexus was clearly delineated (Figure 1). Large numbers of labeled fibers filled the interganglionic connectives, including the secondary and tertiary interganglionic connectives of the primary plexus. Individual axons were identifiable within the densely stained plexus, and were typically smooth in appearance when running within the connectives. Upon entering the ganglia, however, the same axons contained numerous axonal swellings along their length (Figure 2A,C). Within a ganglion, axons immunoreactive for alpha-synuclein formed an elaborate network encircling the myenteric neurons with tightly apposed strings of varicosities. These rings of varicosities occurred around both alpha-synuclein-negative (Figure 2A,D) and alpha-synuclein-positive neurons (Figure 2B,E).
Figure 1.
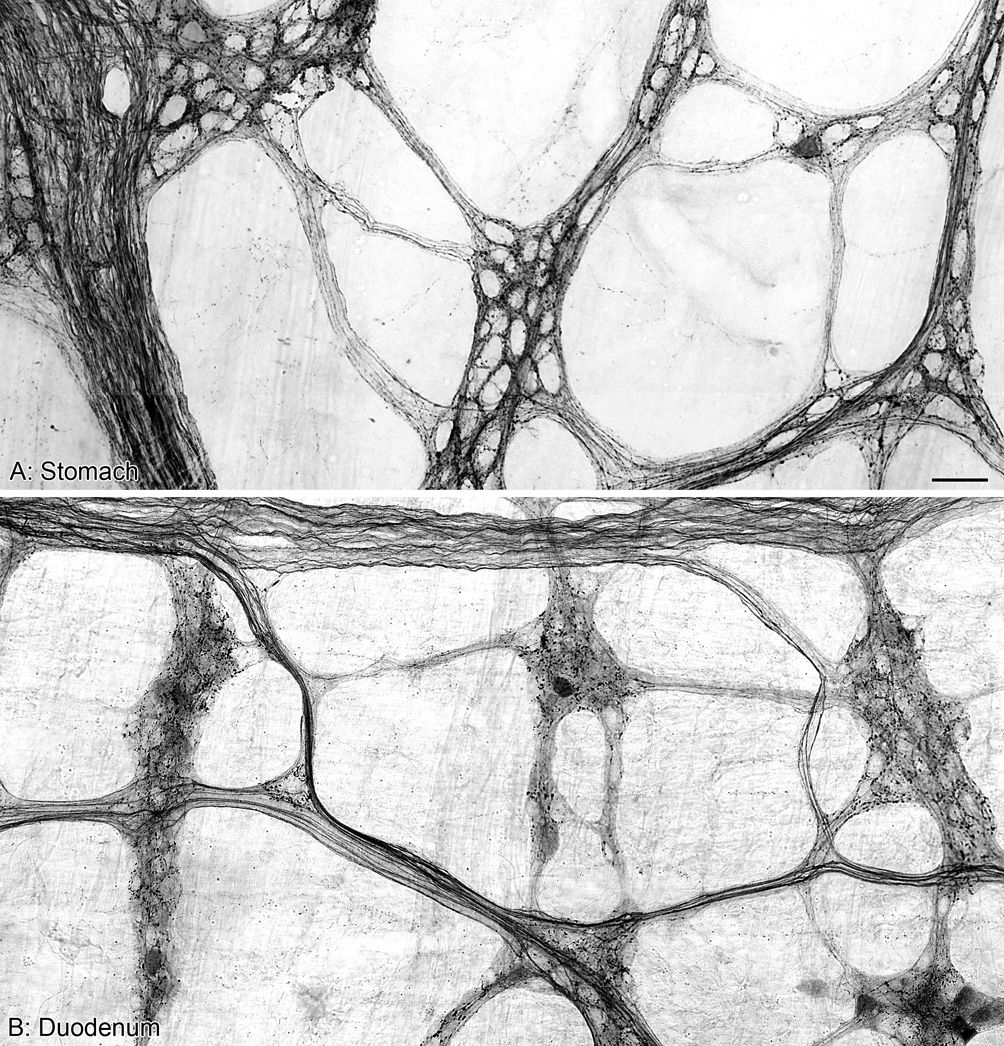
Panels A and B are both photomontages illustrating the innervation of the myenteric plexus by alpha-synuclein-positive fibers and varicosities in the stomach (A) and proximal duodenum (B) of control rats. Scale bar in A = 50 µm for A,B.
Figure 2.
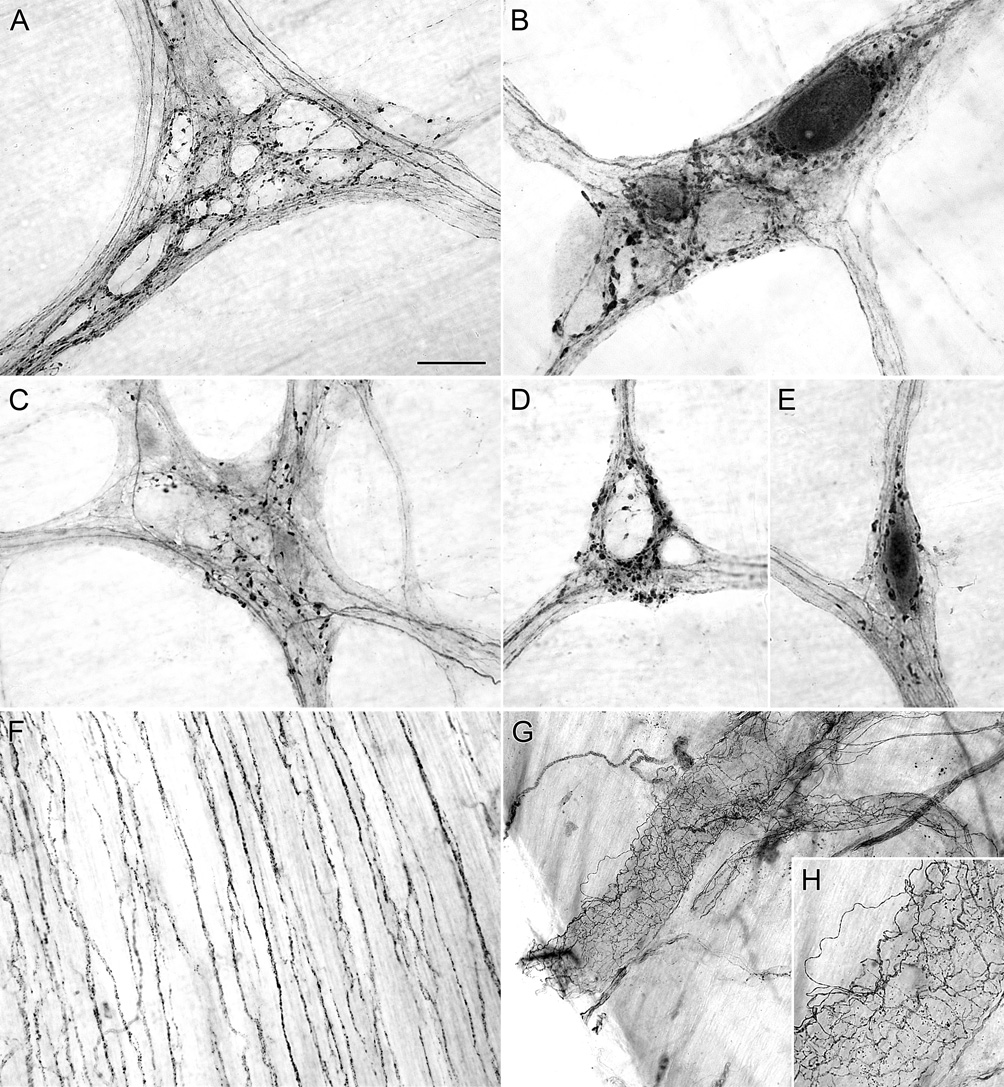
Alpha-synuclein-positive varicosities were observed throughout the myenteric ganglia of the stomach (A,B) and the duodenum (C–E) of control rats. Alpha-synuclein-positive varicosities encircle both darkly stained alpha-synuclein-positive neurons (B,E) and unstained alpha-synuclein-negative neurons (A,D). The outline of unstained neurons within ganglia, which represent alpha-synuclein-negative neurons, are silhouetted by dense innervation of alpha-synuclein-positive fibers and varicosities (A,D). In the stomach, the longitudinal muscle of the forestomach was innervated by alpha-synuclein-positive axons (F). Blood vessels in the stomach were innervated by alpha-synuclein-positive axons (G,H). Scale bar in A = 40 µm for A; 25 µm for B-E, 80 µm for F,H; 160 µm for G.
In addition to projecting to and through the myenteric plexus, axons immunoreactive for alpha-synuclein innervated the longitudinal smooth muscle of the forestomach (Figure 2F), and blood vessels in the stomach (Figure 2G,H) and duodenum. Alpha-synuclein-positive axons in the longitudinal smooth muscle of the forestomach were in close apposition to unstained intramuscular interstitial cells of Cajal whose processes and somas were silhouetted by the stained axons.
Enteric Neurons expressing Alpha-Synuclein
A subset of myenteric neurons expresses alpha-synuclein in both the nucleus and the cytoplasm of the somata. The density of myenteric neuronal somata immunoreactive for alpha-synuclein was low in the stomach, but became progressively greater in the small intestine as we sampled from sites more distal to the pyloric sphincter (Figure 3).
Figure 3.
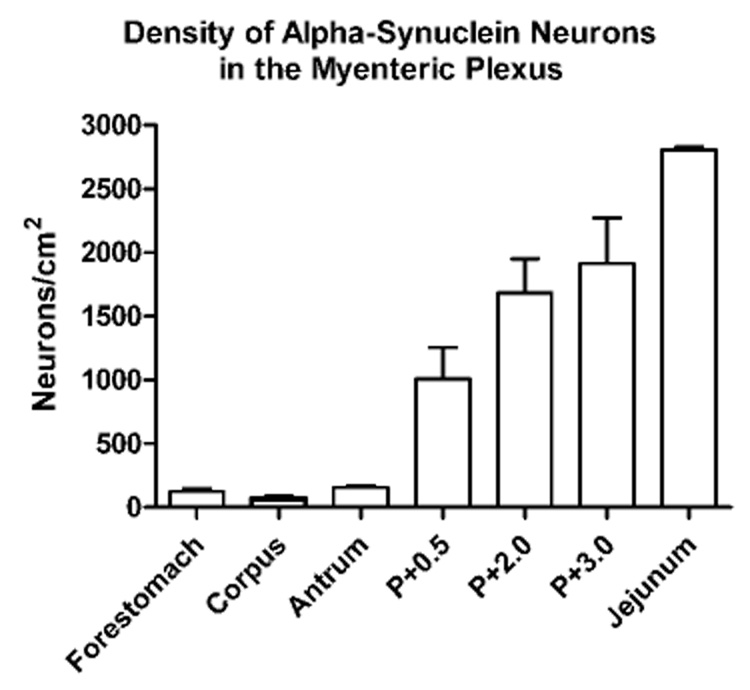
Mean ± S.E.M. density of neurons in the myenteric plexus immunoreactive for alpha-synuclein. The density of neurons positive for alpha-synuclein was greater in the small intestine compared to the stomach, and within the small intestine the density of neurons immunoreactive for alpha-synuclein increased the further we sampled from the pyloric (P) sphincter. The region of the jejunum sampled was at approximately P+52 cm.
Distribution of Alpha-synuclein-positive Myenteric Neurons Co-reactive for either NOS, Calbindin, or Calretinin
Since nitrergic neurons (expressing NOS) and cholinergic neurons (expressing calbindin or calretinin) are the two mutually exclusive chemical phenotypes of enteric neurons that collectively make up the myenteric neuronal population and since hypothetically alpha-synuclein expression might be correlated with one of the phenotypes, the patterns of co-localization were examined quantitatively.
In the survey of stomach whole mounts (ventral; n = 18), 715 myenteric neurons immunoreactive for alpha-synuclein were examined for co-reactivity with either NOS, calbindin, or calretinin (6 whole mounts/antibody). There was an average of 39.7 alpha-synuclein-positive neurons surveyed per ventral whole mount, with positive staining occurring in the nucleus and cytoplasm of both strongly- and weakly-stained cells. The neurons immunoreactive for alpha-synuclein were spread throughout the stomach with 64% located within the boundaries of the nonglandular forestomach, and the remaining 36% contained within the corpus-antrum. In the stomach whole mounts double labeled for alpha-synuclein and NOS, 58% of the alpha-synuclein-positive neurons overall were immunoreactive for NOS. When whole mounts were double labeled for alpha-synuclein and calbindin, 35% of the alpha-synuclein-positive neurons overall were immunoreactive for calbindin. Similarly, in whole mounts double labeled for alpha-synuclein and calretinin, 35% of the alpha-synuclein-positive neurons overall were co-reactive for calretinin.
Expressing co-localization for the stomach as overall averages masked a dramatic and specific pattern of co-expression. By instead plotting the regional organization of double labeled cells in the stomach, we determined that 90% of the alpha-synuclein neurons in the forestomach were co-reactive for NOS, while 92% of the alpha-synuclein neurons in the corpus-antrum were co-reactive for either calbindin or calretinin (Figure 4). This pattern of regional specificity for double labeled cells could not easily be explained away by the distribution of the individual markers because neurons immunoreactive for either NOS, calbindin, or calretinin were distributed throughout the myenteric plexus of the forestomach, corpus, and antrum, and were not localized within one single defined region of the stomach (Figure 5).
Figure 4.
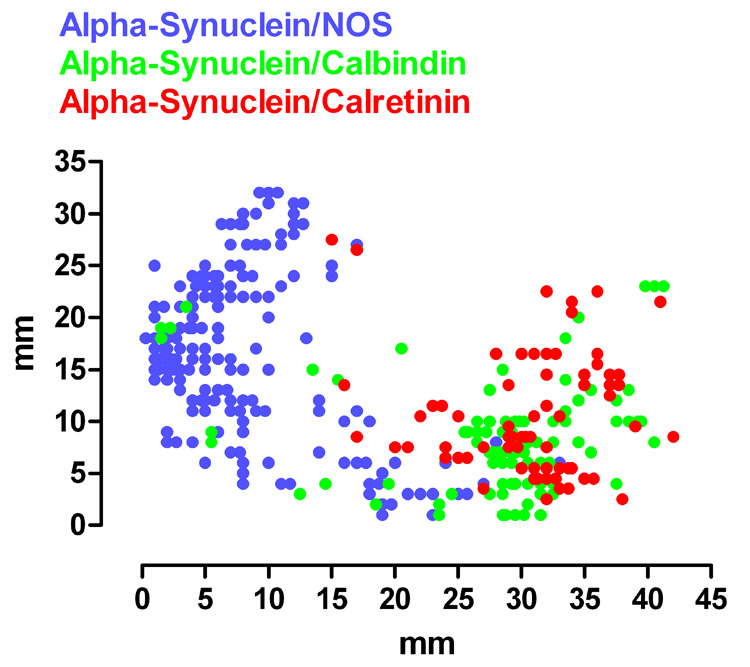
Plotting the distribution of myenteric neurons within the ventral stomach wall that were co-reactive for alpha-synuclein and either NOS, calbindin, or calretinin revealed regional specificity for the different chemical phenotypes. Neurons double labeled for alpha-synuclein and NOS (blue) were located primarily within the boundaries of the forestomach, whereas neurons double labeled for alpha-synuclein and either calbindin (green) or calretinin (red) were localized to the corpus and the antrum. The orientation of the stomach wall is mucosa side up, so for the x-axis 0 mm represents the edge of the forestomach and for the y-axis 0 mm and 35 mm represents the greater and lesser curvatures, respectively.
Figure 5.
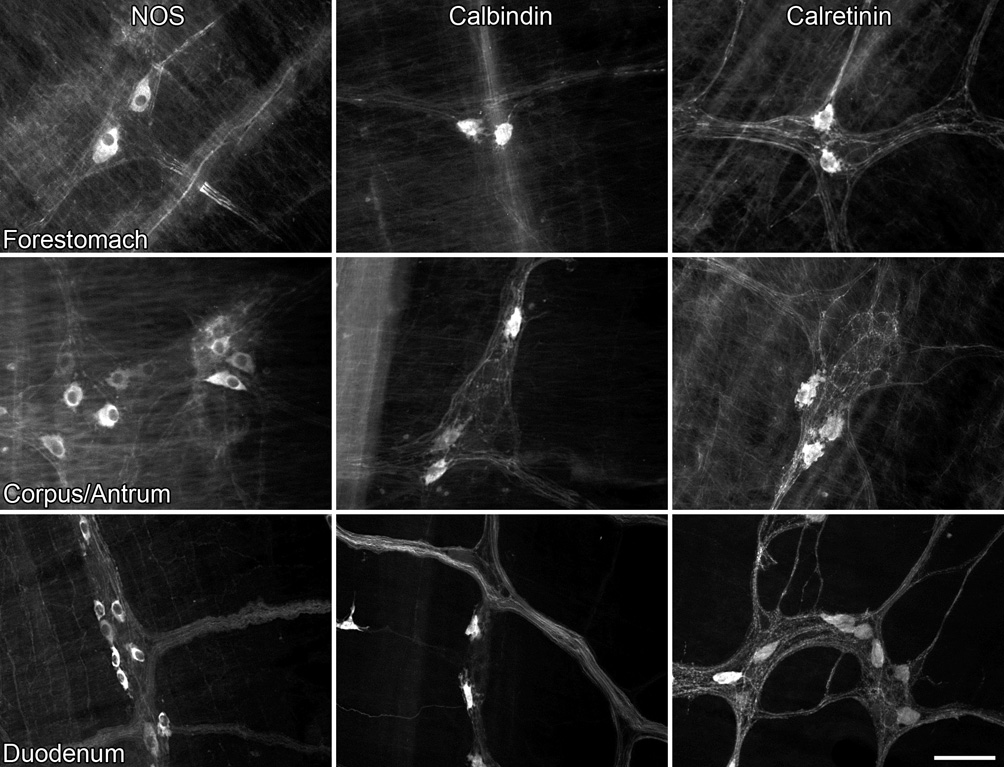
The regional specificity of neurons co-reactive for alpha-synuclein and either NOS, calbindin, or calretinin (illustrated in Figure 4) cannot be explained by the distribution of the three phenotypes throughout the stomach and duodenum. Neurons positive for all three markers were found throughout the forestomach, corpus/antrum, and duodenum. Scale bar = 80 µm.
For the duodenum, a total of 631 neurons immunoreactive for alpha-synuclein were sampled from the 18 whole mounts double labeled for either NOS, calbindin, or calretinin (6 whole mounts/antibody). Similar to stained neurons in the stomach, the nucleus and the cytoplasm of the cells were positive for alpha-synulcein. In whole mounts processed for both alpha-synuclein and NOS, 40% the alpha-synuclein cells were co-reactive for NOS. When whole mounts were double labeled for alpha-synuclein and calbindin, 13% of the alpha-synuclein-positive neurons were immunoreactive for calbindin. Finally, in whole mounts double labeled for alpha-synuclein and calretinin, 38% of the alpha-synuclein-positive neurons were co-reactive for calretinin. Myenteric neurons immunoreactive for each of the three antibodies (i.e., NOS, calbindin, and calretinin) were distributed throughout the ganglia of the duodenal whole mounts (Figure 5), and no regional pattern (such as seen in the stomach) was apparent.
Expression of Alpha-Synuclein within Vagal Efferent but not Vagal Afferent Terminals
Bilateral injections of Dextran-Texas Red into either the dmnX or the nodose ganglia resulted in the selective labeling of, respectively, either vagal efferent or afferent terminals. Within the GI tract, the Dextran-Texas Red labeled vagal efferent fibers were located within the connectives and ganglia of the myenteric plexus, and, upon entering a ganglion, produced endings containing dense terminal-like varicosities around one or more neurons. Immunohistochemistry combined with anterograde labeling revealed that vagal preganglionic axons express a high level of alpha-synuclein. In fact, every Dextran-Texas Red labeled varicosity was unmistakably positive for alpha-synuclein.
The presence, however, of alpha-synuclein-positive, Dextran-Texas Red negative neural elements highlights the fact that a) not all vagal efferents are labeled following typical injections of tracers into the dmnX, and b) the non-vagal origin of some of the alpha-synuclein axons and terminals.
In addition, and importantly in terms of potential pathways for an alpha-synuclein-mediated retrograde transfer of a pathogenic stimulus (as postulated by Braak and co-workers), alpha-synuclein-positive neurons in the myenteric plexus were consistently found to be either completely or partially encircled by dense, varicose vagal efferent endings that were positive for alpha-synuclein (Figure 6). Not all neural elements positive for alpha-synuclein, however, were vagal efferents.
Figure 6.
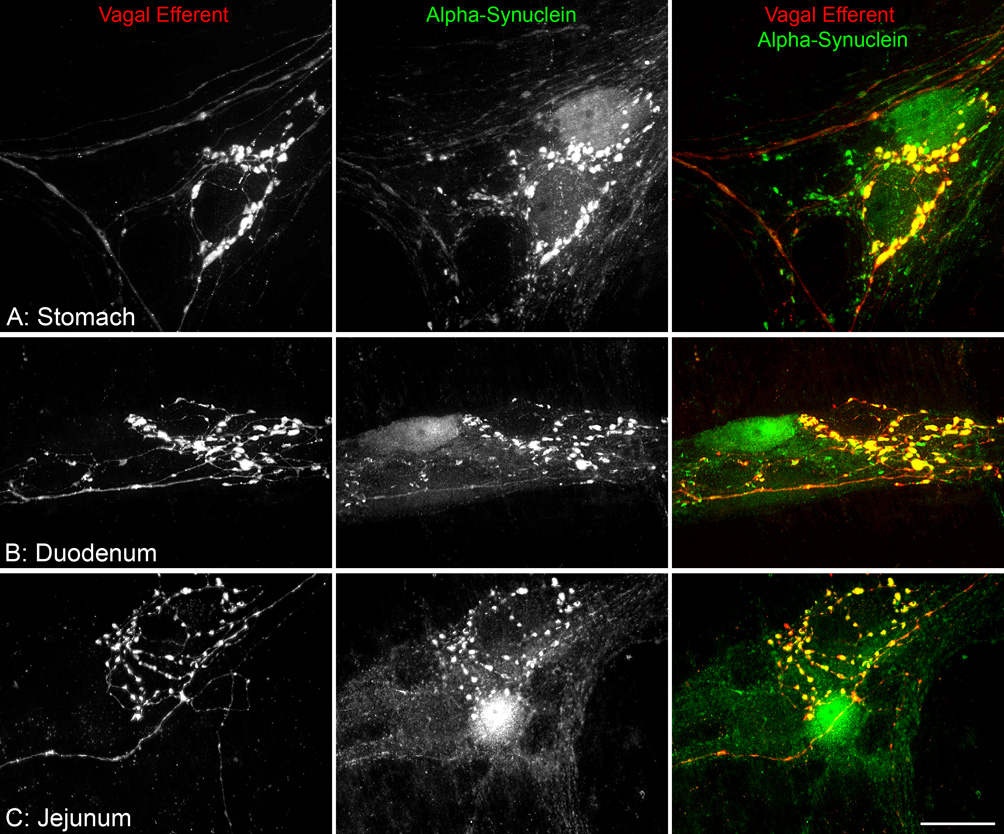
Varicosities along the vagal efferent axons innervating the myenteric ganglia were positive for alpha-synuclein, and in close proximity to myenteric neurons that were also positive for alpha-synuclein. Vagal efferent innervation (red) of the myenteric plexus was selectively labeled by injecting the tracer Dextran-Texas Red into the dorsal motor nucleus of the vagus (dmnX). Varicosities occurring on Texas Red labeled efferent axons terminating within myenteric ganglia are thought to be synaptic vesicles and thus sites of transmitter release. Myenteric neurons positive for alpha-synuclein (green) were typically encircled by alpha-synuclein-positive varicosities (A–C). In the merged images, varicosities along the vagal efferents are positive for alpha-synuclein (yellow). Scale bar in C = 25 µm for A–C.
Following injection of Dextran-Texas Red into the nodose ganglia, the myenteric plexus of the stomach and small intestine and the longitudinal smooth muscle of the forestomach were both innervated by their respective specialized vagal afferent terminals (Phillips and Powley, 2000). Specifically, the myenteric plexus was innervated by intraganglionic laminar endings (IGLEs) consisting of numerous plates of puncta that form a fine laminar covering that encapsulates one or more neurons within a ganglion; whereas, the longitudinal smooth muscle was innervated by intramuscular arrays (IMAs) consisting of a parent axon which typically, upon entering the muscle layer, bifurcates multiple times, creating an array of parallel terminal elements that terminate within one or more adjacent muscle sheets. The circular muscle layer was removed to facilitate access of the primary antibodies to the myenteric plexus, so observations on the innervation of the circular muscle by IMAs were not possible. Anterograde labeling of vagal afferent terminals combined with immunohistochemical labeling of alpha-synuclein did not find any expression of the antigen within the two types of afferent terminals.
IGLEs were, however, in close registration with alpha-synuclein-positive varicosities within the ganglia of the myenteric plexus (Figure 7A), and in some cases the laminae of IGLEs were either superficial or deep to alpha-synuclein-positive neurons (Figure 7B,C). Similarly, IMAs terminating within the longitudinal smooth muscle were intertwined with alpha-synuclein-positive fibers and unstained interstitial cells within the same muscle sheets, but were not themselves positive for alpha-synuclein.
Figure 7.
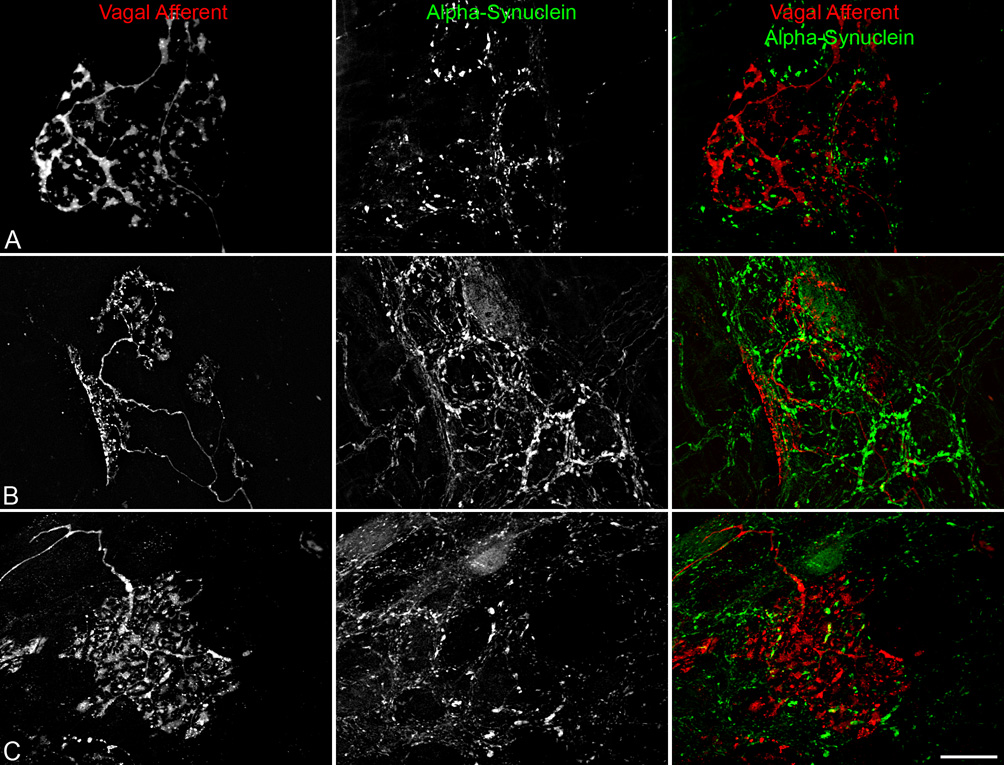
Vagal afferent terminals in the myenteric plexus were not co-localized with alpha-synuclein, though some afferent terminals associated with alpha-synuclein-positive enteric neurons. Vagal afferent innervation (red) of the gastric myenteric plexus was selectively labeled by injecting the tracer Dextran-Texas Red into the nodose ganglia. Panels A–C shows a single vagal afferent axon entering a myenteric ganglion and terminating as highly arborizing laminar endings (i.e., intraganglionic laminar endings; IGLEs). IGLEs were in tight registration with alpha-synuclein-positive (green) varicosities, axons, and neurons (B,C). In some cases, the leafy terminals of an IGLE were draped directly upon the surface of an alpha-synuclein-positive neuron (B). Scale bar in C = 25 µm for A–C.
Verification of Vagotomies with Retrograde Transport of FluoroGold
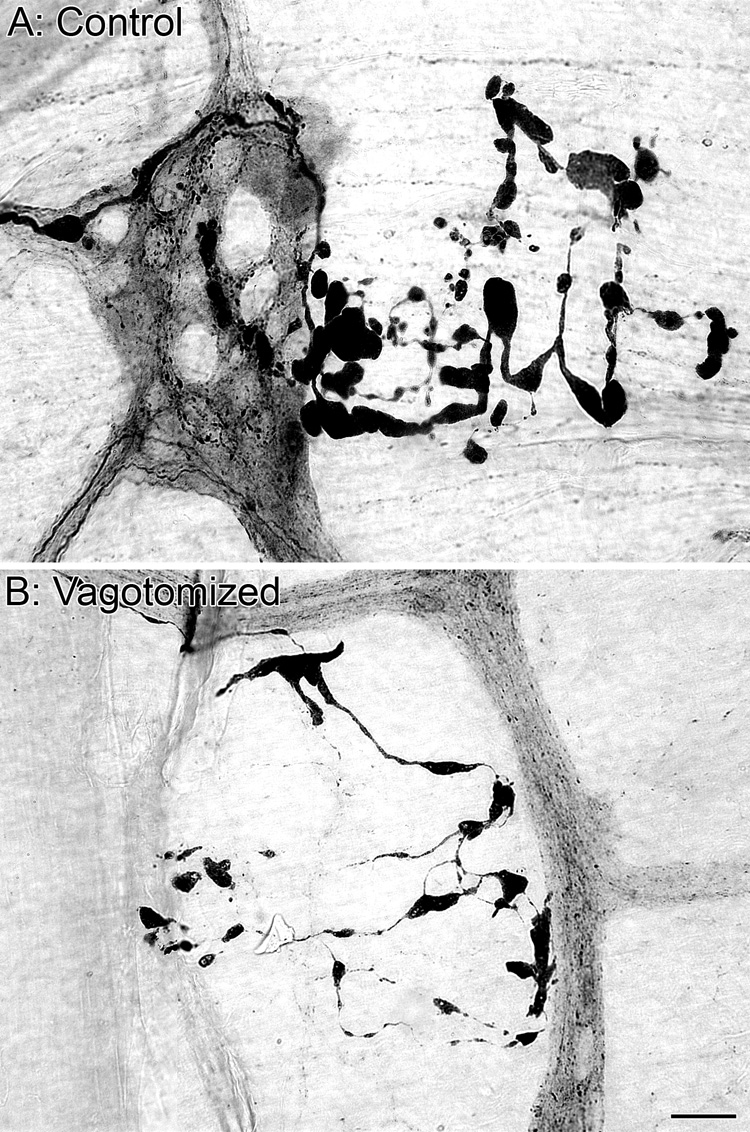
Since vagotomy is the most definitive means of eliminating vagal efferents and thus gauging which subgroups of alpha-synuclein-positive neurites in the GI tract are vagal efferents (see above) and which are intrinsic (or, of course, extrinsic sympathetic efferents or afferents), we evaluated the completeness of vagotomy of our operated animals at the time of euthanasia. Six rats satisfied the stringent criteria used to establish that the subdiaphragmatic vagotomy had eliminated all of the vagal abdominal branches: 1) compared to shams (n = 5) with all vagal branches intact and their corresponding columns in the dmnX heavily labeled with FluoroGold, completely vagotomized rats had very few FluoroGold-labeled motor neurons distributed through the dmnX, and 2) none of the rats judged to have complete vagotomies had concentrations of preganglionic neurons in either the gastric or celiac columns of the left and right dmnX (Figure 8).
Figure 8.
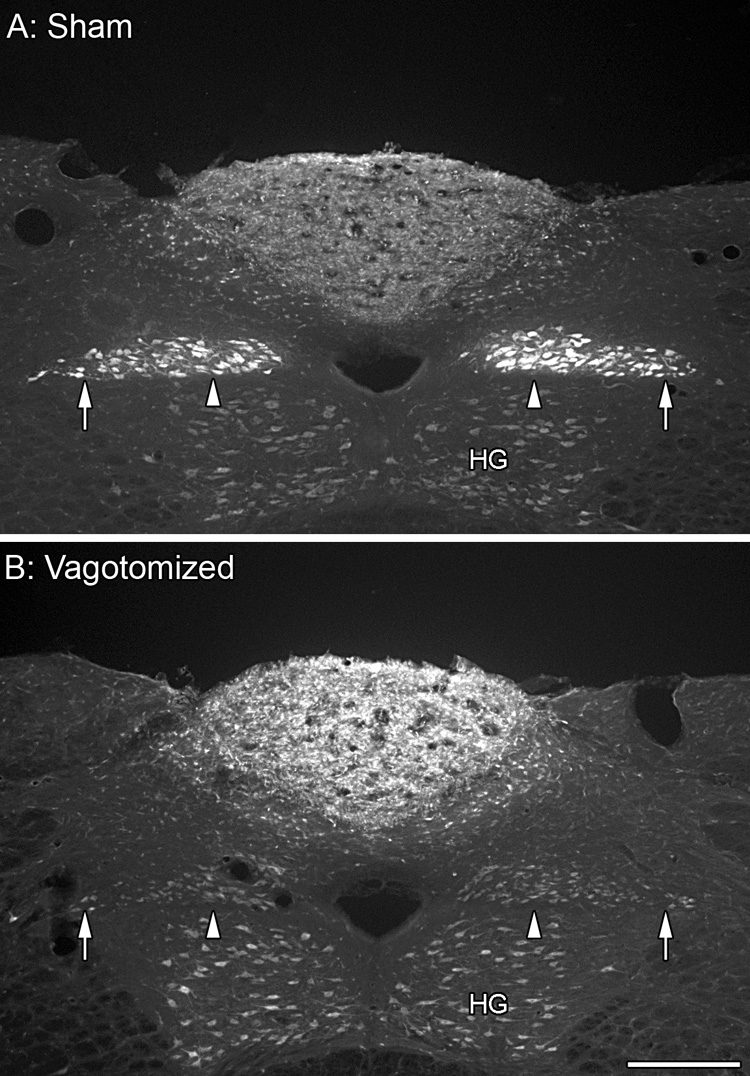
The vagal innervation of the GI tract was eliminated by cutting and cauterizing the left and the right trunks of the nerve at the level of the diaphragm. The medial and lateral columns of the dmnX are both clearly labeled with FluoroGold in the sham rats (A; arrowheads, gastric branch columns; arrows, celiac branch columns). FluoroGold labeling is absent from the medial and lateral columns of the completely vagotomized rats (B) with the remaining dmnX neurons containing only secondary FluoroGold labeling appearing no brighter than neurons in the hypoglossal nucleus (HG). Scale bar in B = 250 µm for A,B.
Pattern of Innervation by Alpha-Synuclein in Vagotomized Rats
Elimination of the vagal innervation to the GI tract resulted in a dramatic reduction in the density of the innervation of the myenteric plexus by axons and varicosities immunoreactive for alpha-synuclein (Figure 9). Whereas in the sham rats the myenteric plexus was well defined by the alpha-synuclein innervation (cf. Fig. 1), the myenteric plexus of vagotomized rats was only discernible because of residual background staining. Ganglia and fiber tracts which were richly innervated in sham rats, consisted of only scattered varicosities and stray fibers, respectively, in the vagotomized rats.
Figure 9.
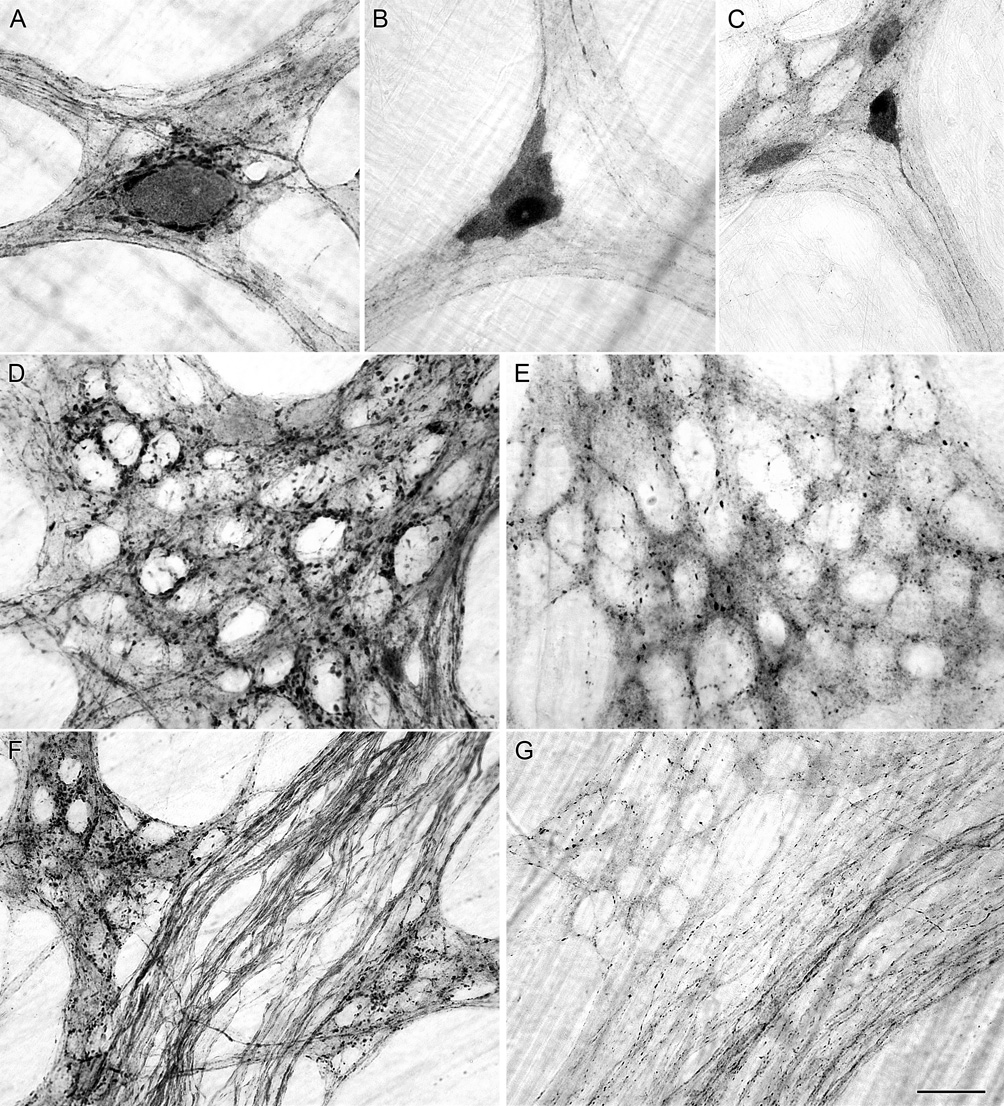
Alpha-synuclein-positive neurons (A), varicosities (D) and axons (F) were present in the myenteric plexus of the stomach of sham rats with their vagal efferent innervation intact. Vagotomy did not eliminate the presence of alpha-synuclein-positive neurons in the myenteric plexus (B,C); however, vagotomy did dramatically reduced the presence of both alpha-synuclein-positive varicosities within ganglia (B,C,E) and alpha-synuclein-positive axons in fiber bundles (G). Innervation of the longitudinal muscle of the forestomach and innervation of blood vessels by alpha-synuclein-positive axons was still present in the stomach of vagotomized rats; images not shown. Scale bar in G = 25 µm for A,B,D,E; 40 µm for C,F,G.
Following vagotomy, myenteric neurons immunoreactive for alpha-synuclein remained. These alpha-synuclein neurons, which in sham operated controls were typically encircled by closely apposed and varicose alpha-synuclein-positive terminals, were frequently found to be bare of any varicose alpha-synuclein-positive contacts in the vagotomized rats. In addition, elimination of the vagal innervation unmasked the axonal processes of the more strongly labeled alpha-synuclein-positive enteric neurons. These axons of enteric neurons, which had been obscured by the dense networks of vagal neurites positive for alpha-synuclein in the control and sham rats, could be followed for long distances as they tapered off within other ganglia or the smooth muscle layers (Figure 9B,C). In addition to intrinsic neurons immunoreactive for alpha-synuclein, the innervation of the longitudinal smooth muscle layer of the forestomach and the blood vessels by fibers immunoreactive for alpha-synuclein remained after vagotomy.
Vagal Efferent Preganglionic Contact with Immunohistochemically Identified Subsets of Neurons
Double-label immunohistochemistry was done in conjunction with anterograde labeling of vagal preganglionics to identify the types of alpha-synuclein-positive myenteric neurons contacted by vagal efferent terminals. NOS-, calbindin-, and calretinin-immunoreactive neurons were common throughout the myenteric plexus of the stomach and duodenum, however calbindin and calretinin neurons were not as abundant as neurons immunoreactive for NOS. The highly varicose vagal efferent fibers either partially or completely encircled alpha-synuclein-positive neurons in the stomach and duodenum co-reactive for either NOS, calbindin, or calretinin (Figures 10). Neurons immunoreactive for either NOS, calbindin, or calretinin but not alpha-synuclein were also routinely observed in close association to vagal efferent varicosities.
Figure 10.
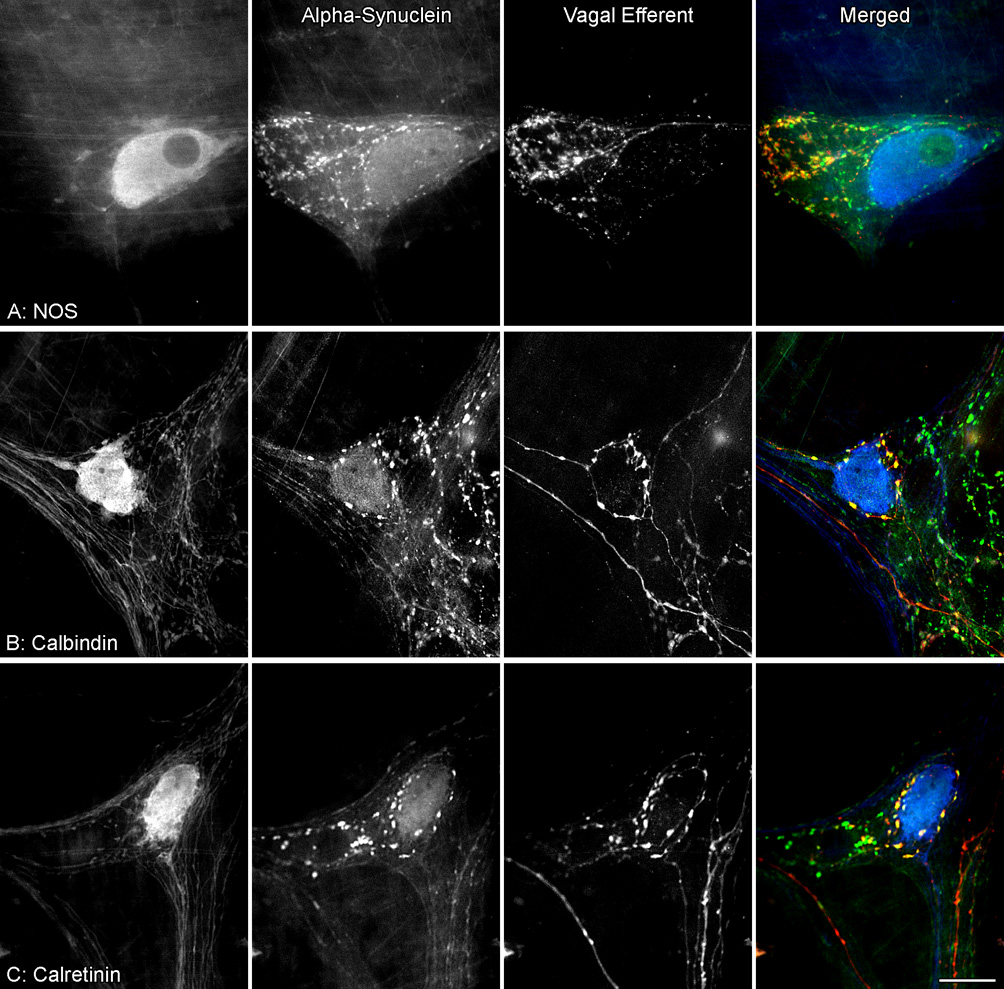
Vagal efferent varicosities positive for alpha-synuclein were in close proximity to myenteric neurons co-reactive for alpha-synuclein and either NOS (A), calbindin (B) or calretinin (C). Dextran-Texas Red tracing of vagal efferents (red) was combined with alpha-synuclein (green) and either NOS, calbindin, or calretinin (blue) double immunohistochemical labeling of myenteric neurons in the stomach. A subpopulation of alpha-synuclein-positive neurons were co-reactive for NOS (green nucleus with a light blue cytoplasm), calbindin or calretinin (light blue nucleus and cytoplasm). Yellow varicosities indicate co-localization of Dextran-Texas Red and alpha-synuclein. Scale bar in C = 25 µm for A–C.
Extrinsic and Intrinsic Origin of Alpha-Synuclein Immunoreactive Inclusions
A distinct axonopathy represented by markedly swollen alpha-synuclein immunoreactive axons and terminals was observed, albeit infrequently, in the stomach and small intestine of adult rats. Such swollen alpha-synuclein immunoreactive axons could be followed for several millimeters as they ran within the connectives and through various ganglia before terminating in close proximity to myenteric neurons (Figure 11A,C). These dystrophic swellings were in some cases as large as the smaller neuronal somata (Figure 11B), and appeared to be packed with unstained or lightly stained organelles, presumably mitochondria. We were able to determine, by anterograde labeling of the vagal efferent innervation of the myenteric plexus with Dextran-Texas Red in combination with immunohistochemical labeling of alpha-synuclein, that some of the markedly swollen varicosities immunoreactive for alpha-synuclein were vagal efferent axons and terminals. These markedly swollen alpha-synuclein-positive vagal efferent terminals were observed encircling myenteric neurons that were also immunoreactive for alpha-synuclein (Figure 11D–F). Not all of the dystrophic axons immunoreactive for alpha-synuclein, however, were vagal efferents because some remained following complete subdiaphragmatic vagotomy (Figure 12).
Figure 11.
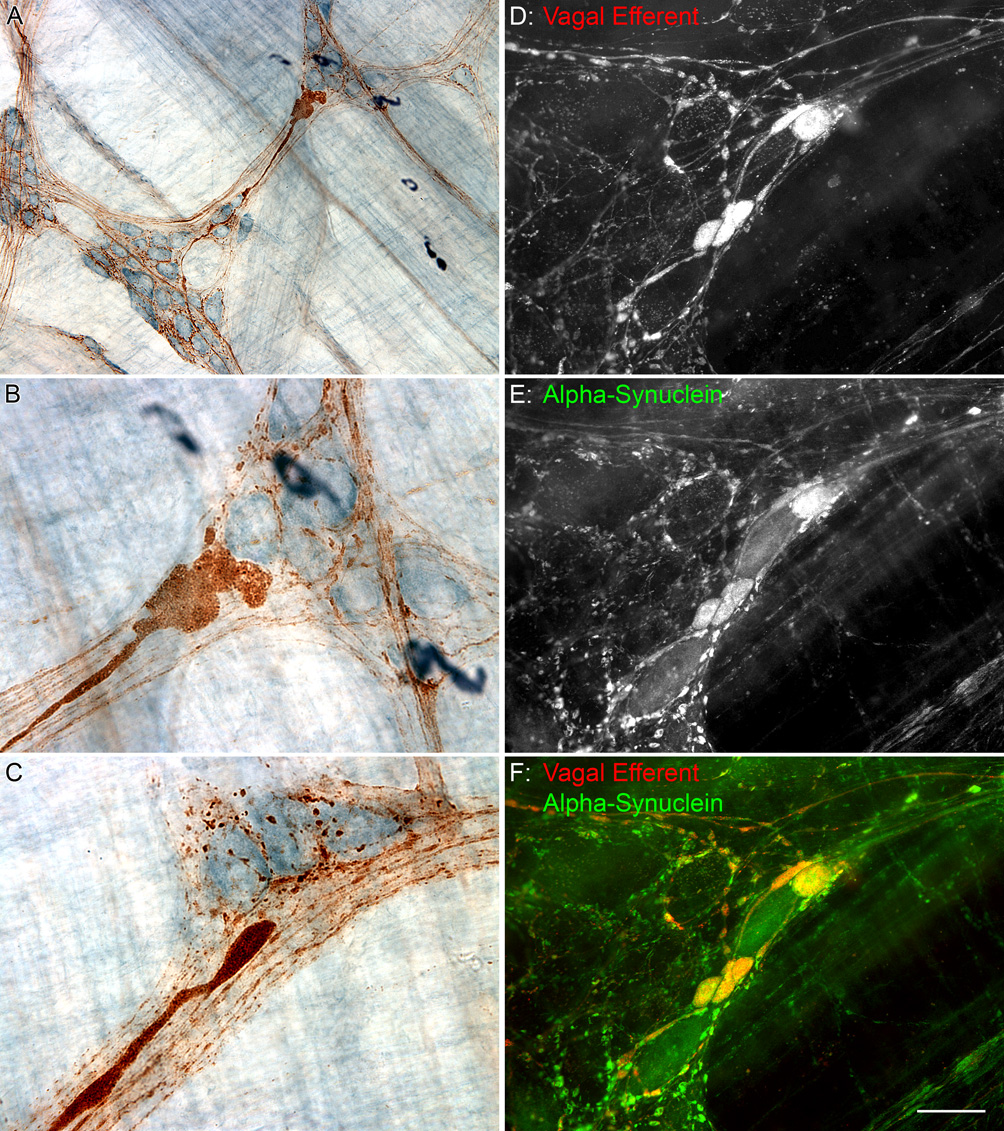
Markedly swollen axons, indicative of dystrophy or degeneration, were positive for alpha-synuclein (brown DAB precipitate) and found to be in close proximity to myenteric neurons (light blue cells labeled with Cuprolinic Blue) in the stomach of a 10 month old rat (A–C). Some of the alpha-synuclein dystrophic swellings that encircled alpha-synuclein-positive myenteric neurons (E) were vagal efferents (D). Yellow swollen varicosities indicate co-localization of Dextran-Texas Red and alpha-synuclein (F). Scale bar in F = 80 for A; 25 µm for B–F.
Figure 12.
Alpha-synuclein-positive axons were observed with dystrophic swellings along their length within the myenteric plexus of the small intestine (A). Similar alpha-synuclein-positive axonopathies were observed in the small intestine of vagotomized rats confirming an origin other than vagal (B), presumably intrinsic. Scale bar in B = 25 µm for A,B.
Discussion
In view of the conspicuous, though incompletely understood, roles of alpha-synuclein in synucleinopathies, including notably Parkinson’s disease (e.g., Trojanowski and Lee, 2002; Marti et al., 2003; Norris et al., 2004), the present experiment was designed to examine the pattern of expression of the protein in the autonomic circuitry of the proximal gut. The results delineate an extensive and complex network of vagal preganglionic axons and myenteric postganglionic neurons positive for alpha-synuclein. In particular, we provide the first direct anatomical demonstrations that vagal motor axons and terminals in the gut are positive for alpha-synuclein and that some of these preganglionic efferents terminate on alpha-synuclein-positive neurons in the myenteric plexus of the stomach and duodenum. These and additional observations address and help focus several postulates included in a provocative proposal, the “Braak model,” offering a mechanism for the development and spread of the Lewy material neuropathy in Parkinson’s disease. Thus, a consideration of the present results within the framework of the Braak explanation for the development of Parkinson’s is useful both for assessing the results and evaluating some of the premises of the Braak model; additional, orthogonal implications of the experiment are then discussed separately.
Alpha-synuclein-positive pathways innervating the gut
Braak and colleagues have suggested (Del Tredici et al., 2002; Braak et al., 2003, 2006; Hawkes et al., 2007) that the progressive pathological process of Parkinson’s disease starts when a putative pathogen breaches the mucosa of the GI tract, compromises or “infects” alpha-synuclein-positive neurons in the intrinsic ENS, and then spreads to the CNS via the vagal preganglionic innervation of the GI tract. Because alpha-synuclein-positive Lewy bodies and Lewy neurites, the diagnostic features of Parkinson’s disease, first occur, during the earliest and pre-symptomatic stage of the disease, in the autonomic pathways in the GI tract, Braak and colleagues elaborated several predictions about the distribution of alpha-synuclein within the myenteric plexus and the relationship of the vagal motor terminals to alpha-synuclein-positive enteric neural elements.
In particular, three postulates of the Braak model are relevant: 1) The hypothesized pathogen is presumed to be transferred from the GI mucosa to the CNS by retrograde transport in a pathway that expresses alpha-synuclein throughout its trajectory. 2) The postganglionic element of this pathway is postulated to be a specific subset or chemical phenotype of myenteric neurons that project to mucosal regions where the pathogen is incorporated. 3) The vulnerable alpha-synuclein circuitry is inferred to be in the stomach.
The first of the premises is that the Parkinson’s pathogen gains access to the CNS through an unbroken chain of alpha-synuclein-expressing projections, including vagal preganglionic neurons and myenteric postganglionic neurons. The present results establish that such a circuit exists in both the stomach and duodenum. Alpha-synuclein-positive myenteric neurons were observed throughout the stomach—as well as the duodenum. Furthermore, alpha-synuclein-expressing vagal efferent axons formed tight rings of varicose appositions around such positive myenteric neurons. Formally, at least, these juxtaposed extrinsic and intrinsic elements could provide the projection pathways that Braak and colleagues have postulated (Braak et al., 2003, 2006).
Additional observations in the present results add a significant footnote to this premise, however. Alpha-synuclein-positive neurons occur in relatively low numbers in the stomach and duodenum (~3% and ~6 to 13% of myenteric neurons, respectively—see below). If the Braak postulate of an “unbroken chain” of alpha-synuclein neurons transferring a pathogen transynaptically by retrograde transport is correct, then there are only a limited number of gastric and duodenal myenteric elements susceptible to being compromised.
In contrast to the limited number of positive enteric neurons, virtually all vagal efferent axons are positive for alpha-synuclein. Previous observations have suggested that most, if not all, myenteric neurons in the stomach are contacted by vagal preganglionic terminals (Berthoud et al., 1991; Holst et al., 1997), and the present results corroborate the conclusion. In addition, though, the present results for the first time indicate that virtually all of these efferent axonal projections are positive for alpha-synuclein. The finding that the protein is ubiquitous in vagal efferent terminals in the gut is consistent with the apparently extensive--and perhaps universal--expression of alpha-synuclein observed in the somata of dmnX neurons (Li et al., 2002; Bloch et al., 2006; Yu et al., 2007).
The present observations also illustrate that many alpha-synuclein-postive vagal preganglionics innervate myenteric neurons that do not express alpha-synuclein, even within a ganglion that contains a separate alpha-synuclein-positive neuron. If one were to assume, contrary to the Braak suggestion, that the putative Parkinson’s pathogen was transferred not merely retrogradely across a synaptic cleft from post- to pre-ganglionic neurons expressing alpha-synuclein, but rather could be distributed extra-synaptically within myenteric ganglia or other gut tissue, then the number of preganglionic pathways for the pathogen to be relayed to the CNS would increase geometrically.
The second Braak premise relevant to the present observations is the proposition that the autonomic vulnerability to the putative Parkinson’s pathogen occurs in a specific chemical phenotype of enteric neurons. Based on the observations of Wakabayashi and co-workers (1990) indicating that Lewy material could be found in vasoactive intestinal polypeptide (VIP)-positive myenteric neurons of Parkinson’s patients, Braak and colleagues (2006) inferred that the phenotype of the vulnerable enteric neurons is VIPergic.
In the present experiment, we observed that some alpha-synuclein-positive myenteric neurons were NOS-positive. Indeed, strikingly, 90% of the alpha-synuclein-positive neurons in the forestomach were NOS-positive. In myenteric neurons, VIP is found in nitrergic neurons (Ekblad et al., 1994; Van Geldre and Lefebvre, 2004). Consistent with this established co-expression of NOS and VIP, we also observed, in a limited series, that 70% of the alpha-synuclein-positive neurons in the forestomach were co-reactive for VIP, and that 16% of the alpha-synuclein neurons in the remainder of the stomach were co-reactive for VIP [unpublished observations; n = 3; i.e., 1 rat (10 months old) from present series as well as 2 older F344 rats from a separate series; double labeling for VIP (rabbit polyclonal antiserum; Antibody Core no. 7913, CURE/Digestive Diseases Research Center, UCLA, Los Angeles, CA) and alpha-synuclein]. Thus, based both on previous co-localization experiments and on our limited observations, it is reasonable to assume that the majority of the NOS/alpha-synuclein neurons in the forestomach are also VIPergic. Co-localization of NOS and alpha-synuclein (in lower percentages) was also observed for the small intestine. Such co-expressing myenteric neurons could, of course, represent—and satisfy the requirement of this second premise of the Braak model--the proposed myenteric elements of the autonomic pathway.
Concurrently, though, the present results suggest that such a proposed NOS/VIP mechanism of susceptibility may be unduly limited. While 58% of gastric alpha-synuclein-positive neurons overall were immunoreactive for NOS, roughly a comparable percentage were immunoreactive for the cholinergic markers calbindin (35%) and calretinin (35%). Similar ratios of cholinergic (i.e. calbindin or calretinin) and alpha-synuclein immunoreactive neurons were also observed in the duodenum. Also, strikingly, in the corpus-antrum, 92% of the alpha-synclein-positive neurons expressed immunoreactivity for one of the cholinergic, rather than the NOS (and presumably rather than the VIP), markers. Notably, too, alpha-synuclein-positive vagal efferents encircled and apparently innervated both nitrergic (i.e., NOS-positive) and cholinergic (calbindin-positive and calretinin-positive) postganglionic enteric neurons expressing alpha-synuclein. Such a pattern does not suggest that NOS/VIP neurons would be the only postganglionic or intrinsic channels contacted by extrinsics expressing alpha-synuclein.
Though it is possible to argue that any pathogen would only travel retrogradely through NOS/VIP neurons, such a model would be restrictive in terms of both numbers of neural channels capable of spreading the pathogen and the particular gastric region most vulnerable to pathogen spread. This restrictiveness would seem particularly limiting in light of the fact that many forestomach NOS neurons appear to project to the smooth muscle coat (cf. Fig 2), not the submucosa or mucosa where their terminals might be compromised by the postulated pathogen. A larger pool of vulnerable neurons would be involved if all alpha-synuclein-expressing neurons, not just NOS/VIP positive neurons, are vulnerable.
The third and final Braak premise addressed by the present results concerns the region of the GI tract in which a breach of the mucosal barrier might lead to transport and spread of a Parkinson’s pathogen. Braak and co-workers (2003) have speculated that the stomach is the most likely site. Furthermore, Braak et al. (2005) examined the stomach of Parkinson’s patients for aggregations of alpha-synuclein and found lesions in samples from the cardia, fundus, and pylorus; they did not mention observing any abnormal pathology in the corpus. Importantly, they did not examine the small intestine for the presence of alpha-synuclein-positive aggregates.
While the stomach may be afflicted in Parkinson’s disease, several observations suggest that either the proposal that the stomach is the primary site of retrograde transport of the putative pathogen (the present Braak premise) or the proposal that the pathogen is passed retrogradely trans-synaptically along an “unbroken chain” of efferent projections expressing alpha-synuclein (the first of the three premises—see above) would likely need to be modified. First, in the present experiment, the stomach had the lowest density of alpha-synuclein-positive myenteric neurons in the proximal GI tract, with higher densities found in the small intestine (i.e., jejunum > duodenum > stomach). Indeed, the proportion of gastric myenteric neurons that are alpha-synuclein-positive is quite low: comparisons of the counts of alpha-synuclein-positive neurons in the present experiment with estimates of the total population of gastric and intestinal myenteric neurons in our earlier experiments using the pan-neuronal marker Cuprolinic Blue (Phillips et al., 2003, 2004a) suggests that, at most, less than 3% of all gastric myenteric neurons express alpha-synuclein. In contrast, moving distally through the small intestine, the proportion of alpha-synuclein neurons increases to 6% of the neurons in the proximal duodenum, 13% of the neurons in the distal duodenum, and 22% of the neurons in the jejunum.
Second, the role of the intestines in absorption and the evidence that the epithelial barrier to luminal materials, including potential toxins or pathogens, is compromised with age suggests that the higher proportion of enteric neurons in the intestines might be vulnerable. There is a significant break down in epithelium barrier function with age (Mullin et al., 2002), and a subsequent increase in intestinal permeability to large molecules (Ma et al., 1992). Thus, increased permeability with age facilitates penetration of toxins through the epithelial lining of the intestines to the large number of alpha-synuclein-positive elements (see also below).
Third, comparisons between the distribution of alpha-synuclein expression and the distributions of the different neurochemical phenotypes appear inconsistent with any rigid phenotypical or regional or organ-specific localization for the spread of an alpha-synuclein misfolding factor. For example, in the stomach, Braak and colleagues (2006) have observed Lewy bodies and neurites throughout most of the organ (i.e., cardia, fundus, and pylorus), though gastric NOS/VIP neurons expressing alpha-synuclein are located, on the basis of the present results, in the forestomach. For another example, both NOS and VIP expressing neurons (e.g., NOS: Aimi et al., 1993; Phillips et al., 2003; e.g. VIP: Van Geldre and Lefebvre, 2004; Ekblad et al., 1994) as well as alpha-synuclein-expressing neurons (the present experiment) are also observed in the small intestines. This suggests that—if the enzyme, peptide, and protein, respectively, are markers for pathways vulnerable to the putative Parkinson’s pathogen--pathological aggregations of Lewy material could originate in the intestines. In keeping with such a prediction, Singaram et al. (1995) observed Lewy bodies in VIP-positive neurons in the ascending colon of Parkinson’s disease patients. A similar argument for an intestinal as well as a gastric locus of origin for the spread of Parkinson’s synucleinopathy would appear consistent with the fact that Lewy material also develops in the sympathetic projections to the gut (Block et al., 2006; Braak et al., 2007).
Significance of cytoplasmic and nuclear expression of alpha-synuclein in myenteric plexus neurons
The strong cytoplasmic and nuclear expression of alpha-synuclein in myenteric neurons positive for the protein is consistent with the proposed role of the molecule in Parkinson ’s disease. Andringa and colleagues (2003) noted that there is a clear correspondence between the regions of the rat brain that demonstrate somal (as opposed to only synaptic) expression of alpha-synuclein and the homologous structures in the human brain that are susceptible to Lewy body formation in Parkinson’s disease. Thus, the investigators made the prediction that neurons with a high expression of alpha-synuclein are “Lewy body-vulnerable”. If correct and if the idea extrapolates to the ENS, then the presence of alpha-synuclein-positive myenteric neuronal somata in the GI tract further supports the proposition that the GI tract is the site of initiation of Parkinson’s pathology. More generally, at least, the strong expression of alpha-synuclein in myenteric neurons suggests that these neurons might be prone to pathological misfolding of proteins or neurodegenerative changes in response to insults to the GI tract.
Nuclear expression of alpha-synuclein was originally thought to be an artifact of the antiserum used to detect the protein; however a recent report by Yu and colleagues (2007) using novel antibodies to the protein showed extensive nuclear localization of alpha-synuclein in the CNS of rodents. Since there appears to be differences among antibodies that are likely to contribute to conflicting reports on the pattern of alpha-synuclein expression in the nervous system, of particular importance to the present study is the fact that we used an antibody that has been thoroughly characterized and shown to be highly specific to alpha-synuclein, and which has previously been used to demonstrate neuronal localization of alpha-synuclein in the brain (Andringa et al., 2003; Bennett, 2005). The nuclear expression of alpha-synuclein immunoreactivity in neurons of both the CNS and ENS raises the question of a role for the protein in nuclear function, and needs to be clarified by future studies.
Dystrophic Swellings in Alpha-synuclein-positive Processes in GI Tract: Aging and Parkinson’s
An intriguing observation in the present experiment is the presence of markedly swollen alpha-synuclein-positive vagal efferent terminals encircling alpha-synuclein-positive myenteric neurons in adult rats. In addition, we observed other dystrophic alpha-synuclein-positive neurites that apparently were axons of enteric neurons, insofar as they occurred in the myenteric plexus of vagotomized rats. In the limited sample, aggregates of alpha-synuclein material or dystrophic enteric neurites were not found in the same ganglia as markedly swollen vagal efferents.
These observations underscore the conclusions that neuropathies occur in the ENS of even “healthy” adults and that (at least some of) these neuropathies occur in neurons that express alpha-synuclein. In earlier reports, we have characterized neuropathies as well as neuronal and glial losses in the standard rat model of aging, the Fischer 344 rat (Phillips et al., 2004b, 2006, 2007; Phillips and Powley, 2007). The neuropathies commonly consist of dystrophic or markedly swollen neurites. Neuronal losses, glial losses, and neuropathies of aging all begin in adulthood and progress or accumulate in number over the lifespan. The age-related neuronal losses are concentrated in cholinergic neurons (the calretinin and calbindin neurons of the present analysis), with nitrergic neurons being spared. Dystrophic and markedly swollen neurites are found in processes with a variety of chemical phenotypes.
The alpha-synuclein-positive swellings and dystrophic neurites observed in the present experiment (using both the F344 rat and the SD rat) may reflect these same aging processes that we have previously characterized and indicate that there is a base-rate of synucleinopathy in GI tract of ostensibly healthy aging individuals. Conceivably, the “spontaneous” synucleinopathies associated with aging and those of Parkinson’s disease are independent. On the other hand, conceivably, as specific elements of the autonomic innervation of the gut become prone, with age, to developing synucleinopathies, they also become susceptible to being compromised by the agent(s) responsible for Parkinson’s disease. If the putative Parkinson’s pathogen is transported retrogradely by those axons that develop age-related synucleinopathies, the pathogen might be selective specifically for those pathways that innervate the mucosa or submucosa and hence that have terminals in the vicinity of the compromised epithelium. Whether normal or healthy aging and Parkinson’s disease operate on such a common subset of vulnerable projections or whether the two processes are independent will require further empirical analysis.
Alpha-synuclein as a marker for vagal efferent axons
Another observation of the present experiment has implications for future work. By separately labeling vagal efferents and afferents to the GI tract with tracer injections, examining these projections for the expression of alpha-synuclein, and employing vagotomies to selectively eliminate these projections to the myenteric plexus and smooth muscle wall of the gut, we were able to draw several inferences: 1) Virtually all vagal efferents express alpha-synuclein in their axons and terminal varicosities within the myenteric plexus. 2) Eliminating vagal efferents by vagotomy dramatically reduces, but does not eliminate all, neurites expressing alpha-synuclein in the myenteric plexus. 3) Virtually no vagal afferents to the smooth muscle wall of the GI tract express alpha-synuclein.
The fact that all vagal efferents to the gut are alpha-synuclein-positive suggests that the protein would be a good marker or label for vagal preganglionic axons, in some situations. In experiments where false negatives are a potential source of confusion and false positives are not a concern (for example, in a case where one needed to decide whether vagal axons were efferent or afferent), alpha-synuclein would be an excellent marker for vagal preganglionic fibers. On the other hand, where false positives would be confusing (for example, in a case where one needed to decide whether a neurite in the myenteric plexus was an extrinsic vagal or intrinsic enteric fiber) alpha-synuclein would not be discriminative marker.
Conclusion
In summary, then, virtually all vagal motor fibers innervating the myenteric plexus of the proximal GI tract express alpha-synuclein in their axons and terminals. The preganglionic endings encircle and form appositions with myenteric postganglionic neurons, including those that express alpha-synuclein. The pathway of alpha-synuclein-positive vagal preganglionics that form appositions on postganglionic neurons that also express the protein satisfies the Braak proposal of a direct alpha-synuclein-positive route for transynaptic retrograde transport from gut to brain. Additional observations on the proportions of alpha-synuclein-expressing neurons in myenteric ganglia, neurochemical phenotypes of the enteric postganglionic neurons, and distributions of the elements throughout the proximal gut, however, suggest several possible amendments or modifications of the premises of the model that Braak and colleagues have proposed to account for the spread of Lewy material accumulations throughout the nervous system in Parkinson’s disease. Additional experiments designed to elucidate the roles of alpha-synuclein—and other synuclein proteins—in both Parkinson’s disease and aging are needed to clarify the different conditions.
Acknowledgements
We thank Michelle Murphy for her help with the SlideBook software, and Nazim Anwar for his expert assistant with the preparation of the medullas for FluoroGold analysis. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIH DK61317 and DK27627).
List of abbreviations
- CNS
central nervous system
- DAB
diaminobenzidine
- dmnX
dorsal motor nucleus of the vagus
- ENS
enteric nervous system
- F344
Fischer 344
- GI
gastrointestinal
- HG
hypoglossal nucleus
- IGLE
intraganglionic laminar ending
- IMA
intramuscular array
- NOS
nitric oxide synthase
- P
pyloric
- SD
Sprague Dawley
- VIP
vasoactive intestinal polypeptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section Editor: Charles R. Gerfen, NIMH, 36 Convent Drive, Bethesda, MD 20892-4075
References
- Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, Launer LJ, Curb JD, White LR. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord. 2007;22:1581–1586. doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- Abbott RD, Ross GW, White LR, Nelson JS, Masaki KH, Tanner CM, Curb JD, Blanchette PL, Popper JS, Petrovitch H. Midlife adiposity and the future risk of Parkinson's disease. Neurology. 2002;59:1051–1057. doi: 10.1212/wnl.59.7.1051. [DOI] [PubMed] [Google Scholar]
- Adamczyk A, Solecka J, Strosznajder JB. Expression of alpha-synuclein in different brain parts of adult and aged rats. J Physiol Pharmacol. 2005;56:29–37. [PubMed] [Google Scholar]
- Aimi Y, Kimura H, Kinoshita T, Minami Y, Fujimura M, Vincent SR. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993;53:553–560. doi: 10.1016/0306-4522(93)90220-a. [DOI] [PubMed] [Google Scholar]
- Andringa G, Du F, Chase TN, Bennett MC. Mapping of rat brain using the Synuclein-1 monoclonal antibody reveals somatodendritic expression of alpha-synuclein in populations of neurons homologous to those vulnerable to Lewy body formation in human synucleopathies. J Neuropathol Exp Neurol. 62:1060–1075. doi: 10.1093/jnen/62.10.1060. [DOI] [PubMed] [Google Scholar]
- Bennett MC. The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther. 2005;105:311–331. doi: 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260:R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol (Berl) 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- Brenz Verca MS, Bahi A, Boyer F, Wagner GC, Dreyer JL. Distribution of alpha- and gamma-synucleins in the adult rat brain and their modification by high-dose cocaine treatment. Eur J Neurosci. 2003;18:1923–1938. doi: 10.1046/j.1460-9568.2003.02913.x. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua CE, Tang BL. alpha-synuclein and Parkinson's disease: the first roadblock. J Cell Mol Med. 2006;10:837–846. doi: 10.1111/j.1582-4934.2006.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Mulder H, Uddman R, Sundler F. NOS-containing neurons in the rat gut and coeliac ganglia. Neuropharmacology. 1994;33:1323–1331. doi: 10.1016/0028-3908(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Espada J, Juarranz A, Galaz S, Canete M, Villanueva A, Pacheco M, Stockert JC. Non-aqueous permanent mounting for immunofluorescence microscopy. Histochem Cell Biol. 2005;123:329–334. doi: 10.1007/s00418-005-0769-2. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Uryu K, Lee VM, Trojanowski JQ. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci U S A. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JM. The synucleins. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3002. REVIEWS3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Shorter J. Prime time for alpha-synuclein. J Neurosci. 2007;27:2433–2434. doi: 10.1523/JNEUROSCI.0094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;381:81–100. doi: 10.1002/(sici)1096-9861(19970428)381:1<81::aid-cne7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Henning Jensen P, Dahlstrom A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002;113:463–478. doi: 10.1016/s0306-4522(02)00143-4. [DOI] [PubMed] [Google Scholar]
- Ma TY, Hollander D, Dadufalza V, Krugliak P. Effect of aging and caloric restriction on intestinal permeability. Exp Gerontol. 1992;27:321–333. doi: 10.1016/0531-5565(92)90059-9. [DOI] [PubMed] [Google Scholar]
- Marti MJ, Tolosa E, Campdelacreu J. Clinical overview of the synucleinopathies. Mov Disord. 2003;18 Suppl 6:S21–S27. doi: 10.1002/mds.10559. [DOI] [PubMed] [Google Scholar]
- McBride PA, Schulz-Schaeffer WJ, Donaldson M, Bruce M, Diringer H, Kretzschmar HA, Beekes M. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J Virol. 2001;75:9320–9327. doi: 10.1128/JVI.75.19.9320-9327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin JM, Valenzano MC, Verrecchio JJ, Kothari R. Age- and diet-related increase in transepithelial colon permeability of Fischer 344 rats. Dig Dis Sci. 2002;47:2262–2270. doi: 10.1023/a:1020191412285. [DOI] [PubMed] [Google Scholar]
- Norris EH, Giasson BI, Lee VM. Alpha-synuclein: normal function and role in neurodegenerative diseases. Curr Top Dev Biol. 2004;60:17–54. doi: 10.1016/S0070-2153(04)60002-0. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Regenerating vagal afferents reinnervate gastrointestinal tract smooth muscle of the rat. J Comp Neurol. 2000;421:325–346. [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004a;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106:69–83. doi: 10.1016/S1566-0702(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Kieffer EJ, Powley TL. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol (Berl) 2004b;209:19–30. doi: 10.1007/s00429-004-0426-x. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Pairitz JC, Powley TL. Age-related neuronal loss in the submucosal plexus of the colon of Fischer 344 rats. Neurobiol Aging. 2007;28:1124–1137. doi: 10.1016/j.neurobiolaging.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434:358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Rhodes BS, Powley TL. Effects of age on sympathetic innervation of the myenteric plexus and gastrointestinal smooth muscle of Fischer 344 rats. Anat Embryol (Berl) 2006;211:673–683. doi: 10.1007/s00429-006-0123-z. [DOI] [PubMed] [Google Scholar]
- Powley TL, Berthoud HR. A fluorescent labeling strategy for staining the enteric nervous system. J Neurosci Methods. 1991;36:9–15. doi: 10.1016/0165-0270(91)90132-j. [DOI] [PubMed] [Google Scholar]
- Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol. 1987;253:R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Advances in neural tracing of vagal afferent nerves and terminals. In: Undem BJ, Weinreich D, editors. Methods and New Frontiers in Neuroscience: Advances in Vagal Afferent Neurobiology. Vol. 28. Boca Raton, Florida: CRC Press; 2005. pp. 123–145. [Google Scholar]
- Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM. Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet. 1995;346:861–864. doi: 10.1016/s0140-6736(95)92707-7. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Parkinson's disease and related synucleinopathies are a new class of nervous system amyloidoses. Neurotoxicology. 2002;23:457–460. doi: 10.1016/s0161-813x(02)00065-7. [DOI] [PubMed] [Google Scholar]
- Ueki A, Otsuka M. Life style risks of Parkinson's disease: association between decreased water intake and constipation. J Neurol. 2004;251 Suppl 7:vII18–vII23. doi: 10.1007/s00415-004-1706-3. [DOI] [PubMed] [Google Scholar]
- Van Geldre LA, Lefebvre RA. Interaction of NO and VIP in gastrointestinal smooth muscle relaxation. Curr Pharm Des. 2004;10:2483–2497. doi: 10.2174/1381612043383890. [DOI] [PubMed] [Google Scholar]
- van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. Pathogenesis of natural scrapie in sheep. Arch Virol Suppl. 2000:57–71. doi: 10.1007/978-3-7091-6308-5_5. [DOI] [PubMed] [Google Scholar]
- Van Nassauw L, Brouns I, Adriaensen D, Burnstock G, Timmermans JP. Neurochemical identification of enteric neurons expressing P2X(3) receptors in the guinea-pig ileum. Histochem Cell Biol. 2002;118:193–203. doi: 10.1007/s00418-002-0447-6. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson's disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol (Berl) 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Burnstock G. P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol. 2004;121:169–179. doi: 10.1007/s00418-004-0620-1. [DOI] [PubMed] [Google Scholar]
- Yu S, Li X, Liu G, Han J, Zhang C, Li Y, Xu S, Liu C, Gao Y, Yang H, Ueda K, Chan P. Extensive nuclear localization of alpha-synuclein in normal rat brain neurons revealed by a novel monoclonal antibody. Neuroscience. 2007;145:539–555. doi: 10.1016/j.neuroscience.2006.12.028. [DOI] [PubMed] [Google Scholar]


