Summary
We have previously shown that human herpesvirus-8 (HHV-8) infected dendritic cells (DC) undergo incomplete maturation and have defective antigen presenting function. Here we examined the effects of HHV-8 infection on cytokine production which is critical to the function of DCs. We detected expression of IL-6, TNF-α, MIP-1α, MIP-1β, RANTES and IL-12p40, 2-6 h post-infection and which peaked by 15-24 h. The expression decreased 24-48 h post-infection, with the exception of TNF-α, which remained high throughout the 72 h. Interestingly, while IL-12p40 expression increased post-infection, bioactive IL-12p70 was not detected in the supernatants. These results suggest an intentional skewing of cytokine production in HHV-8 infected DCs towards induction of a TH2 response.
Human herpesvirus-8 (HHV-8, also known as Kaposi’s sarcoma-associated herpesvirus, KSHV) is a gamma-herpesvirus and is the causative agent of Kaposi’s sarcoma, primary effusion lymphoma and a subset of multicentric Castleman’s disease (Moore & Chang, 2001). We have previously reported that primary infection with HHV-8 in HIV-negative adults does not result in pronounced clinical symptoms despite the presence of cellular and humoral immune responses and a detectable viremia (Wang et al., 2001). Analysis of cytotoxic T lymphocyte (CTL) responses to viral proteins expressed during a primary infection revealed a distinct but relatively non-robust immune response (Wang, Jenkins, Jacobson, Kingsley, Day, Zhang, Meng, Pellet, Kousoulas, Baghian, & Rinaldo, Jr., 2001). The reason for this diminished immune response in normal individuals is not well understood but could reflect an effect of HHV-8 infection on antigen presenting cells. In support of this hypothesis, we have shown that HHV-8 can infect monocyte-derived dendritic cells (MDDC) through the C-type lectin dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN; CD209). While this infection is non-productive, it results in an altered pattern of dendritic cell (DC) function including loss of endocytotic ability, altered surface marker expression, and loss of antigen presentation ability, suggesting a partial rather than full maturation of these cells (Rappocciolo et al., 2006). These effects may directly result in the dampened immune response during a primary HHV-8 infection (as described by a weak T cell response and low antibody titers) that we and others have observed (Brander et al., 2000;Guihot et al., 2006b;Little & Yarchoan, 2006b;Wang, Jenkins, Jacobson, Kingsley, Day, Zhang, Meng, Pellet, Kousoulas, Baghian, & Rinaldo, Jr., 2001). We therefore sought to determine if HHV-8 infection of DCs results in an altered pattern of cytokine production that could be related to the loss of DC function.
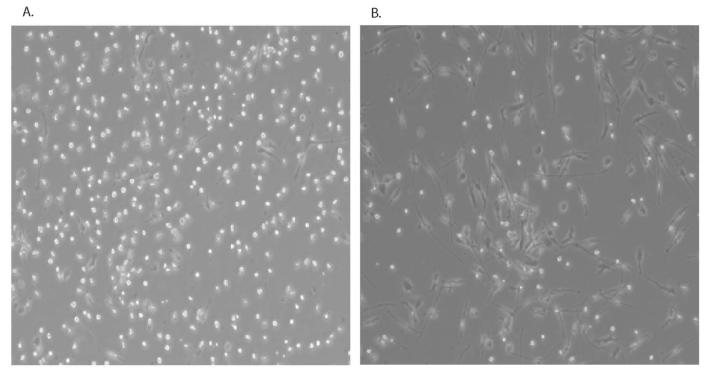
DCs were generated from enriched CD14+ monocytes grown for 6 days in AIM-V media and supplemented with IL-4 (1000U/ml) and GM-CSF (1000U/ml) and infectious HHV-8 was purified from supernatants of TPA-induced BCBL-1 cells as previously described(Rappocciolo, Jenkins, Hensler, Piazza, Jais, Borowski, Watkins, & Rinaldo, Jr., 2006). Cells were infected with gradient-purified virus equivalent to 50 viral DNA copies per cell as previously described(Rappocciolo, Jenkins, Hensler, Piazza, Jais, Borowski, Watkins, & Rinaldo, Jr., 2006). Cells were not washed following absorption, to avoid loss of cytokines released upon initial interaction with the virion. Immature DCs infected with purified HHV-8 for 24hs appear morphologically distinct from uninfected DC as shown in Figure 1. Viral infection resulted in the DCs exhibiting an elongated appearance similar to in vitro-matured DC.
Figure 1. DCs infected with HHV-8 develop a distinct morphology similar to matured DCs.
DC from an HHV-8-negative donor were left uninfected (A) or infected with HHV-8 (B) for 24h and photographed under brightfield microscopy.
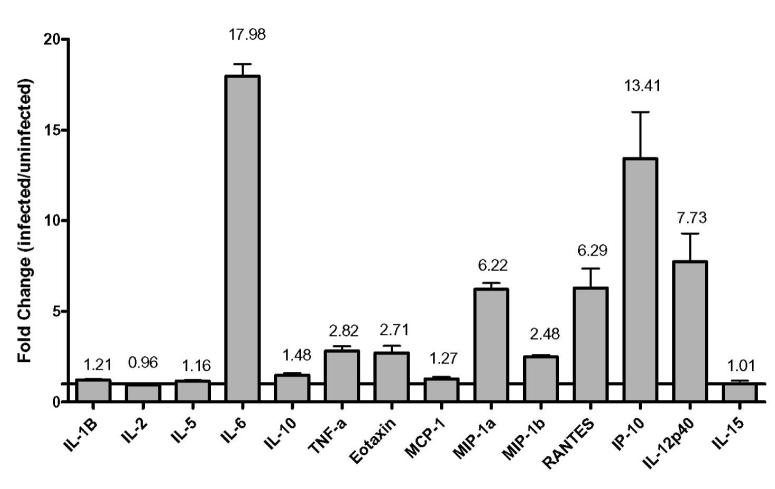
We have previously demonstrated that HHV-8 infection of immature DCs resulted in a loss of endocytosis and a uniform decrease in cell surface receptors even though less than 100% of the cells are infected (Rappocciolo, Jenkins, Hensler, Piazza, Jais, Borowski, Watkins, & Rinaldo, Jr., 2006). Thus the effects of viral infection appear to be global in the culture rather than isolated to infected cells. Therefore, we wanted to determine whether HHV-8 infection could trigger the release of cytokines or chemokines that could be responsible for altering the maturation profile of the cells. To this end, supernatants from DC cultures that had been infected with purified HHV-8 or left uninfected for 48 h were subjected to a comparative screening for cytokine secretion using kits obtained from Biosource and assayed by Luminex technology (Figure 2). As interleukin-4 (IL-4) and granulocyte macrophage colony stimulating factor (GM-CSF) were added exogenously to induce differentiation of monocytes to DC, comparisons of these cytokines between uninfected and infected cultures could not be determined as they exceeded the detection limit. Interferon-γ (IFN-γ), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF-b), granulocyte colony stimulating factor (G-CSF), hepatocyte growth factor (HGF), IL-13, and IL-17 were not detected in either set of cultures (data not shown). IL-1β, IL-2, IL-5, monocyte chemoattractant protein-1 (MCP-1), IL-10, and IL-15 were detectable at low levels in both uninfected and infected cultures (Figure 2). IL-6 showed the greatest difference between uninfected and infected cultures (approximately 18-fold), while tumor necrosis factor-α (TNF-α) (approximately 3-fold), Eotaxin (approximately 3-fold), macrophage inflammatory protein-1α (MIP-1α) (approximately 6-fold), macrophage inflammatory protein-1β (MIP-1β) (approximately 2.5-fold), RANTES (approximately 6-fold), IFN-γ inducible protein-10 (IP-10) (approximately 13.5-fold), and IL-12p40 (approximately 8-fold) all showed greater than 2-fold increases in infected cultures (Figure 2).
Figure 2. Changes in cytokine expression in HHV-8 infected DCs versus uninfected DC.
Supernatant samples from DCs from an HHV-8-negative donor were infected or left uninfected, harvested at 48h and relative cytokine amounts were determined by Luminex. Data are representative of duplicate samples for each of two sample populations.
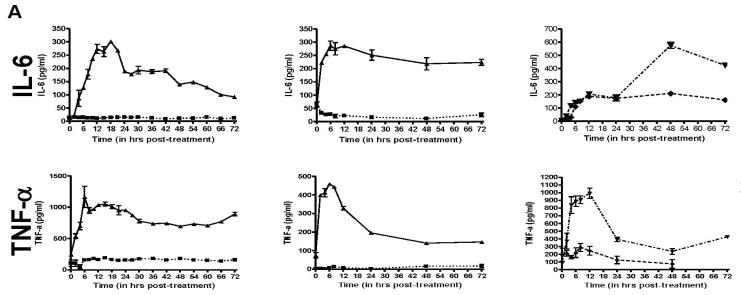
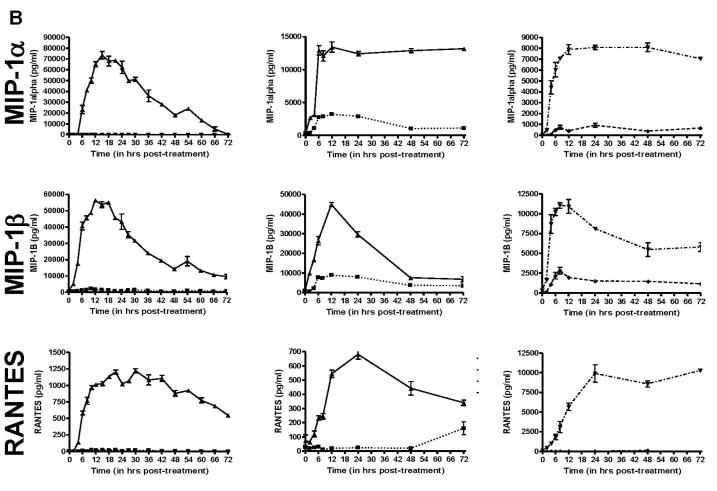
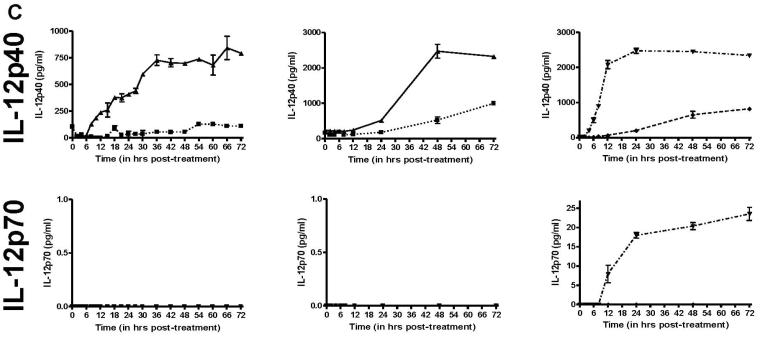
Since morphological changes to HHV-8 infected DCs could be observed as early as 6h post-infection (data not shown), we wanted to determine how quickly these cytokines were released into the media after infection. To this end, we performed ELISA (R&D Systems) for TNF-α, IL-6, MIP-1α, MIP-1β, RANTES and IL-12p40, all which showed a minimum of 2-fold increases in the HHV-8 infected cultures in the previous Luminex screening. We also tested for IFN-α and IL-12p70 by ELISA, neither of which were included in the Luminex screening. For each cytokine, DC from 2 HHV-8-negative donors were infected with HHV-8 or remained uninfected, and supernatant samples were removed from the cells at various times. Additionally, as a positive control to mimic cytokine release from immature DCs stimulated to become mature DCs, the DCs from a third HHV-8-negative donor were treated with LPS (250ng/ml, Calbiochem) or recombinant trimeric CD40L (1 μg/ml, Amgen), and supernatant samples were harvested at corresponding times. In this case, a third donor was used as sufficient cells could not be purified from a single donor to perform the necessary experiments to include infection and protein treatment at one time. At each timepoint, supernatant was removed from a well containing 106 cells and thus represents the total accumulated amount of each cytokine in the supernatant between the time of infection and the time of harvest. As shown in Figure 3, each of the cytokines that showed viral infection-related changes by Luminex also had noticeable differences as determined by ELISA. IFN-α was not detected in uninfected or infected DC (data not shown). IL-6 was induced between 2-6 h post-infection, peaked between 6-18 h post-infection and decreased after 18 h. TNF-α production began within 2 h post-infection, peaked at 6 h and decreased thereafter. The chemokines MIP-1α, MIP-1β, and RANTES had a slower induction with peaks 12-24 h post-infection and decreased to low levels thereafter. Lastly, as shown in the Luminex screening, IL-12p40 was induced to high levels beginning between 12-18 h post-infection and peaking 48-72 h later. However, bioactive IL-12p70 was not detected in either the uninfected or infected cultures at any time point, but was detected when immature DC were treated with trimeric CD40L.
Figure 3. Temporal expression of selected cytokine expression in HHV-8 infected DCs.
A, B, & C. DC were infected with HHV-8 (▲, solid line), untreated (■, dotted line), treated with LPS (◆, dashed line) or trimeric CD40L (▼ ,dot/dash line). Supernatant samples were harvested at selected times post-treatment and analyzed by ELISA for IL-6 and TNF-α (A), MIP-1α, MIP-1β, and RANTES (B) or IL-12p40 and IL-12p70 (C). Each column represents the results from separate DC donors. Error bars represent duplicate samples.
As previously mentioned, we have shown that HHV-8-infected DCs exhibit an intermediate phenotype that is neither immature nor fully mature (CD83low, HLA-DR+, HLA-ABClow, DC-SIGNlow/-). As these effects were not limited to infected cells but also included uninfected cells in the same culture (Rappocciolo, Jenkins, Hensler, Piazza, Jais, Borowski, Watkins, & Rinaldo, Jr., 2006) (data not shown), we suspected a paracrine effect and sought to determine which cytokines were being produced by infected cells. We found that pro-inflammatory cytokines (IL-6 and TNF-α), chemokines (MIP-1α, MIP-1β, RANTES), and potentially TH2-skewing cytokines (IL-10, IL-12p40 but not IL-12p70) were increased after HHV-8 infection. This is consistent with reports of IL-6, IL-10, TNF-α, MIP-1α, MIP-1β, and RANTES expression in cultured PEL and KS cells, and in KS biopsies (Asou et al., 1998;Brockmeyer et al., 1999;Drexler et al., 1999;Uccini et al., 2003). Lack of IL-12 production in response to maturation stimuli has also been observed in PEL cell lines (Asou, Said, Yang, Munker, Park, Kamada, & Koeffler, 1998) and purified peripheral blood myeloid DC from KS patients (Della et al., 2006b). Indeed, exposure to certain stimuli can polarize DC to promote a TH2 rather than a TH1 phenotype (de Jong et al., 2002), and lack of IL-12 expression contributes to this skewing (Hilkens et al., 1997). As it is known that DC polarized towards TH2 induction can contribute to persistence of viruses, (Palucka & Banchereau, 2002), lack of IL-12 production by HHV-8 infected DC suggests this mechanism may be at work in this case.
These findings are of interest in the context of our previous observations that in vitro infected DC show limited functionality after infection with HHV-8. Interestingly, recent studies have shown that both infection of monocytes by HHV-8 or exposure to PEL-derived cytokines results in the inability of these cells to differentiate into DCs, decreases antigen uptake and presentation, and alters their surface marker phenotype (Cirone et al., 2007;Cirone et al., 2008). Moreover, others have shown that peripheral blood myeloid DC from classical KS patients have similar defects that are attributable to a soluble factor (Della et al., 2006a). For instance, TNF-α stimulus in the absence of an accessory signal (i.e., LPS or CD40L) has been implicated in the production of partially or semi-mature DC, which could lead to peripheral tolerance (Lutz & Schuler, 2002;Sallusto & Lanzavecchia, 1994). Moreover, efficient IL-12 p70 production is necessary for generating robust CTL responses (Trinchieri, 2003). Given that HHV-8-infected persons have a dampened CTL response to the virus (Brander, Suscovich, Lee, Nguyen, O’Connor, Seebach, Jones, van Gorder, Walker, & Scadden, 2000;Guihot et al., 2006a;Little & Yarchoan, 2006a;Wang, Jenkins, Jacobson, Kingsley, Day, Zhang, Meng, Pellet, Kousoulas, Baghian, & Rinaldo, Jr., 2001), the lack of IL-12p70 observed in our experiments suggests that HHV-8 could have developed a mechanism to prevent the production of IL-12p70 either through transcriptional repression of the p35 subunit or over-production of potentially antagonistic p40 homodimers (von Grunberg & Plum, 1998). However, a quantitative assay for determination of p40 homodimers in humans is not available.
We postulate that at least some of these cytokine and chemokine responses are caused by viral binding and entry into the cell rather than production of viral proteins, as our lab has shown that HHV-8 infection of DCs is non-productive(Rappocciolo, Jenkins, Hensler, Piazza, Jais, Borowski, Watkins, & Rinaldo, Jr., 2006). Moreover, the cytokine and chemokine responses were induced as early as 2 h post-infection of the DCs using gradient-purified virus, and preliminary experiments using gradient-purified UV-irradiated virus were also able to induce cytokine responses (data not shown). Indeed, the HHV-8 viral OX2 homolog, which has been shown to be incorporated into the virion, is capable of inducing IL-1β, IL-6, MCP-1, and TNF-α in circulating monocytes, macrophages, and DCs (Chung et al., 2002). Recent studies have shown that activating-antibody or HIV gp120 binding to DC-SIGN results in the production of TNF-α, MIP-1α, RANTES, and IL-10 (Caparros et al., 2006;Hodges et al., 2007;Shan et al., 2007). Furthermore, these same studies indicate that DC-SIGN ligation on DCs can result in lack of maturation, lessened T cell stimulatory capacity, and lack of surface receptor upregulation. These responses appear to be dependent on mannosylation of viral glycoproteins. As HHV-8 encodes at least one highly mannosylated glycoprotein (gB) (Baghian et al., 2000) and the virus is known to bind to DC-SIGN (Rappocciolo, Jenkins, Hensler, Piazza, Jais, Borowski, Watkins, & Rinaldo, Jr., 2006;Rappocciolo et al., 2008), it is plausible that DC-SIGN binding to virus is at least partly responsible for the dampened immune responses previously observed in HHV-8 infected DC.
Acknowledgments
The authors would like to thank the Anna Lokshin lab for the use of their Luminex screening technology. We thank Amgen for the gift of CD40L (MTA n.200312724). This research was supported in part from NIH grant 1 R01 CA82053 and funds from the University of Pittsburgh Cancer Institute.
Reference List
- Asou H, Said JW, Yang R, Munker R, Park DJ, Kamada N, Koeffler HP. Mechanisms of growth control of Kaposi’s sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood. 1998;91:2475–2481. [PubMed] [Google Scholar]
- Baghian A, Luftig M, Black JB, Meng YX, Pau CP, Voss T, Pellett PE, Kousoulas KG. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology. 2000;269:18–25. doi: 10.1006/viro.2000.0198. [DOI] [PubMed] [Google Scholar]
- Brander C, Suscovich T, Lee Y. & other authors Impaired CTL recognition of cells latently infected with Kaposi’s sarcoma-associated herpes virus J Immunol 20001652077–2083. [DOI] [PubMed] [Google Scholar]
- Brockmeyer NH, Willers CP, Anders S, Mertins L, Rockstroh JK, Sturzl M. Cytokine profile of HIV-positive Kaposi’s sarcoma derived cells in vitro. Eur J Med Res. 1999;4:95–100. [PubMed] [Google Scholar]
- Caparros E, Munoz P, Sierra-Filardi E. & other authors DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production Blood 20061073950–3958. [DOI] [PubMed] [Google Scholar]
- Chung YH, Means RE, Choi JK, Lee BS, Jung JU. Kaposi’s sarcoma-associated herpesvirus OX2 glycoprotein activates myeloid-lineage cells to induce inflammatory cytokine production. J Virol. 2002;76:4688–4698. doi: 10.1128/JVI.76.10.4688-4698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone M, Lucania G, Aleandri S, Borgia G, Trivedi P, Cuomo L, Frati L, Faggioni A. Suppression of dendritic cell differentiation through cytokines released by Primary Effusion Lymphoma cells. Immunol Lett. 2008 doi: 10.1016/j.imlet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Cirone M, Lucania G, Bergamo P, Trivedi P, Frati L, Faggioni A. Human herpesvirus 8 (HHV-8) inhibits monocyte differentiation into dendritic cells and impairs their immunostimulatory activity. Immunol Lett. 2007;113:40–46. doi: 10.1016/j.imlet.2007.07.013. [DOI] [PubMed] [Google Scholar]
- de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J Immunol. 2002;168:1704–1709. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- Della BS, Nicola S, Brambilla L, Riva A, Ferrucci S, Presicce P, Boneschi V, Berti E, Villa ML. Quantitative and functional defects of dendritic cells in classic Kaposi’s sarcoma. Clin Immunol. 2006a;119:317–329. doi: 10.1016/j.clim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Della BS, Nicola S, Brambilla L, Riva A, Ferrucci S, Presicce P, Boneschi V, Berti E, Villa ML. Quantitative and functional defects of dendritic cells in classic Kaposi’s sarcoma. Clin Immunol. 2006b;119:317–329. doi: 10.1016/j.clim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Drexler HG, Meyer C, Gaidano G, Carbone A. Constitutive cytokine production by primary effusion (body cavity- based) lymphoma-derived cell lines. Leukemia. 1999;13:634–640. doi: 10.1038/sj.leu.2401371. [DOI] [PubMed] [Google Scholar]
- Guihot A, Dupin N, Marcelin AG. & other authors Low T cell responses to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma J Infect Dis 2006a1941078–1088. [DOI] [PubMed] [Google Scholar]
- Guihot A, Dupin N, Marcelin AG. & other authors Low T cell responses to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma J Infect Dis 2006b1941078–1088. [DOI] [PubMed] [Google Scholar]
- Hilkens CM, Kalinski P, de BM, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–1926. [PubMed] [Google Scholar]
- Hodges A, Sharrocks K, Edelmann M. & other authors Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication Nat Immunol 20078569–577. [DOI] [PubMed] [Google Scholar]
- Little RF, Yarchoan R. Poor specific T cell responses to human herpesvirus 8: A key to unleashing Kaposi sarcoma? J Infect Dis. 2006a;194:1030–1031. doi: 10.1086/507649. [DOI] [PubMed] [Google Scholar]
- Little RF, Yarchoan R. Poor specific T cell responses to human herpesvirus 8: A key to unleashing Kaposi sarcoma? J Infect Dis. 2006b;194:1030–1031. doi: 10.1086/507649. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus. In: Knipe DM, Howley PM, editors. Fields Virology. 4th Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 2803–2833. [Google Scholar]
- Palucka K, Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol. 2002;14:420–431. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol. 2008;82:4793–4806. doi: 10.1128/JVI.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR., Jr. DC-SIGN Is a Receptor for Human Herpesvirus 8 on Dendritic Cells and Macrophages. J Immunol. 2006;176:1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Klasse PJ, Banerjee K. & other authors HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells PLoS Pathog 20073e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Uccini S, Scarpino S, Ballarini F, Soriani A, Chilosi M, Montesu MA, Masala MV, Cottoni F, Ruco L. In situ study of chemokine and chemokine-receptor expression in Kaposi sarcoma. Am J Dermatopathol. 2003;25:377–383. doi: 10.1097/00000372-200310000-00003. [DOI] [PubMed] [Google Scholar]
- von Grunberg PW, Plum G. Enhanced induction of interleukin-12(p40) secretion by human macrophages infected with Mycobacterium avium complex isolates from disseminated infection in AIDS patients. J Infect Dis. 1998;178:1209–1212. doi: 10.1086/515687. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Jenkins FJ, Jacobson LP. & other authors Primary human herpesvirus 8 infection generates a broadly specific CD8(+) T-cell response to viral lytic cycle proteins Blood 2001972366–2373. [DOI] [PubMed] [Google Scholar]