Abstract
Background
We studied the relationship between behaviors promoted through the US Environmental Protection Agency Worker Protection Standard (WPS) and other programs and agricultural pesticide exposures in 73 strawberry fieldworkers employed in Monterey County, California.
Methods
Farmworkers’ behaviors were assessed via self-report and organophosphorus (OP) pesticide exposure was measured using dimethyl alkylphosphate (DMAP) and malathion dicarboxylic acid (MDA) urinary metabolite levels.
Results
Wearing WPS-recommended clothing, wearing clean work clothes, and the combination of handwashing with soap and wearing gloves were associated with decreases in DMAP and MDA metabolite levels. Despite these protective behaviors, however, participants had significantly higher levels of exposure as compared with a national reference sample.
Conclusions
Interventions that facilitate compliance with these behaviors may be effective in decreasing fieldworkers’ pesticide exposures. However, further efforts are needed to reduce the exposure disparities experienced by farmworkers and decrease the potential for “take home” exposures to farmworkers’ families.
Keywords: farmworker, pesticides, worker protection standard (WPS), occupational behavior, clothing, urinary metabolites
INTRODUCTION
Pesticide exposure is a significant occupational risk facing the nearly three million farmworkers [Hansen and Donohoe, 2003] employed in U.S. agriculture [Das et al., 2001; McCauley et al., 2001; Villarejo, 2003]. Over the past few decades, governmental and other organizations have developed interventions to reduce U.S. farmworkers’ occupational pesticide exposures. The largest intervention to date is the U.S. EPA Worker Protection Standard (WPS). This federally mandated program requires agricultural employers to follow specified waiting periods or re-entry intervals (REI) before allowing workers to enter pesticide-treated areas; to inform workers of the names and potential health consequences of pesticides used; to supply protective equipment to pesticide handlers (i.e., farmworkers who mix, load, or apply pesticides); and to provide WPS pesticide safety training to farmworkers who will be required to enter fields that recently have been or will be treated with a pesticide subject to an REI [EPA, 1992, 2005]. Workers are supposed to receive WPS training within 5 days of entry into pesticide-treated areas and to be retrained every 5 years [EPA, 1992, 2005].
WPS training promotes occupational behaviors believed to reduce pesticide exposures such as wearing clothing that protects the skin (e.g., a long-sleeved shirt, pants, closed-toe shoes, and a hat); wearing clean work clothes; handwashing with soap; eating outside of the field; washing fruits and vegetables taken from the fields before eating them; and showering or bathing immediately after work [EPA, 1992, 2005]. Other programs to reduce farmworkers’ pesticide exposures also promote these behaviors [Flocks et al., 2001; Quandt et al., 2001; Elkind et al., 2002; Vela Acosta et al., 2005; Bradman et al., 2008].
While behaviors such as glove use and handwashing are endorsed by WPS and other programs as ways for all U.S. farmworkers to reduce their pesticide exposures, there is limited field-based evidence of their effectiveness, especially among U.S. fieldworkers (i.e., farmworkers who are not pesticide handlers). Most U.S. studies examining the effectiveness of WPS-promoted behaviors focus on pesticide handlers, even though they represent a minority of the farmworker population and are granted more protections under WPS than fieldworkers (e.g., WPS requires employers to provide pesticide handlers with protective clothing and equipment). Furthermore, few studies have been conducted with samples larger than 20 workers or have measured the impact of behavior on pesticide exposures using biomarkers such as acetylcholinesterase or urinary metabolites [Davies et al., 1982; Keifer, 2000].
Field-based research conducted with U.S. pesticide handlers indicates that WPS-promoted behaviors can reduce some occupational pesticide exposures. For example, wearing clothing such as long pants and a long-sleeved shirt [Fenske et al., 1987, 1990, 2002], coveralls [Davies et al., 1982; Nigg et al., 1986; Fenske, 1988; Lander and Hinke, 1992; Fenske et al., 2002], a disposable chemical-resistant suit [Nigg et al., 1986; Lander and Hinke, 1992; Fenske et al., 2002], and gloves [Putnam et al., 1983; Nigg et al., 1986; Fenske et al., 1990] have been found to reduce dermal pesticide exposures among pesticide handlers. Handwashing with soap has also been reported to decrease pesticide residues on handlers’ hands [Boeniger and Lushniak, 2000].
A few field-based studies of U.S. fieldworkers have examined the effectiveness of protective clothing [McCurdy et al., 1994; Krieger and Dinoff, 2000], handwashing with soap [Boeniger and Lushniak, 2000; Curwin et al., 2003], and gloves [Bradman et al., 2008]. Their findings suggest that these behaviors can be effective in reducing dermal pesticide exposures for fieldworkers.
In the present study, conducted in Monterey County, California, we evaluated whether reported WPS-recommended behaviors, such as wearing a long-sleeved shirt, pants, closed-toe shoes, and a hat; wearing clean work clothes; using gloves; and handwashing with and without soap were associated with reductions in fieldworkers’ organophosphorus (OP) pesticide exposure, as measured by urinary metabolite levels. We focus on OP pesticides because of their extensive agricultural use and their possible effects on adult neurobehavioral function [Keifer and Mahurin, 1997; Kamel and Hoppin, 2004], and potential adverse effects on neurodevelopment in children [Eskenazi et al., 1999, 2007] who may be exposed to pesticides carried home on workers’ skin and clothing [McCurdy et al., 1994; Bradman et al., 1997; Krieger and Dinoff, 2000; Lu et al., 2000; Curl et al., 2002].
METHODS
Setting
Behavioral and OP urinary metabolite data used in the present study were collected in July 2003 as part of a larger community-based participatory research intervention study conducted at two Monterey County strawberry farms. The aim of this study was to determine the effectiveness of a multi-component worksite intervention in reducing both fieldworkers’ occupational exposures and the potential for children’s pesticide exposures via the “take home” of agricultural pesticides on workers’ clothing and skin [Bradman et al., 2008]. Study partners included the Center for Children’s Environmental Health Research, a community-university partnership between the School of Public Health at the University of California Berkeley and a number of governmental, research, and community organizations located in Monterey County (Clínica de Salud del Valle de Salinas, California Rural Legal Assistance, and the Grower-Shipper Association of Central California). This partnership has been discussed previously [Eskenazi et al., 2003; Israel et al., 2005]. In 2003, the year this study was conducted, 497,383 pounds of OP pesticides were applied in Monterey County [DPR, 2003] and approximately 30,000 farmworkers were employed at county farms [Larson, 2000].
Participants
To be eligible for participation in the intervention study mentioned above, farmworkers had to be at least 18 years old, Spanish-speaking, and planning on working at the same farm until October 2003. The current analysis is limited to farmworkers (n = 73) who were employed at one of the two farms participating in the intervention; farmworkers’ urine samples were not collected at the other participating farm (n = 57). There were few sociodemographic differences, however, between workers who provided samples (n = 73) and those who did not (n = 57). Compared to workers who did not provide samples, sampled workers were more likely to live in a household where other household members worked in agriculture and where there were no children (P<0.05 for both). Sampled workers were also less likely to wash their hands or to wash their hands with soap (P<0.05 for both). The research protocol for this study was approved by the Office for the Protection of Human Subjects at the University of California Berkeley. Written informed consent was obtained from each participant prior to data collection.
Data Collection
Interviews
Participants were interviewed by native Spanish-speaking interviewers using a structured questionnaire that had been reviewed by local study staff and community partners, and pre-tested with members of the Center’s Farmworker Council. Information collected included demographics, occupational history, knowledge about pesticides, prior pesticide safety training, and behaviors at work and at home. For each behavior, fieldworkers were asked to report for what they “usually” did “during the past four weeks at work”. Face-to-face interviews were conducted approximately 1 week prior to specimen collection in the fields in a private location away from employers, farm staff, and other workers.
Urine collection and laboratory analysis
We assessed worker pesticide exposure by measuring pesticide metabolites in urine. Among the pesticides registered for use on strawberries, we focused on malathion because of its widespread agricultural use and the availability of a laboratory method to measure its specific urinary metabolites as well as the presence of the parent compound in environmental samples (see below). Malathion is a dimethyl-substituted OP pesticide. During metabolism, malathion can be carboxylated and excreted as one of two malathion-specific metabolites: malathion monocarboxylic acid (MMA) or malathion dicarboxylic acid (MDA). The phosphoric group undergoes hydrolysis to become one of three dimethyl alkylphosphate (DMAP) urinary metabolites: dimethyl phosphate (DMP); dimethyl thiophosphate (DMTP); or dimethyl dithiophosphate (DMDTP). DMAP metabolites are non-specific, meaning that they may reflect exposures to other dimethyl OP pesticides or to pre-formed DMAP metabolites present in the environment.
We measured MDA and DMAP metabolites from spot urine samples collected from each worker upon re-entry to the fields after the pre-harvest interval (72 hr) for malathion on strawberries had expired. Three days prior to sample collection, malathion had been applied to the fields using a ground application method. Samples were collected from a total of 73 field workers over a period of 2 days. All field workers worked in the fields that had been treated by malathion on the first day of re-entry. Forty participants (54.8%) provided urine samples on the first day of re-entry (~80.5 hr after malathion application; M ± SD = 8.5 ± 0.5 hr after re-entry). The remaining 33 participants (45.2%) provided urine samples on the following day (~100 hr after malathion application; M ± SD = 28.0 ± 0.5 hr after re-entry). DMAP and MDA metabolite levels were available for 73 and 72 participants, respectively.
Specimen collection procedures were the same as those used by the Centers for Disease Control and Prevention (CDC) in the National Health and Nutrition Examination Survey 1999–2000 (NHANES) [CDC, 2003]. Immediately prior to sample collection, which took place in field bathrooms, trained study workers gave each participant verbal instructions for urine collection in Spanish. Farmworkers were provided with sterile collection cups, gloves, and trays to reduce sample contamination. Samples were kept on ice packs until they arrived at the field office in Salinas where they were aliquotted into smaller storage jars and frozen at −80°C. For quality control, frozen field blanks and spikes prepared by the CDC were defrosted, repackaged in the field in an identical manner to those used for sample collection, and shipped blinded on dry ice to the National Center for Environmental Health at the CDC (Atlanta, GA) for analysis.
Urinary metabolites were analyzed by the CDC using either gas-chromatography-tandem mass spectrometry or high performance-liquid chromatography-tandem mass spectrometry with isotope dilution quantification as previously described in Bravo et al. [2004] and Olsson et al. [2004]. We adjusted MDA and DMAP metabolite levels for creatinine, standard practice in occupational studies of adult populations [Lauwerys and Hoet, 1993]. Creatinine concentrations in urine were determined using a commercially available diagnostic enzyme method (Vitros CREA slides, Ortho Clinical Diagnostics, Raritan, NJ).
Laboratory quality control included repeat analysis of in-house urine pools enriched with known amounts of pesticide residues whose target values and confidence limits were previously determined. The validity of each analytical run was determined using the Westgard rules for quality control [Westgard, 2003]. Limits of detection (LODs) for the DMAP metabolites were 0.08 μg/L for DMDTP, 0.4 μg/L for DMP and 0.3 μg/L for DMTP. The LOD for MDA was 0.3 μg/L. We assigned an imputed value of the LOD/√2 to levels below the detection limit [Hornung and Reed, 1990; Barr et al., 2004]. Because malathion may devolve to more than one DMAP urinary metabolite, quantities were converted to molar concentrations (nmol/L) and summed to obtain the total concentrations of DMAP metabolites [Barr et al., 2004]. DMAP concentrations are presented in nanomoles per gram of creatinine and MDA concentrations are presented in micrograms per gram of creatinine.
Of 14 field blank samples collected, MDA metabolites were measured in none and very low levels of DMAP metabolites were measured in two (0.02 and 0.03 μg/L) indicating that virtually no contamination occurred in the field during processing or shipment to the analytical laboratory. The MDA recoveries for 10 low (20 μg/L) and 10 high (60 μg/L) field spikes averaged 95% and 105% respectively.
Dislodgeable foliar residue (DFR) collection and analysis
To determine the potential for fieldworker exposure, we measured malathion in DFR samples collected from a field that participants worked in and had been sprayed with malathion (n = 12) and a field that had not been sprayed (n = 4). Sample collection and analytical methods are described in detail in Bradman et al. [2008]. Briefly, leaf punch samples were collected from equal sized sub-plots of the fields. The DFR samples were immediately placed in a cooler on ice packs and transferred to the field laboratory, where the samples were rinsed in a 0.1% Sur-Ten solution. The rinseate was decanted into sample jars and frozen at −80°C until it was shipped blinded on dry ice to the University of Kentucky Department of Horticulture for analysis. Quality control procedures included the use of field spikes and blanks. The samples were extracted and analyzed by gas chromography with a nitrogen phosphorous detector. No malathion residue was measured in field blank samples (n = 3) indicating that no contamination occurred in the field during processing or handling. The low (n = 2 at 2 μg), medium (n = 2 at 20 μg), and high (n = 3 at 200 μg) spike recoveries were on average 223%, 121% and 117% of the spiked concentrations, respectively. The limit of detection for malathion in the DFR samples = 0.00088 μg/cm2.
Data Analysis
The primary objective of this analysis was to investigate the impact of occupational behaviors on fieldworkers’ OP pesticide exposures. Participants who responded that they wore a long-sleeved shirt, pants, closed-toe shoes, and a hat were coded as “wears all four items of clothing recommended by WPS” (yes/no). Wearing clean work clothes was also dichotomized (yes/no). Glove use was based on the response to “in the past four weeks, did you usually wear gloves at work” (yes/no). Handwashing was dichotomized (yes/no), and if yes, classified as “without soap” or “with soap”.
We calculated summary statistics for demographic characteristics, occupational behaviors, OP pesticide urinary metabolite levels, dislodgeable foliar residues. We then tested to see if there were differences in behaviors of interest by demographic characteristic using Fisher’s exact tests. Interrelationships between median urinary metabolite levels, farmworker characteristics, and behaviors were examined using Pearson correlations. Differences in median urinary metabolite levels by behavioral and demographic characteristics were tested using nonparametric K-sample tests on the equality of medians and Kruskal–Wallis one-way analysis of variance tests. We also compared DMAP and MDA levels among fieldworkers in our study with metabolite levels of adults who participated in the National Health and Nutrition Examination Survey (NHANES) [CDC, 2003]. Differences in median urinary metabolite levels for the NHANES reference population and workers in our study were tested using K-sample tests on the equality of medians.
To assess the relationships between each behavior of interest and urinary metabolite levels, we constructed multivariate regression models with log (base e) transformed DMAP and MDA metabolite levels as dependent variables and behaviors of interest as independent variables. Although we initially investigated the relationship of handwashing without soap on metabolite levels, we retained only handwashing with soap for the multivariate analyses since the two handwashing behaviors were significantly correlated and handwashing without soap was not statistically significant in initial bivariate analyses.
In order to ascertain if the association of glove use with the outcomes differed by handwashing practices, we investigated the interaction of glove use and handwashing with soap. Since there was evidence of interaction (P<0.20), we reclassified glove use and handwashing behavior into four mutually exclusive dichotomous variables: (1) both uses gloves and washes hands with soap versus does not; (2) only uses gloves versus does not; (3) only hand washes with soap versus does not; and (4) neither uses gloves nor washes hands with soap versus does not. Since few workers (n = 3) reported only using gloves, we completed our analysis both with and without these workers. Final multivariate models included these four mutually exclusive glove use and handwashing behavior categories (neither uses gloves nor washes hands with soap as the referent); wearing a long-sleeved shirt, pants, closed-toe shoes, and a hat; and wearing clean work clothes. Models were also adjusted for sampling date and farmworkers’ sex.
In order to down-weight the effect of outliers on our models (>3 SD from the mean), we used robust regression for our analysis [Rousseeuw and Annick, 1987]. Final beta coefficients (β) and 95% confidence intervals were converted into measurements of percent change in DMAP or MDA metabolite levels associated with a one-unit increase in the predictor variable using the formula: percent change = 100 × (eβ−1) [Wooldridge, 2000]. All data analyses were conducted using STTA, version 8.0 (StataCorp LP, College Station, TX).
RESULTS
Demographic Characteristics
Table I summarizes the demographic characteristics of the 73 fieldworkers. All participants were born in Mexico (data not shown) and spoke Spanish. The majority were male, between 20 and 40 years old (mean ± SD, 30.9 ± 10.3 years), married or living as married, lived in the United States an average of 5.9 years (±6.6 years), and worked on U.S. farms an average of 5.3 years (±6.0 years). Most (80%) had a sixth grade education or less and were living at or below the federal poverty level (69.9%) and almost all (97%) were living within 200% of poverty [U.S. and Bureau, 2000]. Participants lived with an average of eight household members (SD = 6.6), most of whom were farmworkers. Over 50% of participants were living in a household with at least one child and 28.8% were living in a household with at least one child younger than 6 years of age. Sixty percent of participants reported “ever” receiving any information or training about how to protect themselves from pesticides and 45% of these reported that they had received this information or training during the current agricultural season. The primary work task of all but two participants was harvesting strawberries; the other two were ponchadoras (card punchers who inspect and record the number of boxes of strawberries picked by other farmworkers).
TABLE I.
Demographic Characteristics of Strawberry Fieldworkers, Monterey County, California, 2003 (n = 73)
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 61 (83.6) |
| Female | 12 (16.4) |
| Age (years) | |
| 18–24 | 25 (34.3) |
| 25–29 | 15 (20.5) |
| 30–39 | 17 (23.3) |
| ≥40 | 16 (21.9) |
| Ethnicity | |
| Mexican | 63 (86.3) |
| Mexican Indian | 9 (12.3) |
| Other Latino | 1 (1.4) |
| Highest level of education | |
| None | 8 (10.9) |
| 1–6th grade | 50 (68.5) |
| 7–9th grade | 14 (19.2) |
| Some high school | 1 (1.4) |
| Marital status | |
| Married/living as married | 56 (76.7) |
| Single | 17 (23.3) |
| Total years lived in the U.S.b | |
| ≤1 | 16 (22.2) |
| 2–4 | 28 (38.9) |
| ≥5 | 28 (38.9) |
| Number of years working in U.S. agriculture | |
| ≤1 | 23 (31.5) |
| 2–4 | 21 (28.8) |
| ≥5 | 29 (39.7) |
| Received information or training about pesticides | |
| Received <1year ago | 33 (45.2) |
| Received >1year ago | 11 (15.1) |
| Never | 29 (39.7) |
| Monthly household income | |
| $750 or less | 14 (19.2) |
| $751–1,500 | 33 (45.2) |
| $1,501–2,000 | 17 (23.3) |
| ≥$2,001 | 9 (12.3) |
| Family income relative to federal poverty levela | |
| ≤Poverty level | 51 (69.9) |
| >Poverty level <200% of poverty | 20 (27.4) |
| ≥200% Poverty | 2 (2.7) |
| Number of household members | |
| ≤4 | 23 (31.5) |
| 5–9 | 34 (46.6) |
| ≥10 | 16 (21.9) |
| Number of other farmworkers in householdb | |
| 0 | 4 (5.5) |
| 1–4 | 45 (61.6) |
| 5–9 | 12 (16.4) |
| ≥10 | 12 (16.4) |
| Number of children (<6 years) in household | |
| None | 52 (71.2) |
| ≥1 | 21 (28.8) |
No., number.
Workers’ poverty levels were calculated using the U.S. Department of Health and Human Services’ thresholds for the year 2003. A family of four with an annual income of $18,400 or less was considered to be at or below the poverty level; the same family earning between $18,400 and $36,800 is within 200% of the poverty level.
n = 72 due to missing data.
The only significant difference in demographic characteristics between farmworkers sampled on the first and second days of sample collection was that a greater percentage of those sampled on the second sampling day were at or below poverty (78.8% vs. 57.5%; P<0.05).
Urinary Metabolites
As shown in Table II, DMAP and MDA levels were detected in nearly all the samples and these levels were correlated (Pearson r = 0.58, P<0.001). The overall geometric mean was 215.4 nmol/g for DMAP metabolites and 44.4 μg/g for MDA. Metabolite levels were higher in the group sampled on the first day of sampling compared to levels in the group that that were sampled on the second day for both DMAP and MDA. Both median DMAP and MDA metabolite levels differed significantly (two-sample test on the equality of medians, P<0.001) by day of collection.
TABLE II.
DMAP and MDA Urinary Metabolite Levels forTotal Sample and by Sampling Day*
| Percentile
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolite | No. | DF (%) | Minimum | 25th | 50th | 75th | Maximum | Geometric Mean | (95% CI) |
| DMAP (nmol/g) | |||||||||
| Day1 | 40 | 100.0 | 61.3 | 203.6 | 300.6 | 637.1 | 2744.0 | 352.6 | (267.1, 465.5) |
| Day 2 | 33 | 100.0 | 10.2 | 74.3 | 123.6 | 222.0 | 1328.3 | 118.5 | (78.3,179.4) |
| Total | 73 | 100.0 | 10.2 | 122.9 | 219.2 | 426.0 | 2744.0 | 215.4 | (164.8, 281.5) |
| MDA (μg/g) | |||||||||
| Day1 | 39 | 92.3 | 0.1 | 100.6 | 152.0 | 312.7 | 971.3 | 104.4 | (52.0, 209.6) |
| Day 2 | 33 | 93.9 | 0.1 | 5.5 | 23.5 | 48.0 | 186.3 | 16.2 | (9.0, 29.1) |
| Total | 72 | 93.1 | 0.1 | 21.6 | 76.4 | 181.0 | 971.3 | 44.4 | (26.9, 73.5) |
No., number; DF, detection frequency; CI, confidence interval.
Urinary metabolite levels are adjusted for creatinine concentration.
Figure 1a and b compare the cumulative distribution of DMAP and MDA levels for our sample with those measured by the same CDC laboratory for adults in NHANES. DMAP levels are compared to those from the 2001–2003 NHANES (n = 1,795). MDA levels are compared to those in the 1999–2000 NHANES (n = 959) because MDA levels were not available for the more recent NHANES. Median DMAP levels in our sample were significantly higher than the NHANES adult sample (219.2 nmol/g vs. 20.8 nmol/g, respectively, P<0.001). Median MDA levels in our sample were also significantly higher than the NHANES adults (76.4 μg/g vs. 0.2 μg/g, P<0.001). Moreover, median levels for both urinary metabolites for participants sampled on the second day, 1 day after re-entry, were also significantly higher than median metabolite levels reported for the NHANES adult sample (123.6 vs. 20.8 nmol/g respectively for DMAP and 23.5 vs. 0.2 μg/g respectively for MDA, P<0.001 for both).
FIGURE 1.
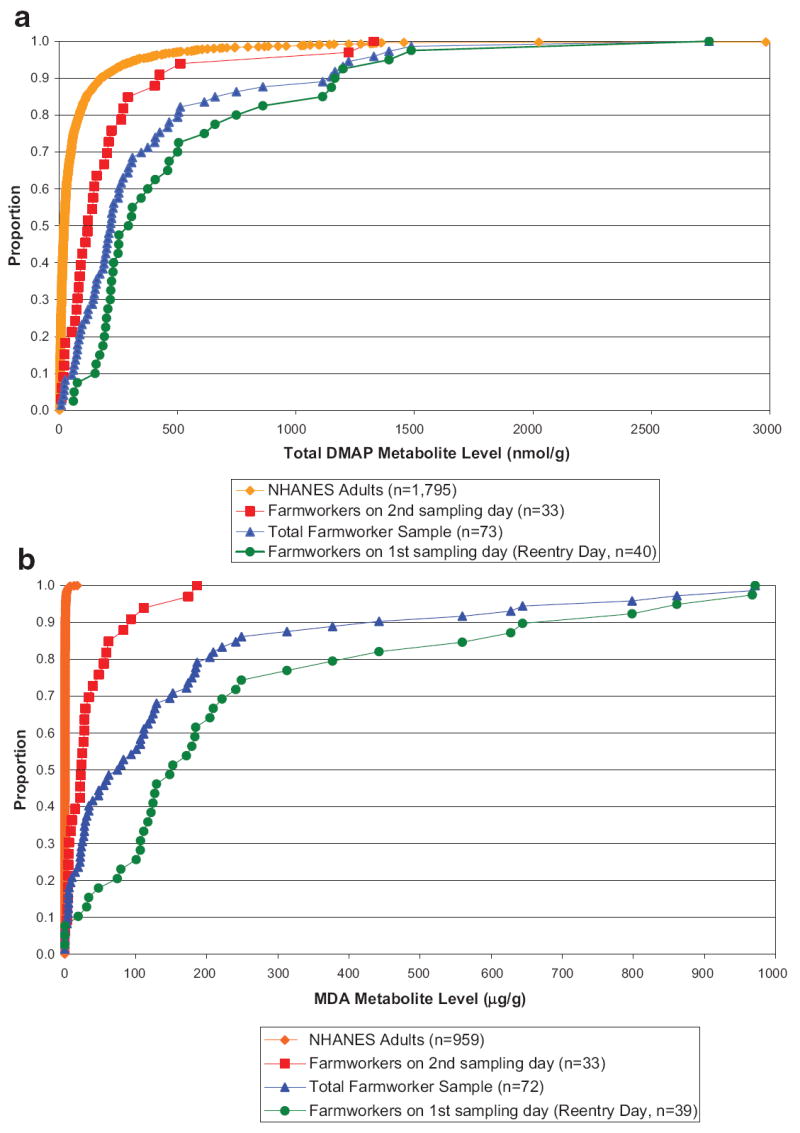
a: Cumulative distribution of DMAP urinary metabolites for farmworker sample and NHANES 2001–2003 adults (four NHANES observations above 3,000 nmol/g were excluded from graph). b: Cumulative distribution of MDA urinary metabolites for farmworker sample and NHANES1999–2000 adults (NHANES2001–2003 data is not available for MDA). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Dislogeable Foliar Residues
Dislodgeable foliar residues of malathion were higher in the sprayed field after expiration of the pre-harvest interval (mean = 0.2 μg/cm2, range = 0.02–0.5, detection frequency = 100%) compared to the unsprayed field (mean = 0.04 μg/cm2, range = 0.0–0.1, detection frequency = 75%).
Occupational Behaviors
Table III presents the frequencies of occupational behaviors reported by participants. Almost all participants (92%) reported wearing the four items of work clothing recommended by WPS training. However, overall, only a quarter of participants reported wearing clean work clothes daily and 43% reported wearing gloves at work. Of the workers who reported using gloves, the majority (93%) reported that they usually used disposable, latex gloves (data not shown). The mean number of times that participants reported washing their hands at work was 3.0 ± 1.1 times/day without soap and 2.6 ± 1.5 times/day with soap. Approximately 38% of participants reported both using gloves and handwashing with soap and 47% reported that they only handwashed with soap (no gloves). Very few workers reported only using gloves (4%) and approximately 10% reported neither using gloves nor handwashing.
TABLE III.
Median DMAP (nmol/g) and MDA (μg/g) Urinary Metabolite Levels by Behaviors and Demographic Characteristics
| Median (IQR)
|
|||
|---|---|---|---|
| Characteristic | No. (%) | DMAP (nmol/g; n = 73)a | MDA (μg/g; n = 72)a |
| Wear long-sleeve shirt, pants, closed-toe shoes, and hatb | |||
| Yes | 67 (91.8)c | 211.3 (112.8, 376.4) | 60.2 (19.0,152.0) |
| No | 6 (8.2) | 703.4 (466.2, 861.8)** | 276.7 (178.9, 442.3)** |
| Wear clean work clothes dailyb | |||
| Yes | 18 (24.7) | 133.7 (79.3, 222.5) | 22.8 (10.2,106.6) |
| No | 55 (75.3)c | 254.1 (156.8, 505.8)*,** | 103.5 (27.7, 208.9)** |
| Neither use gloves nor handwash with soapb | |||
| Yes | 8 (11.0) | 433.5 (240.8, 683.8) | 193.7 (116.2,409.5) |
| No | 65 (89.0)c | 206.4 (112.8, 376.4)* | 56.7 (16.8,161.5)** |
| Only use glovesb | |||
| Yes | 3 (4.1) | 1328.3 (188.4,1486.2) | 186.3 (126.6, 627.6) |
| No | 70 (95.9)c | 218.3 (112.8, 407.1)a,† | 61.8 (21.4,173.6)* |
| Only handwash with soapb | |||
| Yes | 34 (46.6) | 214.3 (150.2, 376.4) | 56.7 (21.8,117.0) |
| No | 39 (53.4)c | 222.5 (74.3, 505.8) | 116.2 (19.0, 204.3)† |
| Both use gloves and handwash with soapb | |||
| Yes | 28 (38.4)c | 155.0 (63.0, 309.2) | 39.3 (5.5,173.6) |
| No | 45 (61.6) | 231.5 (188.4, 460.0)** | 82.6 (24.8,184.2) |
| No. times handwashd | |||
| 0 | 1 (1.4) | 460.0 | 183.2 |
| 1–2 | 21 (28.8) | 397.7 (97.7, 513.3) | 143.8 (27.7,184.2) |
| ≥3 | 51 (69.9)c | 222.0 (122.9, 407.1) | 58.4 (14.6,173.6) |
| No. times hand wash with soapd | |||
| 0 | 11 (15.1) | 744.1 (188.4,1328.3)e,** | 293.0 (111.0, 442.3)f,** |
| 1–2 | 17 (23.3) | 354.0 (79.3, 513.3) | 151.0 (21.4,184.2) |
| ≥3 | 45 (61.6)c | 219.2 (122.9, 347.8) | 43.6 (10.0,138.5) |
| Sexb | |||
| Female | 12 (16.4)c | 222.5 (81.8, 267.5) | 30.9 (4.8,106.6) |
| Male | 61 (83.6) | 218.3 (132.4, 463.1)† | 93.3 (23.5,186.3)* |
| Aged | |||
| 18–24 | 25 (34.3)c | 254.1 (141.3, 505.8)g,† | 99.9 (25.0,194.2) |
| 25–29 | 15 (20.5) | 219.2 (153.2, 502.3) | 129.2 (24.8, 221.4) |
| 30–39 | 17 (23.3) | 172.7 (64.6, 267.5) | 33.7 (4.8, 54.9) |
| >40 | 16 (21.9) | 252.1 (103.7, 418.2) | 60.2 (24.8,184.7) |
No., number; IQR, inter quartile range.
Urinary metabolite levels are adjusted for creatinine concentration.
P values from nonparametric Kruskall–Wallis test.
Frequency one less for MDA.
P values from linear regression.
Difference between not handwashing with soap and handwashing with soap 1–2 times (P<0.05) and 3–4 times (P<0.20).
Difference between not handwashing and handwashing with soap 1–2 times (P<0.20) and 3–4 times (P<0.05).
Difference between farmworkers age18–24 and aged 30–39.
P<0.10.
P<0.05.
P<0.20.
There were no statistically significant differences in the frequencies of behaviors between farmworkers sampled on the first and second days with the exception of only handwashing with soap, the frequency of which was significantly greater among workers sampled on the second day (P<0.05). Female farmworkers, however, were significantly more likely than male farmworkers to wear clean work clothes (P<0.001), to use gloves (P<0.001), to wash hands (P<0.05), to wash hands with soap (P<0.005), and to both use gloves and wash hands with soap (P<0.01) (data not shown). There were almost no significant differences in behaviors based on whether a worker reported having received training or information about how to protect oneself from pesticides, having received this training or information within the current year, or whether the training or information had covered the eleven training points of WPS.
We found several significant interrelationships between our behaviors of interest. The number of times farmworkers washed their hands with and without soap were strongly correlated (Pearson’s r = 0.80, P<0.001). Handwashing without soap was more frequent among workers who wore clean clothes versus not (P<0.05). Washing hands without soap was also more frequent for those wearing gloves, although this relationship was only moderately significant (P<0.10). The frequency of handwashing with soap was significantly greater among workers who wore clean work clothes versus did not (P<0.01) and among farmworkers who wore gloves versus did not (P<0.05). Workers who reported neither using gloves nor handwashing with soap were less likely to use recommended clothing (P<0.10). Farmworkers who reported wearing clean work clothes also wore the four items of recommended clothing.
Metabolite Levels and Behavioral Characteristics
Table III also shows the median urinary metabolite levels by behaviors and demographic characteristics. The relationship between behaviors and metabolite levels were similar for the two urinary metabolite classes. Median DMAP and MDA levels were significantly lower for those wearing a long-sleeved shirt, pants, closed-toe shoes, and a hat versus not (P<0.05 for both) and for those wearing clean work clothes versus not (P<0.05 for both). There were no significant differences in median DMAP or MDA levels between workers who used gloves and those who did not (data not shown). Median DMAP levels were significantly lower among workers who reported using a combination of gloves and handwashing with soap versus those who did not do both (P<0.05). DMAP and MDA metabolite levels were not correlated with the number of times farmworkers handwashed without soap (Pearson r = −0.07 for MDA and r = −0.004 for DMAP), but significantly and negatively correlated with the number of times handwashed with soap (Pearson r = −0.30 and r = −0.24, respectively, P<0.05 for both).
Table IV presents the adjusted multiple regression results for urinary metabolite levels and wearing the four items of recommended clothing; wearing clean work clothes; only using gloves, only handwashing with soap and the combination of using gloves and handwashing with soap (neither using gloves nor handwashing with soap was the referent). After adjustment for date of sampling and farmworkers’ sex, and the other behaviors of interest, the association between the behaviors and urinary metabolite levels were similar for DMAP and MDA. Wearing the four items of protective clothing recommended by WPS was associated with an approximately 50% decrease in DMAP (P<0.01) and 40% decrease in MDA, albeit not significantly. Wearing clean work clothes was associated with decreases of 43% and 41% in DMAP (P<0.04) and MDA (P<0.20), respectively. The combination of using gloves and handwashing with soap was also related to decreases in metabolite levels: a 54% decrease in DMAP levels (P<0.04) and a 46% decrease in MDA (P<0.20).
TABLE IV.
Adjusted Multivariate Associations of Occupational Behaviors With DMAP (nmol/g) and MDA (μg/g) Urinary Metabolite Levelsa,b
| % changec | 95% CI, % changec | P-value | |
|---|---|---|---|
| DMAP (nmol/g; n = 73) | |||
| Wear long-sleeve shirt, pants, closed-toe shoes, and hat versus does not | −49.5 | (−76.6, 9.3)* | 0.08 |
| Wear clean work clothes daily versus does not | −43.1 | (−66.8, −2.4)** | 0.04 |
| Neither use gloves nor hand wash with soap | Referent | — | — |
| Only use gloves | 319.7 | (1280.6, 0.02)** | 0.02 |
| Only hand wash with soap | 20.2 | (−41.3, 146.0) | 0.61 |
| Both use gloves and hand wash with soap | −53.7 | (−78.1, −2.0)** | 0.04 |
| MDA (μg/g; n = 72) | |||
| Wear long-sleeve shirt, pants, closed-toe shoes, and hat versus does not | −40.2 | (−75.7, 47.1) | 0.26 |
| Wear clean work clothes daily versus does not | −40.8 | (−68.8,12.4)† | 0.11 |
| Neither use gloves nor hand wash with soap | Referent | — | — |
| Only use gloves | 115.7 | (−46.2, 764.6) | 0.27 |
| Only hand wash with soap | 3.5 | (−55.1,138.8) | 0.93 |
| Both use gloves and hand wash with soap | −45.7 | (−77.3, 30.2)† | 0.17 |
No., number; CI, confidence interval.
Models adjusted for day of sampling, sex, and the other behaviors of interest.
Urinary metabolite levels are adjusted for creatinine concentration.
Percent change in urinary metabolite levels associated with a unit change in occupational behaviors and 95% confidence interval for percent change in urinary metabolite levels.
P<0.10.
P<0.05.
P<0.01.
P<0.20.
DISCUSSION
Among a group of farmworkers employed as strawberry harvesters in Monterey County, California, we examined the relationship between occupational malathion exposure and several behaviors commonly promoted by the EPAWPS and other programs. Wearing the four items of recommended clothing (long-sleeved shirt, pants, closed-toe shoes, and a hat), wearing clean work clothes, and the combination of using gloves and handwashing with soap were associated with lower DMAP and MDA urinary metabolite levels, although results for the latter metabolite were not statistically significant. The levels of malathion dislodgeable foliar residues (DFRs) we observed were an order of magnitude higher in the sprayed field after expiration of the pre-harvest interval compared to the unsprayed field and are consistent with levels reported in other studies [reviewed in Bradman et al., 2008]. Our findings suggest that wearing all four clothing items recommended by the WPS, wearing clean workclothing, and the combination of using gloves and handwashing with soap reduced exposures from these DFRs.
Fieldworkers in this study had median creatinine-adjusted malathion metabolite levels that were about 395 to 61 times higher (sampling day 1 and 2, respectively) than U.S. national averages for adults who participated in NHANES [Centers for Disease Control and Prevention, 2003]. We sampled farmworkers at the expiration of the pre-harvest interval for malathion on strawberries (72 hr after application) which is 60 hr later than the expiration of the re-entry interval for fieldworkers (12 hr post application). It is likely, therefore, that fieldworkers’ exposure levels would be higher at the time that they legally re-enter fields for non-harvesting field work (e.g., weeding, irrigation, etc.).
Several studies with farmworkers in California and in other states have found low rates of compliance with after work behaviors recommended for decreasing take-home exposures [Arcury et al., 1999, 2002; Hernandez-Valero et al., 2001; McCauley et al., 2001; Thompson et al., 2003; Goldman et al., 2004]. Similarly, only 52% of the fieldworkers in this study reported changing work clothes within 10 min of arriving home and only 6% said that they stored and washed work clothes separately from other family clothes (data not shown). Most were living in a household with at least one child and 28.8% were living in a household with one or more children younger than 6 years old. Thus, residues carried home on work clothes or skin could result in exposures to children living in their homes.
Currently, the standard approach for reducing fieldworker pesticide exposures is education. Many farmworkers, however, do not receive mandated WPS training or do not receive training about all of the topics required by the WPS curriculum [Arcury et al., 1999; Shipp et al., 2007; Strong et al., 2008]. In this study, 40% of participants reported that they had never received any information or training about how to protect themselves from pesticides. Of those who reported having received some training or information (not necessarily WPS training), three-quarters received the training or information during the current agricultural season and a quarter received information on the 11 topics required by the WPS. Furthermore, the effectiveness of educational approaches to changing farmworker behavior is unknown. No rigorous evaluation studies of WPS training or similar educational interventions appear in peer-reviewed publications. In this study, behaviors were not significantly different between fieldworkers who reported that they received information or training and those who did not. Whether WPS training is effective in bringing about consistent protective behavior among fieldworkers has yet to be determined. In separate papers, we will evaluate the efficacy of interventions conducted in this study, including in-field pesticide education, promotion of handwashing, and the provision of gloves and washable and removable outer clothing.
Intervention theory and practice, however, specify that under most circumstances education is not enough to bring about behavior change. A large body of evidence support ecologic theory, which postulates that social, physical, and environmental factors interact to affect health and health behavior [McLeroy et al., 1988; Stokols, 1992; Green et al., 1996]. At the workplace, physical and social characteristics determine whether workers adopt and consistently carry out recommended behaviors [Stokols et al., 1996; Oldenburg et al., 2002]. Accordingly, contextual and structural factors such as whether employers provide fieldworkers with necessary materials and facilities (e.g., soap, water, gloves, showering facilities, laundering), whether break time is sufficiently long (e.g., enough time to wash hands, eat, and rest), employers’ and crew leaders’ commitment to worker safety (e.g., reinforcement of behaviors by farm staff), and how workers are compensated (e.g., piece-rate vs. hourly) likely influence farmworkers’ abilities to carry out WPS-recommended behaviors.
Consistent with an ecological approach to health promotion, interventions that target environmental, policy, and organizational-level barriers are needed to effectively promote farmworkers’ compliance with these and other potentially protective behaviors. Increasing the length and frequency of breaks, eliminating piece-rate compensation, and requiring employers to provide gloves and clean work clothing to fieldworkers are a few examples of possible interventions to evaluate.
This study is one of the first to examine the relationship between WPS-recommended behaviors and occupational OP pesticide exposure. It is also among a few studies to examine these relationships using a biomarker of OP exposure and to focus on fieldworkers rather than pesticide handlers [Keifer, 2000]. Additional strengths of this study include a state-of-the-art methodology for measuring urinary metabolite levels in urine, sample collection timed with re-entry to the field after pesticide application, and a larger sample size than other such studies to date.
One limitation is that we cannot generalize the results of this study, conducted at a single farm and focused on MDA and DMAP metabolites, to the larger fieldworker population or to other pesticide exposures. Additionally, since few farmworkers (n = 3) reported using only gloves (not handwashing), our ability to estimate the sole effect of this practice on pesticide exposure was hampered. Another study limitation is that the reported behaviors may not accurately reflect their practice on the day of sampling. Behaviors were based on fieldworkers’ report approximately 1 week prior to specimen collection (fieldworkers were asked to report for each behavior, what they “usually” did “during the past four weeks at work”), and thus, their behavior may have differed on the day of sampling. In addition, workers may have over-reported behaviors because these behaviors were hygiene-based. Measuring behavior at the time of sampling and verifying self-reported behavior by observation will improve future research in this area.
In summary, based on our findings, occupational behaviors, such as wearing a long-sleeved shirt, pants, closed-toe shoes, and a hat, wearing clean work clothes, and the combination of using gloves and handwashing with soap, could be effective for decreasing fieldworkers’ OP pesticide exposures. In order to enable workers to consistently implement these behaviors, however, interventions must address the most salient behavioral determinants. Furthermore, the effectiveness of other intervention strategies, such as providing additional protective clothing, lengthening re-entry intervals, and using least toxic pest controls, in reducing fieldworkers’ pesticide exposures should be tested. Given the evidence that occupational pesticide exposure experienced by farmworkers may be brought home to their children, intervention effectiveness in preventing take home of agricultural pesticides should be a central outcome of future evaluations.
Acknowledgments
This research was funded by NIEHS grants RO1 ES11352 and PO1 ES009605, and U.S. EPA grant RD 83171001. Additional funding was provided by NIOSH for DFR measurements. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies or CDC. The authors declare that they have no competing financial interests. We gratefully acknowledge the staff, students, community partners, Farmworker Council, and, especially, the farmworkers and growers who participated in this study, for their commitment and contributions. We also thank Mark Boeninger, Nannette Stamm, Diego Vasquez, Lupita Perez-Garnica, Elvia Cabrera, Geri Kavanagh-Baird, Amy Marks, Jeannette Kamen, Jonathan Chevrier, Kelly Birch, Lisa Goldman, Roberto Bravo, Paula Restrepo, Robert Walker, and Richard Thacker for their technical assistance.
Contract grant sponsor: National Institute of Environmental Health Sciences; Contract grant numbers: RO1 ES11352, PO1 ES009605; Contract grant sponsor: U.S. Environmental Protection Agency; Contract grant number: RD 83171001; Contract grant sponsor: National Institute for Occupational Safety and Health (NIOSH).
Footnotes
Published online in Wiley InterScience (www.interscience.wiley.com)
References
- Arcury TA, Quandt SA, Austin CK, Preisser J, Cabrera LF. Implementation of EPA’s Worker Protection Standard training for agricultural laborers: An evaluation using North Carolina data. Public Health Reports. 1999;114:459–468. doi: 10.1093/phr/114.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcury TA, Quandt SA, Russell GB. Pesticide safety among farmworkers: Perceived risk and perceived control as factors reflecting environmental justice. Environ Health Perspect. 2002;110(Suppl 2):233–240. doi: 10.1289/ehp.02110s2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Jr, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ, Jackson RJ, Needham LL. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lushniak BD. Exposure and absorption of hazardous materials through the skin. Int J Occup Environ Health. 2000;6:148–150. doi: 10.1179/oeh.2000.6.2.148. [DOI] [PubMed] [Google Scholar]
- Bradman MA, Harnly ME, Draper W, Seidel S, Teran S, Wakeham D, Neutra R. Pesticide exposures to children from California’s Central Valley: Results of a pilot study. J Expo Anal Environ Epidemiol. 1997;7:217–234. [PubMed] [Google Scholar]
- Bradman A, Salvatore AL, Boeniger M, Castorina R, Snyder J, Barr DB, Jewell NP, Kavanagh-Baird G, Striley C, Eskenazi B. Community-based intervention to reduce pesticide exposure to farmworkers and potential take-home exposure to their families. J Expo Sci Environ Epidemiol. 2008 doi: 10.1038/jes.2008.18. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, Bradman A, Barr DB. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. J Expo Anal Environ Epidemiol. 2004;14:249–259. doi: 10.1038/sj.jea.7500322. [DOI] [PubMed] [Google Scholar]
- CDC; NPN. Second national report on human exposure to environmental chemicals. Centers for Disease Control and Prevention National Center for Environmental Health; 2003. 02-0716. [Google Scholar]
- Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, Coronado G, Thompson B. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ Health Perspect. 2002;110:A787–A792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, Nishioka M, Buhler W. Acephate exposure and decontamination on tobacco harvesters’ hands. J Expo Anal Environ Epidemiol. 2003;13:203–210. doi: 10.1038/sj.jea.7500271. [DOI] [PubMed] [Google Scholar]
- Das R, Steege A, Baron S, Harrison R. Pesticide-related illness among migrant farm workers in the United States. Int J Occup Environ Health. 2001;7:303–312. doi: 10.1179/107735201800339272. [DOI] [PubMed] [Google Scholar]
- Davies JE, Freed VH, Enos HF, Duncan RC, Barquet A, Morgade C, Peters LJ, Danauskas JX. Reduction of pesticide exposure with protective clothing for applicators and mixers. J Occup Med. 1982;24:464–468. [PubMed] [Google Scholar]
- DPR. Analyses of Pesticide Use Trends (1997 to present) California: Department of Pesticide Regulation; 2003. [Google Scholar]
- Elkind PD, Pitts K, Ybarra SL. Theater as a mechanism for increasing farm health and safety knowledge. Am J Ind Med. 2002;42(Suppl 2):28–35. doi: 10.1002/ajim.10053. [DOI] [PubMed] [Google Scholar]
- EPA US. United States Environmental Protection Agency Pesticide Worker Protection Standard Training Part 170.130 1992 [Google Scholar]
- EPA US. The Worker Protection Standard for Agricultural Pesticides How To Comply Manual. EPA 735-B-002. Washington, DC: U. S. Environmental Protection Agency; 2005. [Google Scholar]
- Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107(Suppl 3):409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Gladstone EA, Jaramillo S, Birch K, Holland N. CHAMACOS, A Longitudinal Birth Cohort Study: Lessons from the Fields. J Child Health. 2003;1:3–27. [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA. Comparative assessment of protective clothing performance by measurement of dermal exposure during pesticide applications. Appl Ind Hyg. 1988;3:207. [Google Scholar]
- Fenske RA, Hamburger SJ, Guyton CL. Occupational exposure to fosetyl-Al fungicide during spraying of ornamentals in greenhouses. Arch Environ Contam Toxicol. 1987;16:615–621. doi: 10.1007/BF01055818. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Blacker AM, Hamburger SJ, Simon GS. Worker exposure and protective clothing performance during manual seed treatment with lindane. Arch Environ Contam Toxicol. 1990;19:190–196. doi: 10.1007/BF01056086. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Birnbaum SG, Methner MM, Lu C, Nigg HN. Fluorescent tracer evaluation of chemical protective clothing during pesticide applications in central Florida citrus groves. J Agric Saf Health. 2002;8:319–331. doi: 10.13031/2013.9056. [DOI] [PubMed] [Google Scholar]
- Flocks J, Clarke L, Albrecht S, Bryant C, Monaghan P, Baker H. Implementing a community-based social marketing project to improve agricultural worker health. Environ Health Perspect. 2001;109(Suppl 3):461–468. doi: 10.1289/ehp.01109s3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L, Eskenazi B, Bradman A, Jewell N. Risk behaviors for pesticide exposure among pregnant women living in farmworker households in Salinas, California. Am J Ind Med. 2004;45:491–499. doi: 10.1002/ajim.20012. [DOI] [PubMed] [Google Scholar]
- Green LW, Richard L, Potvin L. Ecological foundations of health promotion. Am J Health Promot. 1996;10:270–281. doi: 10.4278/0890-1171-10.4.270. [DOI] [PubMed] [Google Scholar]
- Hansen E, Donohoe M. Health issues of migrant and seasonal farmworkers. J Health Care Poor Underserved. 2003;14:153–164. doi: 10.1353/hpu.2010.0790. [DOI] [PubMed] [Google Scholar]
- Hernandez-Valero MA, Bondy ML, Spitz MR, Zahm SH. Evaluation of Mexican American migrant farmworker work practices and organochlorine pesticide metabolites. Am J Ind Med. 2001;40:554–560. doi: 10.1002/ajim.10008. [DOI] [PubMed] [Google Scholar]
- Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Env Hyg. 1990;5:46–51. [Google Scholar]
- Israel BA, Parker EA, Rowe Z, Salvatore A, Minkler M, Lopez J, Butz A, Mosley A, Coates L, Lambert G, Potito PA, Brenner B, Rivera M, Romero H, Thompson B, Coronado G, Halstead S. Community-based participatory research: Lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005;113:1463–1471. doi: 10.1289/ehp.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112:950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer MC. Effectiveness of interventions in reducing pesticide overexposure and poisonings. Am J Prev Med. 2000;18:80–89. doi: 10.1016/s0749-3797(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Keifer M, Mahurin R. Chronic neurologic effects of pesticide overexposure. Occup Med. 1997;12:291–304. [PubMed] [Google Scholar]
- Krieger RI, Dinoff TM. Malathion deposition, metabolite clearance, and cholinesterase status of date dusters and harvesters in California. Arch Environ Contam Toxicol. 2000;38:546–553. doi: 10.1007/s002449910071. [DOI] [PubMed] [Google Scholar]
- Lander F, Hinke K. Indoor application of anti-cholinesterase agents and the influence of personal protection on uptake. Arch Environ Contam Toxicol. 1992;22:163–166. doi: 10.1007/BF00213280. [DOI] [PubMed] [Google Scholar]
- Larson A. Migrant and seasonal farmworker enumeration profiles study. California Washington, DC: Migrant Health Program, Bureau of Primary Health Care. Health Resources and Services Administration; 2000. [Google Scholar]
- Lauwerys R, Hoet P. Industrial chemical exposure: Guidelines for biological monitoring. Boca Raton, FL: Lewis Publishers; 1993. [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposure of children in an agricultural community: Evidence of household proximity to farmland and take home exposure pathways. Environ Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- McCauley LA, Lasarev MR, Higgins G, Rothlein J, Muniz J, Ebbert C, Phillips J. Work characteristics and pesticide exposures among migrant agricultural families: A community-based research approach. Environ Health Perspect. 2001;109:533–538. doi: 10.1289/ehp.01109533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy SA, Hansen ME, Weisskopf CP, Lopez RL, Schneider F, Spencer JR, Sanborn JR, Krieger RI, Wilson BW, Goldsmith DF, Schenker MB. Assessment of azinphosmethyl exposure in California peach harvest workers. Arch Environ Health. 1994;49:289–296. doi: 10.1080/00039896.1994.9937482. [DOI] [PubMed] [Google Scholar]
- McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15:351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- Nigg HN, Stamper JH, Queen RM. Dicofol exposure to Florida citrus applicators: Effects of protective clothing. Arch Environ Contam Toxicol. 1986;15:121–134. doi: 10.1007/BF01055257. [DOI] [PubMed] [Google Scholar]
- Oldenburg B, Sallis J, Harris D, Owen N. Checklist of health promotions environments at worksites (CHEW) Am J Health Promot. 2002;16:288–299. doi: 10.4278/0890-1171-16.5.288. [DOI] [PubMed] [Google Scholar]
- Olsson AO, Baker SE, Nguyen JV, Romanoff LC, Udunka SO, Walker RD, Flemmen KL, Barr DB. A liquid chromatography–tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and deet in human urine. Anal Chem. 2004;76:2453–2461. doi: 10.1021/ac0355404. [DOI] [PubMed] [Google Scholar]
- Putnam AR, Willis MD, Binning LK, Boldt PF. Exposure of pesticide applicators to nitrofen: Influence of formulation, handling systems, and protective garments. J Agric Food Chem. 1983;31:645–650. doi: 10.1021/jf00117a042. [DOI] [PubMed] [Google Scholar]
- Quandt SA, Arcury TA, Austin CK, Cabrera LF. Preventing occupational exposure to pesticides: Using participatory research with latino farmworkers to develop an intervention. J Immigr Health. 2001;3:85–96. doi: 10.1023/A:1009513916713. [DOI] [PubMed] [Google Scholar]
- Rousseeuw P, Annick L. Robust regression and outlier detection. Indianapolis, IN: John Wiley and Sons, Inc; 1987. [Google Scholar]
- Shipp EM, Cooper SP, del Junco DJ, Bolin JN, Whitworth RE, Cooper CJ. Pesticide safety training among farmworker adolescents from Starr County, Texas. J Agric Saf Health. 2007;13:311–321. doi: 10.13031/2013.23354. [DOI] [PubMed] [Google Scholar]
- Stokols D. Establishing and Maintaining Healthy Environments: Toward a Social Ecology of Health Promotion. Am Psychol. 1992;47:6–22. doi: 10.1037//0003-066x.47.1.6. [DOI] [PubMed] [Google Scholar]
- Stokols D, Pelletier K, Fielding J. The ecology of work and health: Research and policy directions for the promotion of employee health. Health Educ Q. 1996;23:137–158. doi: 10.1177/109019819602300202. [DOI] [PubMed] [Google Scholar]
- Strong LL, Thompson B, Koepsell TD, Meischke H. Factors associated with pesticide safety practices in farmworkers. Am J Ind Med. 2008;51:69–81. doi: 10.1002/ajim.20519. [DOI] [PubMed] [Google Scholar]
- Thompson B, Coronado GD, Grossman JE, Puschel K, Solomon CC, Islas I, Curl CL, Shirai JH, Kissel JC, Fenske RA. Pesticide take-home pathway among children of agricultural workers: Study design, methods, and baseline findings. J Occup Environ Med. 2003;45:42–53. doi: 10.1097/00043764-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Bureau C, editor. U.S. Bureau C. Poverty Thresholds 2000, Current Population Survey. Washington, DC: U.S.; 2000. [11/5/2006]. http://www.census.gov/hhes/poverty/threshld/thresh03.html. [Google Scholar]
- Vela Acosta MS, Chapman P, Bigelow PL, Kennedy C, Buchan RM. Measuring success in a pesticide risk reduction program among migrant farmworkers in Colorado. Am J Ind Med. 2005;47:237–245. doi: 10.1002/ajim.20136. [DOI] [PubMed] [Google Scholar]
- Villarejo D. The Health of U.S. Hired Farmworkers. Annu Rev Public Health. 2003;24:175–193. doi: 10.1146/annurev.publhealth.24.100901.140901. [DOI] [PubMed] [Google Scholar]
- Westgard J. Westgard QC: Tools, technology, and training for healthcare laboratories. Madison, WI: Westgard, Inc; 2003. [Google Scholar]
- Wooldridge J. Introductory econometrics: A modern approach. Cincinnati, OH: Southwestern College Publishing; 2000. [Google Scholar]


