Summary
The pathology of human influenza has been studied most intensively during the three pandemics of the last century, the last of which occurred in 1968. It is important to revisit this subject because of the recent emergence of avian H5N1 influenza in humans as well as the threat of a new pandemic. Uncomplicated human influenza virus infection causes transient tracheo-bronchitis, corresponding with predominant virus attachment to tracheal and bronchial epithelial cells. The main complication is extension of viral infection to the alveoli, often with secondary bacterial infection, resulting in severe pneumonia. Complications in extra-respiratory tissues such as encephalopathy, myocarditis, and myopathy occur occasionally. Sensitive molecular and immunological techniques allow us to investigate whether these complications are a direct result of virus infection or an indirect result of severe pneumonia. Human disease from avian influenza virus infections is most severe for subtype H5N1, but also has been reported for H7 and H9 subtypes. In contrast to human influenza viruses, avian H5N1 virus attaches predominantly to alveolar and bronchiolar epithelium, corresponding with diffuse alveolar damage as the primary lesion. Viremia and extra-respiratory complications appear to be more common for infections with avian H5N1 virus than with human influenza viruses. Further understanding and comparison of the pathology of human and avian influenza virus infections only can be achieved by directed and careful pathological analysis of additional influenza cases.
Keywords: influenza, human, pathology, pathogenesis
Introduction
An understanding of the pathology of influenza A virus infections in humans is important to improve diagnosis and to understand how these viruses cause disease. This knowledge also is important to evaluate animal models that adequately represent the disease in humans, and so to further unravel the pathogenesis and to test potential antiviral drugs and vaccines. We here review the pathology of human influenza A virus infections, both pandemic and seasonal, as well as that caused by infections with avian influenza A viruses such as H5N1 virus.
Uncomplicated influenza
Human influenza A virus infections for which the pathology is described include H1N1, H2N2, and H3N2, which caused pandemics in 1918, 1957, and 1968, respectively [1]. Each time that a new subtype enters the human population it replaces the previously circulating subtype. The exception is the reintroduction in 1977 of H1N1, which has continued to co-circulate with H3N2.
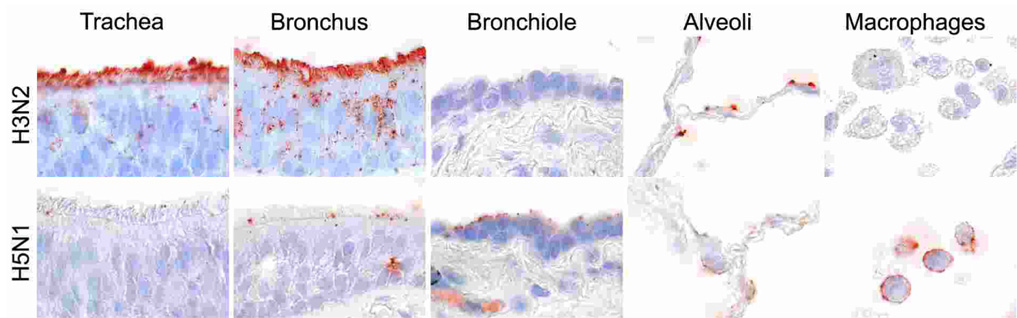
Transmission of human influenza virus occurs by inhalation of infectious droplets or airborne droplet nuclei and, perhaps, by indirect (fomite) contact followed by self-inoculation of the upper respiratory tract or conjunctival mucosa. The relative importance of these routes is still debated [2]. Receptors for which human influenza viruses have a preference are long glycans terminating in sialic acids linked to galactose by an alpha-2,6 linkage [3]. These receptors are expressed on epithelial cells throughout the respiratory tract—nasal mucosa, paranasal sinuses, pharynx, trachea, bronchi, bronchioles, and alveoli—but their abundance varies per site [4]. In the tracheo-bronchial tree, human influenza viruses attach predominantly to ciliated epithelial cells, and attach more abundantly to tracheal and bronchial epithelium than to bronchiolar epithelium (Fig. 1) [5]. In uncomplicated influenza in humans, the cell types in which human influenza virus replicates in vivo only has been determined for the nasal mucosa, where both ciliated and non-ciliated cells are infected [6]. However, ex vivo human tissue cultures have shown that epithelial cells of nasopharynx, adenoids, tonsil [7], bronchus and pulmonary alveolus [4] are permissive. In vitro, primary cell cultures of human tracheal epithelial cells have shown replication in both ciliated and non-ciliated cells [8].
Figure 1.
Attachment of human H3N2 influenza virus (top row) and highly pathogenic avian H5N1 virus (bottom row) in human trachea, lower respiratory tract (bronchus, bronchiole, and alveoli), and alveolar macrophages [5].
One of the only descriptions of histologic lesions associated with uncomplicated influenza in humans is from a study of tracheal and bronchial biopsies obtained from six young adults between 1 and 7 days after onset of symptoms [9]. They had a diffuse, superficial, necrotizing tracheo-bronchitis, which was progressively more severe further down the tracheo-bronchial tree. Lesions were already visible at 1 day after onset of symptoms. Damage to the respiratory epithelium ranged from vacuolization, edema, and absence of cilia to extensive desquamation of epithelial cells. In the lamina propria, there was prominent edema and hyperemia, and infiltration with primarily lymphocytes and histiocytes. Inflammatory cell infiltration was limited compared to the extent of epithelial damage. From 2 days after the onset of symptoms, epithelial repair was visible in the form of epithelial metaplasia.
The changes to the bronchial epithelium from influenza virus infection are short-lasting. In a study of bronchial biopsies from patients between 1 and 6 weeks after onset of symptoms, the only significant differences between influenza patients and healthy controls were thickened surface epithelium and slight increase in lymphocytic infiltration of the lamina propria, corresponding with epithelial regeneration and bronchial inflammation, respectively [10;11].
The typical signs and symptoms of uncomplicated influenza are both local (nasal obstruction, cough, sore throat) and systemic (headache, fever, chills, anorexia, myalgia) [1]. These signs and symptoms are due both to the damage at the site of virus replication and to the local and systemic release of cytokines and other inflammatory mediators [12;13].
Primary complication: viral pneumonia
The most common complication of influenza is extension of the viral infection distally to the lung, resulting in pneumonia. In contrast to damage to the tracheo-bronchial epithelium in uncomplicated influenza, damage to the alveolar epithelium has severe consequences for the gas exchange function of the respiratory tract. This damage to alveolar epithelium—consisting of type I and type II pneumocytes—is due to a combination of the direct cytolytic effect of viral infection and the indirect effect of host response [14]. Type I pneumocytes prevent leakage of fluid across the alveolar-capillary barrier, and type II pneumocytes both resorb fluid from the alveolar lumen and produce lung surfactant that is important for reducing alveolar surface tension. Therefore, damage to these cells allows fluid from the alveolar capillaries to flood into the alveolar lumina. This causes severe, and in some cases fatal, respiratory dysfunction [15].
Risk factors for the development of influenza viral pneumonia include lack of previous exposure to influenza virus with related surface glycoproteins, age greater than 65 years, pulmonary disease, cardiovascular disease, and pregnancy [1]. Individuals who have not been previously exposed to an antigenically related influenza virus lack the protection of the lung against viral infection conferred by specific IgG, which reaches the alveolar lining fluid by transudation from the serum [16–18]. Important chronic underlying pulmonary diseases that predispose influenza patients to hospitalization are chronic obstructive pulmonary disease, asthma, and pulmonary fibrosis [19], which involve remodeling of airways or distal lung parenchyma and thus reduce pulmonary defense against infectious pathogens [20]. There are no clear explanations for the increased risk of influenza viral pneumonia from cardiovascular disease or pregnancy. It has been speculated that pulmonary hypertension secondary to cardiovascular disease or from the increased blood volume in pregnancy may predispose the lung to pulmonary oedema when the alveolar septa are damaged by the virus [21].
Based on attachment studies [5], the primary target cells of human influenza virus in the lower respiratory tract are type I pneumocytes and ciliated bronchiolar epithelial cells, although attachment does occur less frequently to non-ciliated bronchiolar epithelial cells, type II pneumocytes, and alveolar macrophages (Fig. 1). This corresponds with ex vivo infection of alveolar epithelial cells by human influenza virus [4]. In vivo descriptions of the target cells of influenza virus in fatal pneumonia from any of the three influenza pandemics of the last century are very rare. Specific fluorescence was visible in alveolar epithelial cells and alveolar macrophages in lung tissue of two adult women who died with human influenza virus H2N2 pneumonia during or just after the 1957 pandemic [22;23]. Fluorescence-positive interstitial macrophages were detected in the interstitium and alveolar exudate of 7 of 29 lungs from people who died of influenza in Boston during the 1957 pandemic [24].
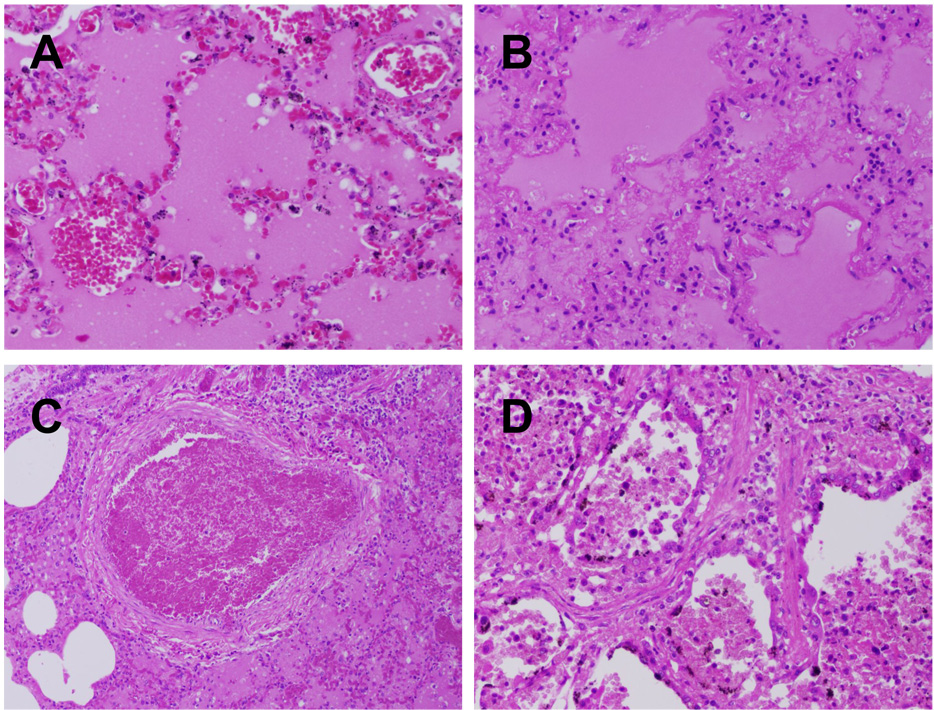
The pathological changes to the lung from influenza viral pneumonia have been most commonly described during pandemics and have been recently been reviewed [25]. The acute alveolar injury (diffuse alveolar damage) caused by influenza virus infection is similar to that caused by many other agents that are noxious for alveoli. In the early stage, there is necrosis of alveolar epithelium, characterized by denudation of the alveolar septum and the presence of desquamated pneumocytes in the alveolar lumen. These desquamated cells are shrunken and show pyknosis or karyorrhexis and cytoplasmic vacuolation or hypereosinophilia. The alveolar lumina are flooded by edema fluid with variable admixture of fibrin and erythrocytes (intra-alveolar hemorrhage) (Fig. 2A). In some alveolar lumina, there are many alveolar macrophages. Characteristically, alveoli and alveolar ducts are lined by hyaline membranes, consisting of fibrin-rich edema fluid mixed with the cytoplasmic and lipid remnants of necrotic epithelial cells (Fig. 2B). The alveolar septa are widened due to hyperemia of alveolar capillaries, interstitial edema, and leukocyte infiltration, mainly neutrophils as well as a few eosinophils. These leukocytes also may be present in alveolar lumina. Fibrinous thrombi may be present in the capillaries of alveolar septa and alveolar ducts, as well as in small pulmonary blood vessels (Fig. 2C). Possibly as a result of these thrombi, alveolar septa may be necrotic. The late stage of influenza viral pneumonia is characterized by re-epithelization of the alveoli by type II pneumocytes (type II pneumocyte hyperplasia), interstitial fibrosis of alveolar septa, and infiltration by mononuclear leukocytes, predominantly lymphocytes and plasma cells (Fig. 2D).
Figure 2.
Charastic lesions of human influenza virus infection in the lung. (A) Acute massive alveolar edema and congestion (1957 pandemic autopsy case, original magnification 200X). (B) Acute massive alveolar edema with hyaline membrane formation and interstitial inflammation (1918 pandemic autopsy case, original magnification 200X). (C) Thrombus in a small pulmonary vessel (1918 pandemic autopsy case (original magnification 40X). (D) Regeneration as evidenced by alveolar type II pneumocyte hyperplasia and interstitial fibrosis (1918 pandemic autopsy case, original magnification 200X).
In addition to the above alveolar changes, the bronchioles show a necrotizing bronchiolitis, characterized by epithelial necrosis, the formation of hyaline membranes, and infiltration by variable numbers of neutrophils. Changes to the trachea and bronchi are similar to those of uncomplicated influenza. Chronic changes of influenza pneumonia may include squamous metaplasia and interstitial fibrosis [25].
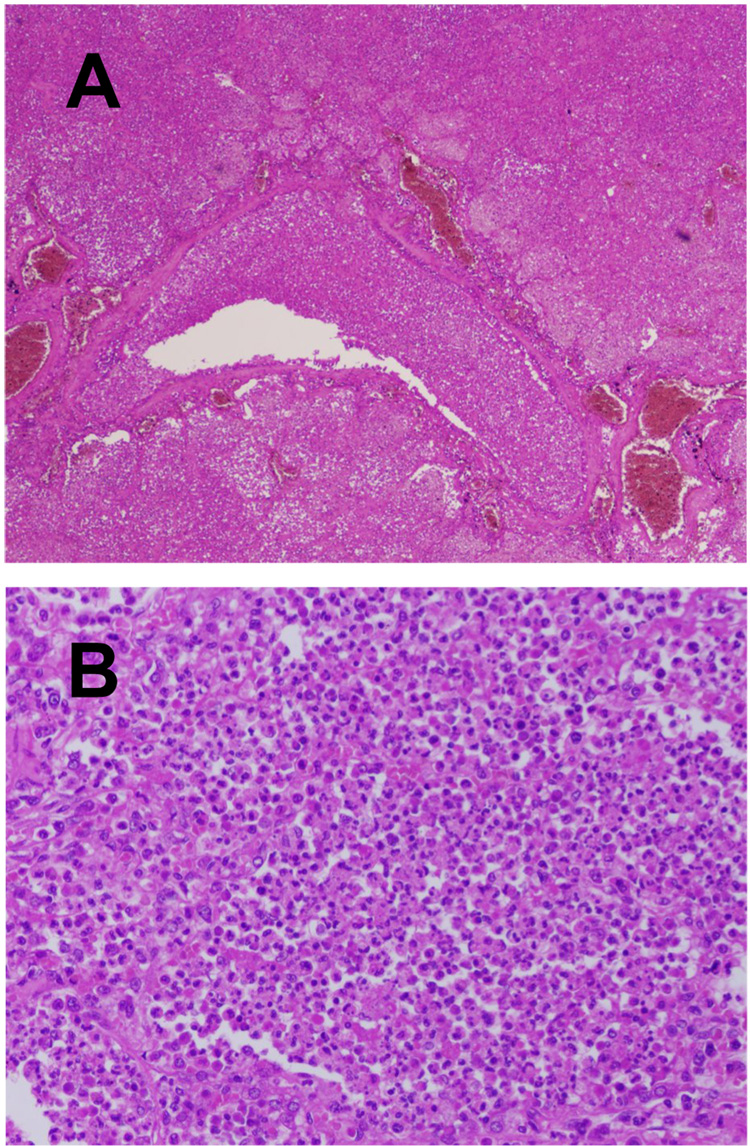
Influenza viral pneumonia often occurs together with, or is followed by, bacterial pneumonia. Prior influenza virus infection may predispose the respiratory tract to bacterial infection by different mechanisms and, vice versa, bacterial infection may enhance influenza virus infection [26]. The bacterial infection results in a different type of inflammation than that caused by influenza virus, with a more prominent infiltration of neutrophils and production of pus: suppurative bronchopneumonia (Fig. 3). A recent review of over 8,000 published autopsy case results from the 1918 pandemic found that the majority of deaths (96%) likely resulted from secondary bacterial pneumonia (Morens D.M., Taubenberger, J.K., Fauci, A.S., unpublished data). As in 1918, most deaths in the 1957 pandemic were due to secondary bacterial pneumonia, although negative autopsy lung cultures were more common than in 1918, possibly due to the widespread administration of antibiotics [27;28]. In one study of the 1957 pandemic, 111/148 (75%) of confirmed fatal cases of influenza had bacteriological and histological evidence of a bacterial pneumonia, mainly due to Staphylococcus aureus or pneumococci [29]. In the same study, 30/148 (20%) of fatal cases were considered due to influenza viral pneumonia.
Figure 3.
Lesions of secondary bacterial infection in fatal human influenza cases. (A) Secondary bacterial bronchopneumonia with neutrophils in the lumen of a bronchiole with transmural infiltration of wall and into surrounding lung tissue (1918 pandemic autopsy case, original magnification 40X). (B) Secondary bacterial bronchopneumonia with neutrophils filling the lumen of an alveolus (1918 pandemic autopsy case, original magnification 40X).
Complications outside the respiratory tract
Human influenza virus primarily infects and causes disease in the respiratory tract. However, human influenza virus infection also is associated with disease in other organs, albeit to a lesser extent. Given the recent reports of extra-respiratory disease from highly pathogenic avian influenza H5N1 virus infection (see below), it is important to revisit these complications of human influenza virus infection.
In general, there are two explanations for the pathogenesis of influenza-associated extra-respiratory complications. The first is that influenza virus spreads via blood to these tissues and replicates there. A likely route for influenza virus to reach blood is by crossing the alveolar-capillary barrier damaged by influenza viral pneumonia. It remains controversial whether viremia routinely occurs during pandemic or seasonal influenza infection. As recently reviewed [30], viremia has been previously reported in influenza virus infection of humans [31–35]. However, several other studies [36–38] failed to detect viremia after onset of illness, suggesting that influenza viremia is rare after onset of symptoms and, if it occurs, is not sustained for long periods [30].
Evidence for replication of influenza virus in extra-respiratory tissues usually comes from detection of virus in these tissues by virus isolation or fully-nested RT-PCR. However, these methods do not exclude the possibility that detected virus originated from blood. The only proof is in situ detection of virus by direct immunofluorescence, immunohistochemistry, or in situ hybridisation in the tissue concerned. Such reports are rare (e.g., brain: [39;40]; heart: [41]) and further confirmation of the ability of human influenza virus to replicate in extra-respiratory human tissues in vivo is badly needed.
The second explanation for the pathogenesis of influenza-associated extra-respiratory complications is suggested by the link between acute respiratory distress syndrome (ARDS) and multi-organ dysfunction syndrome (MODS). ARDS, which may be caused by a variety of insults to the lungs, including influenza virus infection, commonly progresses to MODS [42]. The hepatic, renal, central nervous, gastrointestinal, hematologic, and cardiac systems are most commonly affected [43]. The pathogenesis of MODS has not been elucidated, but is thought to involve the microcirculation and mitochondrial metabolism. Mechanisms may include the release of cytokines into the circulation [44].
Central nervous system disease
An important complication of influenza A virus infection is central nervous system (CNS) dysfunction, that can take a number of forms [45], including influenza-associated acute encephalopathy (IAAE). This is an uncommon neurological syndrome generally of children and adolescents that typically presents during the early phase of influenza virus infection [45].
There are several hypotheses regarding pathogenesis of IAAE. The most straightforward one is that it is caused by viral infection of the CNS. In support of this hypothesis, influenza virus has been detected occasionally by virus isolation or nested RT-PCR in CSF of patients [46–50] and in brain tissue from fatal cases [39;51]. Virus has been detected in neuropil and ependyma of the brain by direct immunofluorescence [39] and in Purkinje cells of the cerebellum and neurons of pontine nuclei by immunohistochemistry [40]. However, the frequent failure to detect virus in CSF and brain of affected patients despite thorough attempts, as well as the lack of inflammation in brain tissue of fatal cases, suggest that virus infection is, at most, only one of the possible pathogeneses. A second hypothesis for the pathogenesis of IAAE is hypercytokinemia, which does not require extra-respiratory virus infection. The severity of CNS dysfunction is correlated with the concentration of pro-inflammatory cytokines in blood and cerebrospinal fluid [45]. However, some patients with severe influenza-associated acute encephalopathy do not have elevated cytokine levels [47]. A third hypothesis that has been proposed is renal and hepatic dysfunction from influenza virus infection, although it is unclear how this occurs [49].
Grossly, the brain in patients with IAAE shows diffuse swelling, which may be severe [28]. Histologically, this corresponds with severe diffuse cerebral congestion and edema, with the notable absence of inflammatory cell infiltrate [28;39;48]. Vascular changes such as hyalinization of the blood vessel wall and thrombosis may be present [50]. The clinical consequences of these lesions include altered consciousness and convulsions. The outcome is highly variable but may result in persistent neurological sequelae or death [45].
Other rare CNS complications of influenza include post-influenza encephalopathy, Reye’s syndrome, Klein-Levin syndrome, post-encephalitic Parkinson’s disease, and encephalitis lethargica [45;52;53]. These are not further discussed here.
Myocarditis
Myocarditis has been observed in association with fatal influenza in each of the three pandemics of the previous century (e.g., [28;54;55]), and in interpandemic periods (e.g., [56;57]) but its pathogenesis is poorly understood. The advent of endomyocardial biopsies at the time of acute disease together with sensitive (in situ) RT-PCR techniques have made it possible to detect the presence of influenza viral RNA in inflamed myocardial tissue in some cases [41;58] but not in others [59;60;60]. It is not clear what the target cells of influenza virus in human heart tissue are: Cioc and Nuovo [41] detected influenza viral RNA in lymphocytes and macrophages within the myocardium of a person who died suddenly and unexpectedly with marked diffuse myocarditis and marked cardiomyocyte necrosis. Ray et al. [56] detected influenza viral antigen throughout the myocardium (cell types showing antigen expression not stated) of a patient with massive myocardial necrosis and associated lymphocytic and mononuclear infiltrates. The necrosis and inflammatory process in the myocardium could be explained by a combination of direct cytolytic effect of viral infection and the host immune response.
The myocarditis consists of cardiomyocyte necrosis associated with variable infiltration of predominantly mononuclear inflammatory cells. There may be interstitial hemorrhage and edema [28;41;54;61;62]. The clinical outcome differs dependent on the duration of the myocardial disease. If the patient dies acutely of fulminant influenza, the main lesion is in the lungs. If the patient dies later, it may be from heart failure. If the patient survives, the resulting myocardial fibrosis may result in heart block due to problems with electrical conduction [60;63].
Myositis or myopathy
Myositis or myopathy is sporadically reported as a complication of both influenza A virus and influenza B virus infections [64]. Myopathy is a better term than myositis, because the majority of muscle biopsies from such cases do not show infiltration by inflammatory cells [64]. The pathogenesis of influenza-associated myopathy is poorly understood. The first hypothesis is direct viral invasion of the muscle. This is supported by the isolation of influenza A virus from muscle biopsies of two patients with influenza A virus infection. However, they were unusual cases. One was a 4-year-old boy with Reye’s syndrome [65], the other was a 72-year-old man with muscle weakness [66]. Also, direct infection of myocytes has not been proven by immunohistochemistry. The second hypothesis is an immune-mediated process. However, the absence of inflammatory cell infiltrates in the majority of muscle biopsies argue against this [64].
Histologic examination of affected muscle biopsies shows muscle degeneration, necrosis, and regeneration, in some cases associated with inflammatory cells [65–69]. The main clinical symptom of influenza-associated myopathy is transient muscle pain in the lower extremities. Most cases resolve completely. Rarely, severe muscle damage develops that results in myoglobinuria and acute renal failure [64].
Differences between pandemic and interpandemic influenza
Influenza pandemics cause higher morbidity and mortality rates than seasonal epidemics during interpandemic periods. This is mainly due the lack of specific immunity to the new virus, so that infection is more likely to result in complicated disease, in particular pneumonia [1]. This raises the question whether the character of the lesions caused by a pandemic virus are qualitatively different from those caused by an interpandemic virus. Unfortunately, it is difficult to compare the pathology of pandemic and interpandemic influenza, because the vast majority of pathological reports are from pandemic periods, and because pathological reports typically describe the late stage of disease and may be complicated by the effects of therapeutic intervention, so that subtle differences may be masked.
Taubenberger and Morens [25] reviewed the pathology of influenza viral pneumonia in interpandemic periods. The observed lesions were similar to those found during pandemic periods. An interesting observation comes from two studies during an interpandemic period comprising a total of 55 fatal influenza virus infection [70;71]. In these studies, influenza viral antigen was detected in tracheal, bronchial, and bronchiolar epithelial cells, but not in alveolar epithelial cells or alveolar macrophages, even in cases showing diffuse alveolar damage. This contrasts with the findings from the 1957 pandemic [22;23], where viral antigen was detected in alveolar epithelial cells (probably both type I and type II pneumocytes) and alveolar macrophages.
Extra-respiratory complications of influenza described during pandemics, including encephalopathy (reviewed in [52] and [45]), myocarditis (e.g., [56]), and myopathy (reviewed in [64]) also have been reported in interpandemic periods. Based on the available information, the character of these complications does not appear to differ in pandemic and interpandemic periods. Together, these studies indicate that, although the proportion of infected people who develop complicated influenza is lower during interpandemic periods, the same types of complications occur and are similar in character to those in pandemic periods.
Special features of human infection with avian influenza viruses
Until 1997, direct human infection with avian influenza viruses was considered to be rare and of little consequence to human health. Highly pathogenic avian influenza (HPAI) virus had been isolated from the blood of a man with clinical symptoms of infectious hepatitis ([72;73], and there had been rare reports of transient conjunctivitis from avian influenza virus infection [74;75]. In 1997, this changed when infection with HPAI virus of the subtype H5N1 was diagnosed in people in Hong Kong, resulting in 6 deaths out of 18 confirmed infections despite intensive care [76–78]. Subsequently, one person died of HPAI virus infection of the subtype H7N7 [79], and a low pathogenic avian influenza (LPAI) virus of the subtype H9N2 was identified as the cause of respiratory disease—albeit mild—in humans [80]. Furthermore, sequencing and phylogenetic analysis of the reconstructed influenza virus of the subtype H1N1 that caused the 1918 pandemic indicates that its genes were derived from avian-like influenza strains [81]. Together, these findings indicate that transmission of avian influenza virus from birds to humans might be rare, but is by no means impossible and has potential severe disease consequences, both for the individual infected and, if the virus is able to adapt to its new host, for the whole population.
H5N1 virus
HPAI H5N1 virus continues to circulate among poultry in many countries of Asia, Africa, and Europe and occasionally spreads to humans with often fatal consequences. Understanding of the pathology of H5N1 virus infection in humans is critically hampered by the few autopsies performed on people who have died of the infection. A recent review identified only nine full autopsies, including one of a fetus, out of 216 laboratory-confirmed fatal cases at the time of publication [82].
Based on clinical evaluation of infected people, the primary disease is centred on the lungs [83]. However, the pattern of attachment of H5N1 virus differs markedly from that for human influenza virus, with important consequences for subsequent disease [5]. In the tracheo-bronchial tree, attachment of human influenza virus is strongest in the trachea and progressively decreases lower down in the tracheo-bronchial tree. In contrast, H5N1 virus shows the strongest attachment in the distal part of the tracheo-bronchial tree—the bronchioles—with progressively less attachment towards the trachea (Fig. 1). The pattern of viral attachment also is distinct within the alveoli. Whereas human influenza virus has a preference for type I pneumocytes, H5N1 virus preferentially attaches to type II pneumocytes and alveolar macrophages (Fig. 1). It has been hypothesized that infection of these cell types might explain the high pathogenicity of H5N1 virus: type II pneumocytes are important for surfactant production, fluid transport out of the alveolar lumen, and re-epithelialization after damage, while alveolar macrophages are important for phagocytosis of pathogens and regulation of the inflammatory response in the alveoli [5;84]. The preference of H5N1 virus for attachment to type II pneumocytes is corroborated by studies that show that these cells have avian-type receptors for influenza virus and can be infected by H5N1 virus in vitro [4] and in vivo [82]. This pattern of viral attachment may also explain why the rare autopsies have shown lesions centred on the alveoli and bronchioles, without reported lesions in trachea or bronchi [82].
The respiratory tract lesions of H5N1 avian influenza in humans are consistent with exudative and proliferative phases of diffuse alveolar damage [82] and thus resemble the lesions of pneumonia from human influenza virus infection. Characteristic features include type II pneumocyte hyperplasia, interstitial infiltration of lymphocytes and in some cases neutrophils, and predominance of macrophages— some showing hemophagocytosis—in alveolar lumina. Additional histologic features include desquamation of epithelial cells into alveolar lumina, hemorrhage, and bronchiolitis. By immunohistochemistry and in situ hybridisation, viral antigens and RNA have been found in type II pneumocytes, as well as ciliated and non-ciliated tracheal epithelial cells [82].
The isolation of the virus from the blood of two patients [85;86] and the detection of H5N1 viral RNA by RT-PCR in 9 of 16 patients [87] suggests that viremia can occur at reasonably high levels and for prolonged periods in people with symptomatic H5N1 virus infection [30]. Such viremia would allow H5N1 virus to spread to extra-respiratory tissues. Indeed, pathological investigations provide evidence for the presence of H5N1 virus in multiple extra-respiratory tissues by immunohistochemistry, in situ hybridisation, or both, often in association with lesions. The brain, where H5N1 virus has been found in neurons, is edematous without significant histologic lesions, or with demyelination, necrosis, and accumulation of reactive histiocytes. The intestine, where H5N1 virus has been found in intestinal epithelial cells and in mononuclear cells in the mucosa, has no abnormalities except lymphocytic apoptosis. The liver, where H5N1 virus has been found in Kupffer cells, shows hepatic necrosis, hepatic lipidosis, cholestasis, and Kupffer cell activation. Lymph nodes, where H5N1 virus has been found in lymphocytes, have reactive histiocytes with hemophagocytotic activity. Such evidence of hemophagocytosis also is present in spleen, bone marrow, lungs, and liver. The placenta, where H5N1 virus has been found in Hofbauer cells (fetal macrophages) and cytotrophoblasts, has syncytiocytotrophoblast necrosis, necrotizing deciduitis, and diffuse villitis. The fetus, where H5N1 virus has been found in lung tissue, shows no specific histologic lesions except edema and scant neutrophil infiltration in the lung. The kidney has acute tubular necrosis in absence of the presence of H5N1 virus [82].
The clinical consequences of these lesions typically manifest as severe pneumonia that often progresses rapidly to acute respiratory distress syndrome. Clinical features outside the respiratory tract include vomiting, diarrhea, myalgia, and—rarely— seizures. Nonspecific clinical presentation or atypical presentation (e.g., encephalopathy and gastroenteritis) often result in initial misdiagnosis of subsequently confirmed cases [83;88].
Together, these studies indicate that the primary lesion in fatal cases of both H5N1 virus infection and human influenza virus infection is the same, namely diffuse alveolar damage. The main difference in respiratory disease is the absence of reports of uncomplicated tracheo-bronchitis in H5N1 virus infection, which is the most common manifestation of human influenza virus infection. This may be due to differences in the attachment preferences—upper respiratory tract for human influenza virus, lower respiratory tract for H5N1 virus—or due to incomplete reporting of less severe H5N1 virus infections.
The level and duration of viremia and the extent of extra-respiratory spread appear to be greater for infections with H5N1 virus than with human influenza virus. It is not clear whether this difference is real or an artifact of more detailed pathologic examination with more up-to-date methods of the few H5N1 influenza cases studied.
Other avian influenza viruses (H7N7, H7N3, H7N2, and H9N2)
Between 1959 and 1996, infections with either high or low pathogenic forms of avian influenza virus (H7N7) infection were reported in six people ([72;74;75;89]. The presumed routes of infection were direct exposure to highly pathogenic avian influenza in poultry [72], accidental laboratory infection [89], pre-and post-mortem examination of infected seals [74], and a piece of straw entering the eye while cleaning out a duck house [75]. In five of six cases, a conjunctivitis developed at 1 to 3 days after inoculation and resolved after 4 days to 2 weeks [74;75;89]. Additionally, one person developed an asymptomatic intraepithelial keratitis one week after inoculation that resolved over the next three weeks [89]. In one of six cases, the virus was isolated from the blood of the patient one month after presumed exposure. The patient had clinical symptoms of an infectious hepatitis, including yellow sclera, dark urine, and loss of appetite. The relationship between these symptoms and isolation of the virus were not clear [72].
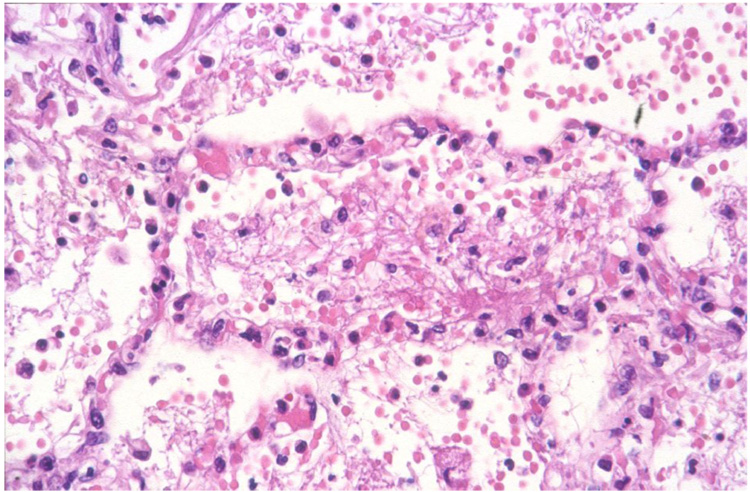
In 2003, an outbreak of HPAI H7N7 virus infection in poultry occurred in the Netherlands, and the virus was detected in 86 people who handled affected poultry and three of their family members. The majority of these people (78/89, 88%) presented with conjunctivitis alone, while a smaller proportion had conjunctivitis and influenza-like illness (5/89, 6%) or influenza-like illness alone (2/89, 2%). Six of seven cases of influenza-like illness were mild. However, one patient developed severe pneumonia and died from acute respiratory distress syndrome and related complications. On autopsy, significant pathological changes were limited to the respiratory tract. Grossly, the lungs were edematous, emphysematous, firm, and about three times the normal weight. Histologically, there was severe diffuse alveolar damage, characterized by flooding of the alveolar lumina with serosanguineous fluid mixed with fibrin and neutrophils (Fig. 4). Although the virus was isolated from postmortem lung samples, viral antigen could not be detected in lung tissue by immunohistochemistry [79;90].
Figure 4.
Lesions of highly pathogenic avian influenza H7N7 virus infection in the lung [79]. There is diffuse alveolar damage, with serosanguineous fluid mixed with fibrin and neutrophils in alveolar lumina.
In 2004, an outbreak of HPAI H7N3 virus infection in poultry occurred in Canada. Two people who had direct conjunctival exposure to infected poultry were infected and developed conjunctivitis and mild influenza-like illness. Disease developed one to 3 days after inoculation and resolved fully [91]. In 2006, one person who was exposed to infected poultry from a U.K. farm with a LPAI H7N3 virus outbreak became infected and developed conjunctivitis [92].
Between 1999 and 2003, at least four separate human cases of LPAI H9N2 virus infection have been confirmed in China [80;93]. One of these cases had a history of probable contact with live chickens before illness; the others had no history of contact with animals. All four were children between 1 and 5 years of age and presented with influenza-like illness. In two children, symptoms included fever, anorexia, inflamed pharynx, and vomiting. In the other two, they included fever and cough. Three of four children recovered after two to six days, the outcome for the last child was not stated.
Influenza hemagglutinin receptor binding preferences for either alpha-2,3 or alpha-2,6 receptors clearly play a role in host-virus interaction but changes in receptor specificity alone are not adequate to account for host adaptation and transmissibility [4;94–96]. Infections with avian influenza viruses of H7 subtype have been associated predominantly with conjunctivitis, even though most H7 and H5 viruses share a predominant alpha-2,3 receptor specificity. Thus, other factors must account for the conjunctival tropism of H7 influenza viruses. Some of the human infections with H9N2 viruses were associated with increased specificity for alpha-2,6 receptors prevalent in human upper respiratory tract [4;97].
Perspectives
Influenza remains a major public health concern, both for its pandemic potential and for the impact of seasonal influenza. Furthermore, direct bird-to-human transmission of avian influenza viruses, particularly of the H5 and H7 subtypes, have caused human disease and mortality. There are many gaps in our knowledge of the pathogenesis and pathology of influenza in humans, despite published pathology studies of influenza virus infection going back at least to 1889 [25]. Because the majority of these studies by necessity took place at the time of pandemics, the last of which occurred in 1968, they lacked the benefit of advanced immunological and molecular biological techniques at our disposal today. This precluded accuracy in both localization of virus in tissues and identification of cell types involved.
Therefore, directed pathology studies, based both on biopsies of influenza patients and autopsies of fatal cases, need to be performed to fill in these gaps. These studies ideally should cover the broad scale of presentation of both human and avian influenza virus infections in humans, from uncomplicated disease to pneumonia and extra-respiratory complications. Also, these pathology studies need to be integrated with virological, immunological, and clinical aspects of influenza virus infection. The knowledge gained can be used to compare and contrast human and avian influenza virus infections in humans. It can also supplement knowledge from laboratory, clinical, and population studies to gain a better overall picture of influenza in humans, in order to guide strategies to combat this many-faceted disease.
Acknowledgments
We thank F. van der Panne for assistance with preparation of figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott, Williams and Wilkins; 2007. pp. 1691–1740. [Google Scholar]
- 2.Hayden F, Croisier A. Transmission of avian influenza viruses to and between humans. J Infect Dis. 2005;192:1311–1314. doi: 10.1086/444399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasan A, Viswanathan K, Raman R, et al. Quantitative biochemical rationale for differences in transmissibility of 1918 pandemic influenza A viruses. Proc Natl Acad Sci U S A. 2008;105:2800–2805. doi: 10.1073/pnas.0711963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinya K, Ebina M, Yamada S, et al. Influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 5.van Riel D, Munster VJ, de Wit E, et al. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol. 2007;171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tateno I, Kitamoto O, Kawamura A., Jr Diverse immunocytologic findings of nasal smears in influenza. N Engl J Med. 1966;274:237–242. doi: 10.1056/NEJM196602032740502. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls JM, Chan MCW, Chan WY, et al. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat Med. 2007;13:147–149. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- 8.Ibricevic A, Pekosz A, Walter MJ, et al. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J Virol. 2006;80:7469–7480. doi: 10.1128/JVI.02677-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh JJ, Dietlein LF, Low FN, Burch GE, Mogabgab WJ. Bronchotracheal response in human influenza: Type A, Asian strain, as studied by light and electron microscopic examination of bronchoscopic biopsies. Arch Intern Med. 1961;108:376–388. doi: 10.1001/archinte.1961.03620090048006. [DOI] [PubMed] [Google Scholar]
- 10.Camner P, Jarstrand C, Philipson K. Tracheobronchial clearance in patients with influenza. Am Rev Respir Dis. 1973;108:131–135. doi: 10.1164/arrd.1973.108.1.131. [DOI] [PubMed] [Google Scholar]
- 11.Levandowski RA, Gerrity TR, Garrard CS. Modifications of lung clearance mechanisms by acute influenza A infection. J Lab Clin Med. 1985;106:428–432. [PubMed] [Google Scholar]
- 12.Hayden FG, Fritz RS, Lobo MC, et al. Local and systemic cytokine responses during experimental human influenza A virus infection: Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5:718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruder D, Srikiatkhachorn A, Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006;19:147–155. doi: 10.1089/vim.2006.19.147. [DOI] [PubMed] [Google Scholar]
- 15.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 16.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 17.Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 18.Ito R, Ozaki YA, Yoshikawa T, et al. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine. 2003;21:2362–2371. doi: 10.1016/s0264-410x(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 19.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 20.Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28:346–351. doi: 10.1183/09031936.06.00131905. [DOI] [PubMed] [Google Scholar]
- 21.Craighead JE. Pathology and pathogenesis of human viral disease. San Diego: Academic Press; 2000. Influenza viruses; pp. 35–46. [Google Scholar]
- 22.Hers JFP, Mulder J. Broad aspects of the pathology and pathogenesis of human influenza. Am Rev Respir Dis. 1961;83:84–97. doi: 10.1164/arrd.1961.83.2P2.84. [DOI] [PubMed] [Google Scholar]
- 23.Mulder J, Hers JFP. Influenza. Groningen, The Netherlands: Wolters-Noordhoff Publishing; 1972. [Google Scholar]
- 24.Martin CM, Kunin CM, Gottlieb LS, et al. Asian influenza A in Boston, 1957–1958. I. Observations in thirty-two influenza-associated fatal cases. AMA Arch Intern Med. 1959;103:515–531. doi: 10.1001/archinte.1959.00270040001001. [DOI] [PubMed] [Google Scholar]
- 25.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louria DB, Blumenfeld HL, Ellis JT, Kilbourne ED, Rogers DE. Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest. 1959;38:213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oseasohn R, Adelson L, Kaji M. Clinicopathologic study of thirty-three fatal cases of Asian influenza. N Engl J Med. 1959;260:509–518. doi: 10.1056/NEJM195903122601101. [DOI] [PubMed] [Google Scholar]
- 29.Hers JFP, Masurel N, Mulder J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet. 1958;2:1141–1143. doi: 10.1016/s0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- 30.Likos AM, Kelvin DJ, Cameron CM, et al. Influenza viremia and the potential for blood-borne transmission. Transfusion. 2007;47:1080–1088. doi: 10.1111/j.1537-2995.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 31.Naficy K. Human influenza infection with proved viremia. Report of a case. N Engl J Med. 1963;269:964–966. doi: 10.1056/NEJM196310312691807. [DOI] [PubMed] [Google Scholar]
- 32.Khakpour M, Saidi A, Naficy K. Proved viraemia in Asian influenza (Hong Kong variant) during incubation period. Br Med J. 1969;4:208–209. doi: 10.1136/bmj.4.5677.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poliakova TG, Ketiladze ES, Zhilina NN, Stakhanova VM. Viremia in influenza A2 (Hong Kong) Vopr Virusol. 1970;15:724–728. [PubMed] [Google Scholar]
- 34.Lehmann NI, Gust ID. Viraemia in influenza: A report of two cases. Med J Aust. 1971;2:1166–1169. doi: 10.5694/j.1326-5377.1971.tb92768.x. [DOI] [PubMed] [Google Scholar]
- 35.Roberts GT, Roberts JT. Postsplenectomy sepsis due to influenzal viremia and pneumococcemia. Can Med Assoc J. 1976;115:435–437. [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley ED, Jackson GG. Viremia in Asian influenza. Trans Assoc Am Physicians. 1966;79:376–387. [PubMed] [Google Scholar]
- 37.Mori I, Nagafuji H, Matsumoto K, Kimura Y. Use of the polymerase chain reaction for demonstration of influenza virus dissemination in children. Clin Infect Dis. 1997;24:736–737. [PubMed] [Google Scholar]
- 38.Kawada J, Kimura H, Ito Y, et al. Systemic cytokine responses in patients with influenza-associated encephalopathy. J Infect Dis. 2003;188:690–698. doi: 10.1086/377101. [DOI] [PubMed] [Google Scholar]
- 39.Franková V, Jirásek A, Tùmová B. Type A influenza: Postmortem virus isolations from different organs in human lethal cases. Arch Virol. 1977;53:265–268. doi: 10.1007/BF01314671. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi M, Yamada T, Nakashita Y, et al. Influenza virus-induced encephalopathy: Clinicopathologic study of an autopsied case. Pediatr Int. 2000;42:204–214. doi: 10.1046/j.1442-200x.2000.01203.x. [DOI] [PubMed] [Google Scholar]
- 41.Cioc AM, Nuovo GJ. Histologic and in situ viral findings in the myocardium in cases of sudden, unexpected death. Mod Pathol. 2001;15:914–922. doi: 10.1097/01.MP.0000024291.37651.CD. [DOI] [PubMed] [Google Scholar]
- 42.Beal AL, Cerra FB. Multiple organ failure syndrome in the 1990s: systemic inflammatory response and organ dysfunction. JAMA. 1994;271:226–233. [PubMed] [Google Scholar]
- 43.Dorinsky PM, Gadek JE. Multiple organ failure. Clin Chest Med. 1990;11:581–591. [PubMed] [Google Scholar]
- 44.Deutschman CS. The systemic inflammatory response syndrome and the multiple organ dysfunction syndrome. In: Fishman AP, editor. Fishman's pulmonary diseases and disorders. 3rdth ed. New York: McGraw-Hill; 1998. pp. 2567–2574. [Google Scholar]
- 45.Toovey S. Influenza-associated central nervous system dysfunction: A literature review. Travel Med Infect Dis. 2008;6:114–124. doi: 10.1016/j.tmaid.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto S, Kobayashi M, Uemura O, et al. PCR on cerebrospinal fluid to show influenza-associated acute encephalopathy or encephalitis. Lancet. 1998;352:873–875. doi: 10.1016/S0140-6736(98)12449-2. [DOI] [PubMed] [Google Scholar]
- 47.Ito Y, Ichiyama T, Kimura H, et al. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol. 1999;58:420–425. doi: 10.1002/(sici)1096-9071(199908)58:4<420::aid-jmv16>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 48.Morishima T, Togashi T, Yokota S, et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35:512–517. doi: 10.1086/341407. [DOI] [PubMed] [Google Scholar]
- 49.Steininger C, Popow-Kraupp T, Laferl H, et al. Acute encephalopathy associated with influenza A virus infection. Clin Infect Dis. 2003;36:567–574. doi: 10.1086/367623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Togashi T, Matsuzono Y, Narita M, Morishima T. Influenza-associated acute encephalopathy in Japanese children in 1994–2002. Virus Res. 2004;103:75–78. doi: 10.1016/j.virusres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Flewett TH, Hoult JG. Influenzal encephalopathy and postinfluenzal encephalitis. Lancet. 1958;2:11–15. doi: 10.1016/s0140-6736(58)90003-5. [DOI] [PubMed] [Google Scholar]
- 52.Studahl M. Influenza virus and CNS manifestations. J Clin Virol. 2003;28:225–232. doi: 10.1016/s1386-6532(03)00119-7. [DOI] [PubMed] [Google Scholar]
- 53.Reid AH, McCall S, Henry JM, Taubenberger JK. Experimenting on the past: The enigma of von Economo's encephalitis lethargica. J Neuropathol Exp Neurol. 2001;60:663–670. doi: 10.1093/jnen/60.7.663. [DOI] [PubMed] [Google Scholar]
- 54.Lucke B, Wight T, Kime E. Pathologic anatomy and bacteriology of influenza: Epidemic of autumn, 1918. Arch Intern Med. 1919;24:154–237. [Google Scholar]
- 55.Tesarová-Mágrova J, Havlík J. Myocarditis as a complication of A 2 Hong-Kong influenza. Cas Lek Cesk. 1972;111:344–346. [PubMed] [Google Scholar]
- 56.Ray CG, Icenogle TB, Minnich LL, Copeland JG, Grogan TM. The use of intravenous ribavirin to treat influenza virus-associated acute myocarditis. J Infect Dis. 1989;159:829–836. doi: 10.1093/infdis/159.5.829. [DOI] [PubMed] [Google Scholar]
- 57.Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW. Influenza and cardiovascular disease: Is there a causal relationship? Tex Heart Inst J. 2004;31:4–13. [PMC free article] [PubMed] [Google Scholar]
- 58.Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 59.Nolte KB, Alakija P, Oty G, et al. Influenza A virus infection complicated by fatal myocarditis. Am J Forensic Med Pathol. 2000;21:375–379. doi: 10.1097/00000433-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 60.McGovern PC, Chambers S, Blumberg EA, et al. Successful explantation of a ventricular assist device following fulminant influenza type A-associated myocarditis. J Heart Lung Transplant. 2002;21:290–293. doi: 10.1016/s1053-2498(01)00336-9. [DOI] [PubMed] [Google Scholar]
- 61.Verel D, Warrack AJ, Potter CW, Ward C, Rickards DF. Observations on the A2 England influenza epidemic: A clinicopathological study. Am Heart J. 1976;92:290–296. doi: 10.1016/s0002-8703(76)80109-3. [DOI] [PubMed] [Google Scholar]
- 62.Engblom E, Ekfors TO, Meurman OH, Toivanen A, Nikoskelainen J. Fatal influenza A myocarditis with isolation of virus from the myocardium. Acta Med Scand. 1983;213:75–78. doi: 10.1111/j.0954-6820.1983.tb03693.x. [DOI] [PubMed] [Google Scholar]
- 63.Onitsuka H, Imamura T, Miyamoto N, et al. Clinical manifestations of influenza A myocarditis during the influenza epidemic of winter 1998–1999. J Cardiol. 2001;37:315–323. [PubMed] [Google Scholar]
- 64.Agyeman P, Duppenthaler A, Heininger U, Aebi C. Influenza-associated myositis in children. Infection. 2004;32:199–203. doi: 10.1007/s15010-004-4003-2. [DOI] [PubMed] [Google Scholar]
- 65.Partin JC, Partin JS, Schubert WK, Jacobs R, Saalfeld K. Isolation of influenza virus from liver and muscle biopsy specimens from a surviving case of Reye's syndrome. Lancet. 1976;2:599–602. doi: 10.1016/s0140-6736(76)90667-x. [DOI] [PubMed] [Google Scholar]
- 66.Kessler HA, Trenholme GM, Harris AA, Levin S. Acute myopathy associated with influenza A/Texas/1/77 infection: Isolation of virus from a muscle biopsy specimen. JAMA. 1980;243:461–462. [PubMed] [Google Scholar]
- 67.Mejlszenkier JD, Safran AP, Healy JJ, Embree L, Ouellette EM. The myositis of influenza. Arch Neurol. 1973;29:441–443. doi: 10.1001/archneur.1973.00490300103017. [DOI] [PubMed] [Google Scholar]
- 68.DiBona FJ, Morens DM. Rhabdomyolysis associated with influenza A: Report of a case with unusual fluid and electrolyte abnormalities. J Pediatr. 1977;91:943–945. doi: 10.1016/s0022-3476(77)80896-2. [DOI] [PubMed] [Google Scholar]
- 69.Ruff RL, Secrist D. Viral studies in benign acute childhood myositis. Arch Neurol. 1982;39:261–263. doi: 10.1001/archneur.1982.00510170003001. [DOI] [PubMed] [Google Scholar]
- 70.Guarner J, Shieh WJ, Dawson J, et al. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am J Clin Pathol. 2000;114:227–233. doi: 10.1309/HV74-N24T-2K2C-3E8Q. [DOI] [PubMed] [Google Scholar]
- 71.Guarner J, Paddock CD, Shieh WJ, et al. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clin Infect Dis. 2006;43:132–140. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- 72.DeLay PD, Casey HL, Tubiash HS. Comparative study of fowl plague virus and a virus isolated from man. Public Health Rep. 1967;82:615–620. [PMC free article] [PubMed] [Google Scholar]
- 73.Campbell CH, Webster RG, Breese SS., Jr Fowl plague virus from man. J Infect Dis. 1970;122:513–516. doi: 10.1093/infdis/122.6.513. [DOI] [PubMed] [Google Scholar]
- 74.Webster RG, Hinshaw VS, Bean WJ, et al. Characterization of an influenza A virus from seals. Virol. 1981;113:712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- 75.Kurtz J, Manvell RJ, Banks J. Avian influenza virus isolated from a woman with conjunctivitis. Lancet. 1996;348:901–902. doi: 10.1016/S0140-6736(05)64783-6. [DOI] [PubMed] [Google Scholar]
- 76.de Jong JC, Claas ECJ, Osterhaus ADME, Webster RG, Lim WL. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Claas ECJ, Osterhaus ADME, van Beek R, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 78.Subbarao K, Klimov A, Katz J, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 79.Fouchier RAM, Schneeberger PM, Rozendaal FW, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peiris M, Yuen KY, Leung CW, et al. Human infection with influenza H9N2. Lancet. 1999;354:916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 81.Taubenberger JK. The origin and virulence of the 1918 "Spanish" influenza virus. Proc Am Philos Soc. 2006;150:86–112. [PMC free article] [PubMed] [Google Scholar]
- 82.Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol. 2008;172:1155–1170. doi: 10.2353/ajpath.2008.070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beigel JH, Farrar J, Han AM, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 84.van Riel D, Munster VJ, de Wit E, et al. H5N1 virus attachment to lower respiratory tract. Science. 2006;311:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 85.de Jong MD, Bach VC, Phan TQ, et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 86.Chutinimitkul S, Bhattarakosol P, Srisuratanon S, et al. H5N1 influenza A virus and infected human plasma. Emerg Infect Dis. 2006;12:1041–1043. doi: 10.3201/eid1206.060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Jong MD, Simmons CP, Tran TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358:261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 89.Taylor HR, Turner AJ. A case report of fowl plague keratoconjunctivitis. Br J Ophthalmol. 1977;61:86–88. doi: 10.1136/bjo.61.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koopmans M, Wilbrink B, Conyn M, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 91.Tweed SA, Skowronski DM, David ST, et al. Human illness from avian influenza H7N3, British Columbia. Emerg Infect Dis. 2004;10:2196–2199. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen-Van-Tam JS, Nair P, Acheson P, et al. Outbreak of low pathogenicity H7N3 avian influenza in UK, including associated case of human conjunctivitis. Euro Surveill. 2006;11:pii=2952. doi: 10.2807/esw.11.18.02952-en. [DOI] [PubMed] [Google Scholar]
- 93.Butt KM, Smith GJD, Chen H, et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol. 2005;43:5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Belser JA, Blixt O, Chen LM, et al. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proc Natl Acad Sci U S A. 2008;105:7558–7563. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chandrasekaran A, Srinivasan A, Raman R, et al. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat Biotechnol. 2008;26:107–113. doi: 10.1038/nbt1375. [DOI] [PubMed] [Google Scholar]
- 96.Taubenberger JK. Influenza hemagglutinin attachment to target cells: 'birds do it, we do it…'. Future Virol. 2006;1:415–418. doi: 10.2217/17460794.1.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wan H, Perez DR. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and replication in human airway epithelial cells. J Virol. 2007;81:5181–5191. doi: 10.1128/JVI.02827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]