Abstract
To better define the relationship between vascular calcification and bone mass/structure, we assessed abdominal aortic calcification (AAC), BMD, and bone microstructure in an age-stratified, random sample of 693 Rochester, MN, residents. Participants underwent QCT of the spine and hip and high-resolution pQCT (HRpQCT) of the radius to define volumetric BMD (vBMD) and microstructural parameters. AAC was quantified with the Agatston scoring method. In men, AAC correlated with lower vertebral trabecular and femoral neck vBMD (p < 0.001), but not after age or multivariable (age, body mass index, smoking status) adjustment. Separation into <50 and ≥50 yr showed this pattern only in the older men. BV/TV and Tb.Th inversely correlated with AAC in all men (p < 0.001), and Tb.Th remained significantly correlated after age adjustment (p < 0.05). Tb.N positively correlated with AAC in younger men (p < 0.001) but negatively correlated in older men (p < 0.001). The opposite was true with Tb.Sp (p = 0.01 and p < 0.001, respectively). Lower Tb.N and higher Tb.Sp correlated with AAC in older men even after multivariable adjustment. Among all women and postmenopausal women, AAC correlated with lower vertebral and femoral neck vBMD (p < 0.001) but not after adjustment. Lower BV/TV and Tb.Th correlated with AAC (p = 0.03 and p = 0.04, respectively) in women, but not after adjustment. Our findings support an age-dependent association between AAC and vBMD. We also found that AAC correlates with specific bone microstructural parameters in older men, suggesting a possible common pathogenesis for vascular calcification and deterioration in bone structure. However, sex-specific differences exist.
Key words: bone composition, bone QCT, osteoporosis, population studies, association
INTRODUCTION
An association between atherosclerosis and osteoporosis, two complex and progressive disorders with significant morbidity and mortality, has long been documented.(1–4) Whereas the existence of this relationship has been well established, its significance has been controversial. Some have contended that the association represents confounding by age, insofar as both conditions are more common in the elderly.(3,5–8) Alternatively, it has been suggested that age-related bone loss results in excess calcium in the blood, which is deposited in the vasculature (dystrophic calcification).(2,9,10) Finally, there is growing interest in the possibility that vascular calcification and bone loss have a similar underlying pathogenesis.(11–26) Most previous studies have been cross-sectional analyses of postmenopausal women.(5–7,11,18,20,26–33) A few have been longitudinal, but examined women selected for the presence of one of these disorders or referred for clinical testing, based presumably on suggestive symptoms or risk factors.(28,34,35) Only a few studies have been performed on unselected populations of women.(23,24,36) Studies involving men have been even less frequent.(9,24,29,30,32,33,37–40)
Abdominal aortic calcification (AAC) is a marker of subclinical atherosclerosis and a predictor of subsequent vascular-associated morbidity and mortality.(41–43) Our group has previously reported that, in an age-stratified random sample of 200 women ≥50 yr of age, the positive association of AAC with fracture and the negative association of AAC with bone mass can be primarily accounted by age.(36) That study, like most others, did not use a quantitative means to measure AAC, and only areal BMD (aBMD) was assessed. Recently, QCT has been used to quantitatively examine calcified plaques in the aorta in a preselected group of postmenopausal women who also had previous QCT BMD determinations.(28) AAC was found to be inversely related to BMD but directly related to vertebral and hip fractures identified from QCT scout radiographs. Further analysis of a small subset followed longitudinally also showed a graded correlation between progression of AAC and bone loss.
Among its many limitations, aBMD, as provided by DXA, cannot distinguish the cortical and trabecular bone compartments. Whereas QCT is able to provide a volumetric assess- ment of BMD, it is also limited by resolution to fully assess bone microstructure. This is significant because the cortical and trabecular bone compartments seem to respond differently to hormonal influences and thus are associated with different levels of metabolic activity. Trabecular microstructure has been shown to contribute to the mechanical properties of bone, separate from absolute bone mass.(44,45) The recent introduction and validation of high-resolution pQCT (HRpQCT) has overcome the limitation of these other imaging modalities,(46) allowing a more complete assessment of bone structure. Our group has previously found specific sex and age effects on bone microstructure in a population-based study(47) and thus hypothesize that AAC can be correlated with bone microstructural parameters in an age-independent manner. Using the advanced imaging techniques of QCT and HRpQCT, we have now conducted a cross-sectional analysis of men and women spanning a wide age range to better define the link between AAC and BMD, with an emphasis on bone microstructure and bone compartments. To our knowledge, this study represents the first application of noninvasive, high-resolution in vivo imaging to assess the relationship between bone microstructure and vascular calcifications.
MATERIALS AND METHODS
Study subjects
In 1999–2001, an age-stratified, random sample of Rochester, MN, residents was recruited using the medical records linkage system of the Rochester Epidemiology Project (REP).(48) This population, which our group has previously studied,(47,49–52) is highly characteristic of the white population in the United States, although it is under-represented with respect to persons of African, Hispanic, and Asian descent. In all, 375 women and 325 men were enrolled into the cohort. The sample spanned the ages of 21–97 yr, with a mean age of 57.4 ± 18.2 (SD) yr. Of the women, 127 were premenopausal (mean age, 38.4 ± 9.0 yr; range, 21–55 yr), whereas 248 were postmenopausal (mean age, 67.5 ± 12.0 yr; range, 39–97 yr). The men ranged in age from 22 to 93 yr, with a mean of 57.1 ± 18.7 (SD) yr. Baseline body mass index (BMI) calculations identified 32% of the men and 29% of the women as obese, using the World Health Organization (WHO) definition.(53)
Informed consent was obtained from all study subjects with the use of a consent form approved by the Institutional Review Board at the Mayo Foundation.
As in previous studies with this cohort, subjects were divided into two groups: one younger (127 premenopausal women and 125 men age <50 yr) and one older (248 postmenopausal women and 200 men age 50+ yr). Menopause was defined as the absence of menses for >6 mo. Seven subjects were not included in analyses because of artifacts noted during AAC quantification (i.e., readings that were unreliable either because of imperfections in scanner function or the presence of metallic material within the body). Those treated with bisphosphonates were excluded beginning 6 mo into the study. The reason for this was the observation of an increase use of these medications on chart review, thus causing concern that this would give distort age-related bone changes. However, three women and two men were recruited before this but were subsequently excluded from our analyses (see below). There were a total of 693 subjects (127 premenopausal women, 245 postmenopausal women, 125 men age <50 yr, and 196 men age 50+ yr) remaining in the data set.
For analyses using hormonal variables or bone turnover markers, certain other subjects were excluded. Of the 372 women, we excluded 84 on hormone therapy, 3 on bisphosphonates, 8 on selective estrogen receptor modulators (SERMs), 1 woman on all three therapies, 41 on oral contraceptives, and 2 who had primary hyperparathyroidism. Of the 321 men, we excluded 2 subjects on testosterone, 1 on hormone therapy, 2 on bisphosphonates, 1 on a SERM, 4 with serum creatinine >2 mg/dl, 1 with an unexplained bioavailable (bio) estradiol (E2) level >60 pg/ml, and 1 subject who died soon after imaging and had multiple laboratory abnormalities before death.
Central QCT
As described elsewhere,(49,54) single-energy CT scans were made at the lumbar spine and proximal femur with a multidetector Light Speed QX-I scanner (GE Medical Systems, Wakesha, WI, USA). For all scanning sites, the slice width was 2.5 mm, and the in-plane voxel size was 0.74 mm. Calibration standards scanned with the patient were used to convert CT numbers directly to equivalent volumetric BMD (vBMD; mg/cm3).(55) To study age- and sex-specific changes in bone distribution and structure, we developed software for the analysis of bone structure, geometry, and volumetric density from the CT images, specific details of which have been previously described.(54) To validate our image-processing algorithm, we made 10 scans of the European Spine Phantom, which is composed of hydroxyapatite.(56) The correlation between BMD results determined by our algorithm and that of the spine phantom was r = 0.998; using scans of L2 from the phantom over 10 days, vBMD was estimated to have a CV of 0.7%.
pQCT
As also previously described,(47) the nondominant wrist (or in the case of a prior wrist fracture, the nonfractured wrist) was scanned using a HRpQCT device (a prototype of the Xtreme CT; Scanco Medical AG, Bassersdorf, Switzerland). The slice width was 89 μm, and the inplane voxel size was 89 μm. The processing and analysis of the images have been extensively described and validated(57–60) and are summarized briefly here. The first step involved the determination of trabecular vBMD as the average mineral density within the trabecular region. From this, the trabecular bone volume/tissue volume (BV/TV) was derived, assuming a mineral density of fully mineralized bone of 1.2 g hydroxyapatite/cm3. Recognizing that individual trabeculae would not be resolved at their correct thickness because of partial volume effects, a thickness-independent structure extraction was used to assess trabecular microarchitecture. To this end, the 3D ridges (the center points of the trabeculae) were detected in the gray-level images, as described before.(58) Trabecular number (Tb.N, 1/mm) was taken as the inverse of the mean spacing of the ridges. Combining Tb.N and BV/TV, trabecular thickness (Tb.Th, mm) was then derived as (BV/TV)/Tb.N, and trabecular separation (Tb.Sp, mm) was derived as (1 − BV/TV)/Tb.N, as done in standard histomorphometry.(61) The validity of this approach has been rigorously tested in comparison with 28-μm resolution μCT.(60) In this comparison, the correlation coefficients between the HRpQCT values for BV/TV, Tb.N, Tb.Th, and Tb.Sp and the respective measures using μCT (n = 15 specimens) were 0.99, 0.96, 0.97, and 0.98, respectively (all p < 0.0001). Note that the older pQCT device used in that analysis had a voxel size of 165 μm compared with the newer scanner used in this study, which had a voxel size of 89 μm, so those results are conservative. In addition to the trabecular parameters, cortical measures, including cortical vBMD (mg/cm3) and cortical thickness (C.Th, mm), were also obtained, as well as bone area (BA, mm2) and endocortical area (En.A, mm2). To assess the short-term precision of the measurements, 20 volunteers (age, 19–40 yr) were scanned twice on the same day after repositioning, and the following CVs were observed: cortical vBMD, 1.3%; C.Th, 5.1%; BV/TV, 1.2%; Tb.N, 2.2%; Tb.Th, 1.8%; and Tb.Sp, 3.4%.
AAC quantification
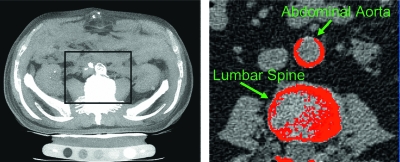
Analysis of AAC was performed with Analyze (version 7.0; AnalyzeDirect, Lenexa, KS, USA) in collaboration with the Mayo Biomedical Imaging Resource. Quantification was performed using the Agatston scoring system, a widely used standard in assessing calcified coronary atherosclerotic plaques.(62) Under this system, a score is generated on the basis of calcified plaque area (Fig. 1), multiplied by a scaling co-factor, which is estimated using the peak attenuation of a calcified lesion. The entire viewing area (middle of the lowest thoracic vertebra through the top of the fourth lumbar vertebra) was studied, while excluding the spine and any potential confounding areas of calcification not related to the aorta. A region of interest was placed around all visible lesions found in the transverse CT slices, and automated measurements of these lesions were made. A threshold attenuation of 130 Hounsfields units (HUs) was used, as in traditional coronary calcification studies.(63) A calcification score was determined by the product of the lesion area (at least two contiguous pixels with a CT density at least 130 HU) and a co-factor dependent on the peak CT density (1, 130–200 HU; 2, 201–300 HU; 3, 301–400 HU; 4, >400 HU) of the lesion. The total Agatston score was determined by the sum of the individual scores from each slice. Agatston scores were normalized per 2.5 mm CT slice to account for slight variability in the number of CT slices obtained in each individual (mean, 56.3 ± 5.0 slices). Excellent intra- and interobserver agreement for calcium quantification has been previously described,(64) and a single trained reader (JTC) performed the analysis on the study population.
FIG. 1.
Abdominal CT (left) with analysis of vascular calcification (right) in the abdominal aorta. An Agatston score is determined by the product of the calcified lesion area (at least two contiguous pixels with a CT density at least 130 HU) and a co-factor dependent on the peak CT density (1, 130–200 HU; 2, 201–300 HU; 3, 301–400 HU; 4, >400 HU) of the lesion.
Laboratory analyses
Fasting serum samples were obtained on all subjects at the time of QCT measurements. Total E2 was measured using a double-antibody RIA (Diagnostic Products, Los Angeles, CA, USA) with an interassay CV of <8% and lower limit of detection of 18 pM (5 pg/ml). Cross-reactivity of this assay was 12% with estrone and 6% or less for other estrogen metabolites. Total testosterone was measured by a modified competitive immunoassay using direct, chemiluminescent technology (ACS 180; Bayer, Tarrytown, NY, USA) with an interassay CV of <15%. The sensitivity of this assay was increased to 0.17 nM (5 ng/dl) using an in-house assay protocol where the volume of standards, controls, and samples was increased to release bound T from endogenous binding proteins. Cross-reactivity of this assay was 5.4% with dihydroxytestosterone and <1% for all other metabolites. The non–sex hormone binding globulin (SHBG)-bound (bioavailable) fractions of E2 and T were measured using a modification of the techniques of O'Conner et al.(65) and Tremblay and Dube,(66) as previously described in our work with this cohort.(67) The interassay CVs for bio E2 and bio T were each <12%.
Serum PTH was measured using an immunochemiluminometric assay for intact PTH (Nichols Institute Diagnostics, San Clemente, CA, USA) with an interassay CV of <13%. Serum 25-hydroxyvitamin D and 1,25-dihydroxy vitamin D were measured by a radioimmunoassay (DiaSorin, Stillwater, MN, USA) with interassay CVs of <15%. Serum IGF-1 and IGF binding protein-3 (IGFBP-3) were measured by immunoradiometric assays (Diagnostic Systems Laboratories, Webster, TX, USA) with interassay CVs of 6% and <14%, respectively. Serum bone-specific alkaline phosphatase (BSALP) was measured by ELISA (Quidel, San Diego, CA, USA) with an interassay CV of <11%, and serum osteocalcin was measured using a two-site immunoradiometric assay (CIS-US, Bedford, MA, USA) with an interassay CV of <6%. Serum amino-terminal propeptide of type I collagen (PINP) was measured by RIA (DiaSorin, Stillwater, MN, USA) with an interassay CV of <9%. Serum C-telopeptide of type I collagen (CTX) was measured by one-step ELISA (Osteometer BioTech, Herlev, Denmark) with an interassay CV of 2.7–3.7% at 0.36–0.52 ng/ml. Urine cross-linked N-telopeptide of type I collagen (NTX) was measured by an automated immunoassay (Ortho-Clinical Diagnostics, Rochester, NY, USA) with an interassay CV of <13% in 24-h urine collections, and the results were expressed per liter of glomerular filtrate (GF; estimate based on serum and urine creatinine).
Statistical analysis
Some analyses were stratified by age (<50 versus 50+ yr) in the men and by menopausal status in the women. Continuous variables were summarized using medians and interquartile ranges, whereas categorical variables were summarized as frequencies and percentages. Respectively, the Wilcoxon rank sum and the χ2 tests were used to perform group comparisons.
Spearman correlations were used to examine relationships between the Agatston Score per CT slice with vBMD, bone structural and microstructural parameters, hormonal variables, and bone turnover markers. Correlations are reported as unadjusted and age adjusted, as well as adjusted for age, BMI, and smoking status (defined as “ever” or “never” smoker).
All analyses were performed using SAS (SAS Institute, Cary, NC, USA).
RESULTS
Baseline characteristics
The baseline characteristics of the younger (<50 yr old) and older (≥50 yr old) men are shown in Table 1. Younger men were taller, heavier, had more calcium intake, and were more physically active than older men. No significant differences were seen in BMI despite the weight difference. Although more older subjects smoked at some time in their lifetime, more younger subjects were active smokers. The prevalence of hypertension, hypercholesterolemia, coronary artery disease, peripheral vascular disease, type 2 diabetes mellitus, and history of osteoporotic fractures (prior hip, vertebral, or distal forearm fracture caused by moderate trauma after age 35 yr) was higher in the men ≥50 yr of age. Also, older men showed lower vBMD at the vertebrae and femoral neck (p < 0.001), lower BV/TV (p < 0.001), higher Tb.N (p = 0.03), and lower Tb.Th (p < 0.001). Tb.N correlated with BMI in younger and older men and remained significant with age adjustment (p = 0.003, p < 0.001) in the two groups.
Table 1.
Clinical Parameters of Rochester, MN Men Stratified by Age
| <50 yr | 50+ yr | p | |
| n | 125 | 196 | |
| Clinical parameters | |||
| Age (yr) | 37 (30.8–43.6) | 69.8 (60.1–79.6) | — |
| Height (cm) | 179.8 (175.7–183.6) | 175 (170–179.5) | <0.001 |
| Weight (kg) | 90.5 (79.2–102) | 85 (77 –95.1) | 0.010 |
| Body mass index (kg/m2) | 27.7 (25–31.3) | 28 (25.8–30.7) | 0.874 |
| Calcium intake (mg/d) | 1144 (799–1494) | 959.7 (732–1308) | 0.023 |
| Physically active, n (%) | 125 (100) | 187 (95) | 0.015 |
| Ever smoker, n (%) | 40 (32) | 108 (55) | <0.001 |
| Current smoking, n (%) | 18 (15) | 14 (7) | 0.032 |
| Former smoking, n (%) | 22 (18) | 94 (48) | <0.001 |
| Medical history | |||
| Hypertension, n (%) | 12 (10) | 93 (47) | <0.001 |
| Hypercholesterolemia, n, (%) | 27 (22) | 100 (51) | <0.001 |
| Coronary artery disease, n (%) | 1 (1) | 62 (32) | <0.001 |
| Peripheral vascular disease, n (%) | 1 (1) | 24 (12) | <0.001 |
| Diabetes mellitus (type 2), n (%) | 0 (0) | 21 (11) | <0.001 |
| Osteoporotic fracture, n (%) | 10 (8) | 58 (30) | <0.001 |
| vBMD parameters | |||
| Vertebral trabecular (mg/cm3) | 179.9 (160.2–200.0) | 136.6 (109.2–163.9) | <0.001 |
| Femoral neck | |||
| Total (mg/cm3) | 337.9 (297.6–363.0) | 272.3 (235.1–316.7) | <0.001 |
| Trabecular (mg/cm3) | 232.6 (208.0–258.5) | 175.7 (149.3–214.1) | <0.001 |
| Cortical (mg/cm3) | 569.7 (526.7–619.0) | 541.6 (490.1–579.7) | <0.001 |
| Microstructural parameters | |||
| Waist | <0.001 | ||
| BV/TV | 0.18 (0.15–0.19) | 0.15 (0.13–0.17) | |
| TbN (1/mm) | 2.70 (2.48–2.91) | 2.79 (2.59–2.94) | 0.032 |
| TbTh (mm) | 0.06 (0.06–0.07) | 0.06 (0.05–0.06) | <0.001 |
| TbSp (mm) | 0.31 (0.28–0.33) | 0.30 (0.29–0.33) | 0.988 |
| Aortic calcification (Agatston score per CT slice) | 0.26 (0.04–1.17) | 38.13 (5.11–119.24) | <0.001 |
| Agatston score > 0, n (%) | 95 (76) | 190 (97) | <0.001 |
Continuous variables are summarized with median (IQR) and a rank sum p value. Categorical variables are summarized with counts (%) and a χ2 p value.
The baseline characteristics of the two groups of women, divided by menopausal status, are shown in Table 2. BMI was statistically different between menopausal status (p = 0.001), although weight and smoking status (ever/never) were not. In addition to lower vBMD at the vertebrae and femoral neck (p < 0.001), postmenopausal women also had lower BV/TV (p < 0.001), Tb.N (p = 0.003), and Tb.Th (p < 0.001), as well as higher Tb.Sp (p = 0.001). Tb.N correlated with BMI in premenopausal and postmenopausal women and remained significant with age adjustment (p < 0.001, p < 0.001) in the two groups.
Table 2.
Clinical Parameters of Rochester, MN, Women Stratified by Menopausal Status
| Premenopausal | Postmenopausal | p | |
| n | 127 | 245 | |
| Clinical parameters | |||
| Age (yr) | 38.5 (31.6–45.2) | 66.9 (58.4–76) | — |
| Height (cm) | 164.8 (160.8–169.2) | 161.7 (157–165.5) | <0.001 |
| Weight (kg) | 67 (59.2–83.4) | 72.9 (63 .1–81.3) | 0.231 |
| Body mass index (kg/m2) | 25 (22.1–29.8) | 27.8 (24.4–31.1) | 0.001 |
| Calcium intake (mg/d) | 1136 (716.5–1500) | 1244.8 (812.1–1660) | 0.045 |
| Physically active, n (%) | 125 (98) | 226 (93) | 0.019 |
| Ever smoker, n (%) | 54 (43) | 105 (43) | 0.925 |
| Current smoking, n (%) | 19 (15) | 20 (8) | 0.044 |
| Former smoking, n (%) | 35 (28) | 85 (35) | 0.155 |
| Current therapy, n (%) | |||
| BCP use | 41 (32) | 0 (0) | |
| ET use | 3 (2) | 81 (33) | |
| SERM use | 0 (0) | 8 (3) | |
| BSP use | 0 (0) | 3 (1) | |
| ET+SERM+BSP use | 0 (0) | 1 (0.4) | |
| Medical history | |||
| Hypertension, n (%) | 11 (9) | 113 (46) | <0.001 |
| Hypercholesterolemia, n (%) | 26 (20) | 126 (51) | <0.001 |
| Coronary artery disease, n (%) | 1 (1) | 44 (18) | <0.001 |
| Peripheral vascular disease, n (%) | 2 (2) | 27 (11) | 0.001 |
| Diabetes mellitus (type 2), n (%) | 1 (1) | 27 (11) | <0.001 |
| Osteoporotic fracture, n (%) | 3 (2) | 68 (28) | <0.001 |
| vBMD parameters | |||
| Vertebral trabecular (mg/cm3) | 189.7 (174.9–210.5) | 132.5 (103.2–167.3) | <0.001 |
| Femoral neck | |||
| Total (mg/cm3) | 389.0 (354.5–439.1) | 304.7 (261.4–342.3) | <0.001 |
| Trabecular (mg/cm3) | 263.0 (227.0–297.8) | 189.5 (149.6–221.9) | <0.001 |
| Cortical (mg/cm3) | 633.8 (584.8–679.8) | 558.6 (497.5–600.2 | <0.001 |
| Microstructural parameters | |||
| Waist | |||
| BV/TV | 0.14 (0.12–0.16) | 0.12 (0.10–0.14) | <0.001 |
| TbN (1/mm) | 2.58 (2.44–2.73) | 2.50 (2.30–2.66) | 0.003 |
| TbTh (mm) | 0.05 (0.05–0.06) | 0.05 (0.04–0.06) | <0.001 |
| TbSp (mm) | 0.34 (0.31–0.36) | 0.35 (0.32–0.39) | 0.001 |
| Aortic calcification (Agatston score per CT slice) | 0 (0–0.28) | 18.41 (2.45–69.77) | <0.001 |
| Agatston score > 0, n (%) | 48 (38) | 217 (89) | <0.001 |
Continuous variables are summarized with median (IQR) and a rank sum p value. Categorical variables are summarized with counts (%) and a χ2 p value.
BCP, birth control pill; ERT, estrogen therapy; SERM, selective estrogen receptor modulator; BSP, bisphosphonate.
Aortic calcification
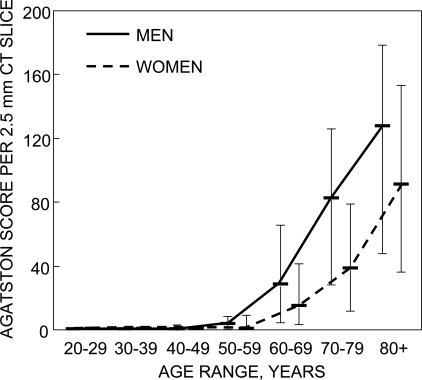
Of the 693 subjects analyzed, 143 (21%) had no evidence of AAC. This group included 36 of 321 males (11%) with a median age of 37 yr (range, 22–66 yr) and 107 of 372 females (29%) with a median age of 39 yr (range, 21–76 yr). The remaining 550 subjects had some degree of AAC. The median AAC score was 246 (range, 0–35,436), and the median AAC score per 2.5-mm CT slice was 4.3 (range, 0–622). As expected, the severity of AAC, as determined by QCT analysis, increased with age (Fig. 2), as did the proportion of individuals with any AAC. Men had more AAC at every age (p < 0.001), and the increase with age was greater in men than women. AAC was present in 95 of 125 of the younger men (76%) and 190 of 196 of the older men (97%). In women, AAC was present in 48 of 127 of premenopausal women (38%) and 217 of 245 of postmenopausal women (89%).
FIG. 2.
Distribution of aortic calcifications (Agatston score) by sex and age among Rochester, MN, residents. Bars represent the 25th and 75th percentiles.
Aortic calcification, vBMD, and bone microstructure
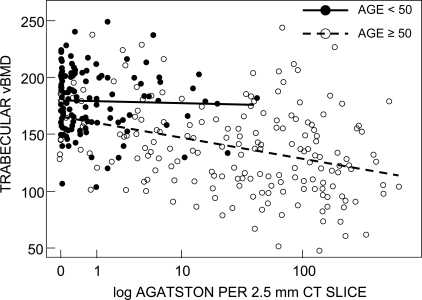
The correlations of vBMD and structural parameters with AAC scores are shown in Tables 3 and 4. Table 3 shows these correlations in men, unadjusted, adjusted for age, and adjusted for age, BMI, and cigarette smoking (ever/never). Higher AAC scores correlated with lower vertebral trabecular and femoral neck total, trabecular, and cortical vBMD (p < 0.001) when combining all men. The association lost its significance on age and multivariable adjustment. Analyzing the age groups separately, this pattern persisted for the older men. A representative scatter plot, with regression lines, highlighting vertebral vBMD is shown in Fig. 3. There were no significant trends noted in younger men other than an isolated association between higher vertebral vBMD and higher AAC, but only after multivariable adjustment (p = 0.04). AAC was also correlated with lower BV/TV (p < 0.001) and Tb.Th (p < 0.001) when combining all men (Table 3). After adjustment for age, Tb.Th remained negatively correlated with AAC (p = 0.03), although this did not persist after multivariable adjustment. In younger men, AAC was correlated with higher Tb.N (p < 0.001), as well as lower Tb.Th (p = 0.001) and Tb.Sp (p = 0.008). These relationships remained significant after age adjustment but not multivariable adjustment. In older men, AAC was associated with lower BV/TV (p = 0.003) and Tb.N (p < 0.001), as well as higher Tb.Sp (p < 0.001). Lower Tb.N and higher Tb.Sp remained significantly correlated with AAC even after multivariable adjustment (p = 0.025 and p = 0.020, respectively).
Table 3.
Unadjusted/Univariate-Adjusted/Multivariable-Adjusted Spearman Correlation Coefficients Between vBMD and Structural Parameters With Aortic Calcification per 2.5-mm CT Slice in Rochester, MN, Men Combined and Stratified by Age
|
All men |
<50 yr |
≥50 yr |
|||||||
| U | A | A/B/C | U | A | A/B/C | U | A | A/B/C | |
| vBMD | |||||||||
| Vertebral trabecular | −0.52* | 0.01 | 0.03 | −0.02 | 0.08 | 0.19† | −0.36* | −0.01 | 0.00 |
| Femoral neck | |||||||||
| Total | −0.41* | 0.06 | 0.06 | −0.01 | 0.09 | 0.07 | −0.26* | −0.01 | 0.01 |
| Trabecular | −0.47* | 0.09 | 0.06 | −0.04 | 0.10 | 0.03 | −0.29* | 0.02 | 0.03 |
| Cortical | −0.24* | −0.02 | 0.02 | −0.00 | 0.04 | 0.16 | −0.18* | −0.10 | −0.08 |
| Microstructure | |||||||||
| BV/TV | −0.37* | −0.10 | −0.08 | −0.15 | −0.07 | −0.03 | −0.22‡ | −0.13 | −0.12 |
| TbN (1/mm) | 0.07 | 0.03 | −0.10 | 0.34* | 0.28‡ | 0.13 | −0.26* | −0.12 | −0.17† |
| TbTh (mm) | −0.42* | −0.13† | −0.05 | −0.30‡ | −0.20† | −0.09 | −0.12 | −0.08 | −0.04 |
| TbSp (mm) | 0.07 | 0.01 | 0.11 | −0.25‡ | −0.22† | −0.09 | 0.29* | 0.15 | 0.18† |
* p < 0.001.
† p < 0.05.
‡ p < 0.01.
U, unadjusted; A, age adjusted; A/B/C, age, BMI, cigarette smoking status (ever) adjusted.
Table 4.
Unadjusted/Univariate-Adjusted/Multivariable-Adjusted Spearman Correlation Coefficients Between vBMD and Structural Parameters with Aortic Calcification per 2.5-mm CT Slice in Rochester, MN Women Combined and Stratified by Menopausal Status
|
All women |
Premenopausal |
Postmenopausal |
|||||||
| U | A | A/B/C | U | A | A/B/C | U | A | A/B/C | |
| vBMD | |||||||||
| Vertebral trabecular | −0.54* | 0.08 | 0.06 | 0.05 | 0.17 | 0.08 | −0.35* | 0.02 | 0.04 |
| Femoral neck | |||||||||
| Total | −0.45* | 0.11† | 0.08 | 0.06 | 0.20† | 0.15 | −0.23* | 0.06 | 0.07 |
| Trabecular | −0.47* | 0.14‡ | 0.08 | 0.06 | 0.28‡ | 0.13 | −0.28* | 0.05 | 0.05 |
| Cortical | −0.35* | 0.05 | 0.08 | −0.05 | −0.01 | 0.09 | −0.12 | 0.06 | 0.09 |
| Microstructure | |||||||||
| BV/TV | −0.12† | 0.10 | 0.03 | 0.23† | 0.29‡ | 0.16 | −0.04 | 0.03 | 0.02 |
| TbN (1/mm) | −0.08 | 0.17† | 0.05 | 0.27‡ | 0.33* | 0.03 | −0.06 | 0.10 | 0.09 |
| TbTh (mm) | −0.12† | 0.04 | 0.03 | 0.18 | 0.23† | 0.21† | −0.02 | −0.01 | −0.01 |
| TbSp (mm) | 0.10 | −0.16† | −0.05 | −0.26‡ | −0.33* | −0.06 | 0.06 | −0.09 | −0.07 |
* p < 0.001.
† p < 0.05.
‡ p < 0.01.
U, unadjusted; A, age adjusted; A/B/C, age, BMI, cigarette smoking status (ever) adjusted.
FIG. 3.
Scatter plot showing relationship of aortic calcifications (Agatston score) and vertebral trabecular vBMD in Rochester, MN, men.
In women (Table 4), AAC also correlated with lower vBMD at all sites measured (p < 0.001). The correlations between total and trabecular BMD in the femoral neck remained significant after age adjustment (p = 0.04 and p = 0.009, respectively), but not after multivariable adjustment. Lower vertebral trabecular, total femoral neck, and femoral neck trabecular vBMD were also correlated with AAC (p < 0.001) in postmenopausal women before age and multivariable adjustment, but not after. In premenopausal subjects, AAC was correlated with higher total (p = 0.03) and trabecular (p = 0.002) vBMD at the femoral neck, but only after age adjustment. Although lower BV/TV and Tb.Th were correlated with AAC (p = 0.03 and p = 0.04, respectively) in all women before adjustment, these statistically significant differences could not be seen in the postmenopausal group. In premenopausal women, AAC was associated with higher BV/TV (p = 0.02) and Tb.N (p = 0.004), as well as lower Tb.Sp (p = 0.006). All three of these associations remained significant after age but not multivariable adjustment.
Aortic calcification, sex steroids, and bone turnover
Hormonal parameters, including E2, T, PTH, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, IGF-1, and IGFBP-3, are reported in 542 subjects who were not on treatment, with results outlined for the 309 men and 233 women in Tables 5 and 6, respectively. Also listed are markers of bone formation (BSALP, osteocalcin, and P1NP), as well as markers of bone resorption (serum CTX and urine NTX). In men spanning all ages, lower IGF-1 and osteocalcin levels were associated with AAC (p < 0.001); these relationships were noted after age, but not multivariable, adjustment. In women, lower 25-hydroxyvitamin D and higher BSALP levels correlated with higher AAC (p = 0.008 and p < 0.001, respectively), and these effects persisted after adjustment.
Table 5.
Unadjusted/Univariate-Adjusted/Multivariable-Adjusted Spearman Correlation Coefficients Between Hormonal Variables, Bone Formation Markers, and Bone Resorption Markers With Aortic Calcification per 2.5-mm CT Slice in Rochester, MN, Men Combined and Stratified by Age
|
All men |
<50 yr |
≥50 yr |
|||||||
| U | A | A/B/C | U | A | A/B/C | U | A | A/B/C | |
| Hormonal variables | |||||||||
| Bio E2 (pg/ml) | −0.41* | 0.02 | −0.00 | −0.11 | −0.03 | −0.12 | −0.32* | −0.02 | −0.00 |
| Bio T (ng/dl) | −0.60* | −0.09 | −0.01 | −0.19† | −0.10 | −0.02 | −0.42* | −0.09 | −0.06 |
| PTH (pM) | 0.19‡ | −0.05 | −0.08 | 0.06 | −0.03 | −0.09 | 0.01 | −0.10 | −0.10 |
| 25-Hydroxyvitamin D (ng/ml) | −0.04 | −0.04 | 0.02 | −0.19† | −0.19† | −0.07 | 0.02 | 0.10 | 0.10 |
| 1,25-Dihydroxyvitamin D (pg/ml) | −0.18‡ | 0.02 | 0.06 | −0.10 | −0.11 | 0.04 | −0.08 | 0.16† | 0.15† |
| IGF-1 (ng/ml) | −0.47* | −0.11† | −0.11 | −0.20† | −0.13 | −0.08 | −0.35* | −0.11 | −0.11 |
| IGFBP-3 (ng/ml) | −0.41* | −0.05 | −0.08 | −0.14 | −0.08 | −0.17 | −0.35* | −0.09 | −0.10 |
| Bone formation markers | |||||||||
| Bone alkaline phosphatase (U/liter) | −0.06 | 0.01 | −0.01 | 0.08 | 0.07 | 0.01 | −0.04 | −0.07 | −0.09 |
| Osteocalcin (ng/ml) | −0.30* | −0.13† | −0.05 | −0.27‡ | −0.22† | −0.11 | −0.08 | −0.14 | −0.13 |
| PINP (μg/liter) | −0.38* | −0.07 | −0.06 | −0.19† | −0.13 | −0.11 | −0.18† | −0.14 | −0.13 |
| Bone resorption markers | |||||||||
| Serum CTX (ng/ml) | −0.27* | −0.04 | −0.00 | −0.17 | −0.12 | −0.02 | −0.08 | −0.07 | −0.07 |
| Urine NTX (nM GF) | −0.07 | −0.11 | −0.09 | −0.12 | −0.10 | −0.05 | −0.02 | −0.20‡ | −0.21‡ |
* p < 0.001.
† p < 0.05.
‡ p < 0.01.
U, unadjusted; A, age adjusted; A/B/C, age BMI, cigarette smoking status (ever) adjusted.
Table 6.
Unadjusted/Univariate-Adjusted/Multivariable-Adjusted Spearman Correlation Coefficients Between Hormonal Variables, Bone Formation Markers, and Bone Resorption Markers With Aortic Calcification per 2.5-mm CT Slice in Rochester, Minnesota, Women Combined and Stratified by Menopausal Status
|
All women |
Premenopausal |
Postmenopausal |
|||||||
| U | A | A/B/C | U | A | A/B/C | U | A | A/B/C | |
| Hormonal variables | |||||||||
| Bio E2 (pg/ml) | −0.49* | 0.13 | 0.08 | 0.23† | 0.22† | 0.12 | −0.17† | 0.06 | 0.09 |
| Bio T (ng/dl) | 0.04 | 0.22* | 0.16† | 0.32‡ | 0.42* | 0.21 | −0.07 | 0.10 | 0.12 |
| PTH (pM) | 0.12 | −0.00 | −0.05 | 0.14 | 0.09 | −0.06 | −0.02 | −0.03 | −0.02 |
| 25-Hydroxyvitamin D (ng/ml) | −0.17‡ | −0.19‡ | −0.16† | −0.19 | −0.21 | −0.17 | −0.14 | −0.17† | −0.18† |
| 1,25-Dihydroxyvitamin D (pg/ml) | −0.20‡ | −0.09 | −0.07 | −0.07 | −0.11 | −0.04 | −0.15 | −0.11 | −0.12 |
| IGF-1 (ng/ml) | −0.42* | −0.09 | −0.07 | −0.30‡ | −0.16 | −0.09 | −0.29* | −0.13 | −0.12 |
| IGFBP-3 (ng/ml) | −0.41† | 0.07 | 0.07 | −0.05 | 0.03 | 0.04 | −0.14 | 0.01 | 0.03 |
| Bone formation markers | |||||||||
| Bone alkaline phosphatase (U/liter) | 0.37* | 0.21‡ | 0.19‡ | 0.31‡ | 0.35‡ | 0.24† | 0.11 | 0.11 | 0.12 |
| Osteocalcin (ng/ml) | 0.07 | −0.03 | 0.02 | −0.24† | −0.12 | −0.08 | −0.02 | −0.00 | 0.02 |
| PINP (μg/liter) | −0.01 | 0.01 | 0.02 | −0.09 | 0.01 | 0.03 | −0.14 | 0.02 | 0.01 |
| Bone resorption markers | |||||||||
| Serum CTX (ng/ml) | 0.13† | −0.03 | 0.00 | −0.14 | −0.05 | −0.04 | −0.05 | −0.01 | −0.01 |
| Urine NTX (nM GF) | 0.20‡ | 0.02 | 0.03 | 0.04 | 0.09 | −0.02 | 0.01 | 0.02 | 0.03 |
* p < 0.001.
† p < 0.05.
‡ p < 0.01.
U, unadjusted; A, age adjusted; A/B/C, age, BMI, cigarette smoking status (ever) adjusted.
DISCUSSION
In a population-based, cross-sectional study of men and women spanning a wide age range, we found an age-dependent association between vBMD and AAC. We also identified an age-dependent association between bone microstructural parameters and AAC in women, although some relationships remained significant in older men even after multivariable adjustment.
Our correlations between BMD and AAC are consistent with previous reports suggesting that the link between atherosclerosis and osteoporosis is age dependent.(3,6,7,36) Others have reported an age-independent link,(20,23,24) although methodologic differences may explain some of the findings. These differences include smaller sample sizes consisting primarily of postmenopausal women,(20) preselection of study participants from a bone trial or bone clinic,(20) and the determination of AAC and bone mass from spine and hand radiographs, respectively.(23,24) This is in contrast with this study, in which we evaluated a larger population of men and women from the community, thus limiting selection bias. Because both osteoporosis and atherosclerosis begin and progress before complications (fractures and cardiovascular events, respectively) become clinically apparent, imaging methods are essential in the early detection of these disease processes. In our study, we not only used QCT technology to detect AAC, but we also quantified the amount of AAC present using the Agatston scoring system. Additionally, we assessed central vBMD by QCT, which has many advantages over DXA, including the ability to adjust for bone size and isolate the trabecular bone compartment. vBMD has been assessed in only a few previous studies, including an analysis of a preselected cohort of postmenopausal women,(28) as well as a population-based study of men and women 68–80 yr of age.(68)
Despite an inverse correlation between vBMD and AAC within each sex overall, we found that the relationship was only significant in the older men and the postmenopausal women. We have previously reported HRpQCT data in this cohort, indicating that different bone microstructural processes occur with aging in men and women.(47) Specifically, young adult men seem to have thicker trabeculae than those present in young women; before age 50, these thick trabeculae seem to be converted to more numerous, thinner trabeculae in men. In this study, lower Tb.Th seemed to correlate with AAC in younger men in an age-independent manner, although the opposite seemed to be true in premenopausal women. Although counterintuitive, higher Tb.N and lower Tb.Sp also seemed to correlate with AAC in younger men and premenopausal women, despite age adjustment. No significant correlations between bone microstructure and AAC were present in postmenopausal women, but lower Tb.N and higher Tb.Sp were associated with AAC in older men, an effect that survived despite multivariable adjustment.
It remains unclear why there seems to be such a sharp contrast between the younger and older age groups, as well as between the sexes. Indeed, many factors have been implicated in the concurrent development of vascular calcification and osteoporosis, including chronic inflammation, estrogen deficiency, hypovitaminosis D, hypovitaminosis K, and oxidative stress.(69) For instance, it has been shown that inflammatory cytokines and lipids seem to promote the differentiation of osteoblasts in the vasculature while inhibiting their differentiation in bone.(16) Conversely, in vitro studies have shown increased activity of osteoclasts through the action of inflammatory lipids,(70) whereas TNF-α has been shown to promote calcification while inhibiting osteoblast differentiation.(71) The osteoprotegerin/RANKL/RANK system has also been implicated.(25,72) We measured several hormones and bone turnover markers, but found no consistent links to AAC across both sexes. An interesting finding was the relationship between serum 1,25-dihydroxyvitamin D (calcitriol) levels with AAC in older men. In vitro studies have shown calcitriol to promote vascular smooth muscle cell calcification through a PTH-related protein pathway,(73,74) and in vivo, medial artery calcification is promoted by calcitriol.(75) Also, we found in older men that lower urine NTX levels are associated with higher AAC. Others have observed that low bone turnover determined from histomorphometry is associated with arterial calcifications in end-stage renal disease.(76) Altogether, these data suggest a need for additional studies to better evaluate risk/benefits of calcitriol in light of the effects it may have on bone and vascular disease.
An important point that continues to be further elucidated is the clinical relevance of the relationship between vascular disease and osteoporosis. Schulz et al.(28) reported a risk of fragility fractures (vertebral and hip) that was significantly greater in postmenopausal women with AAC than in those without AAC. Such an increased risk of fracture was also shown at the hip by Bagger et al.,(35) but not by Samelson et al.(77) in the Framingham Study. However, varying patient selection, technique of AAC determination, and follow-up methods may explain these differences. Low BMD has also been shown to predict all-cause and cardiovascular mortality(78–80) and stroke.(81,82) However, as this study shows, there are differences between sexes. For instance, Varosy et al.(34) from the HERS investigators showed that coronary events decrease in postmenopausal women with known coronary disease after fracture. Also, despite a higher incidence of atherosclerosis and lower incidence of osteoporosis in men compared with women, the risk of death after fracture is actually higher in men.(83)
Another area of uncertainty is the relationship of components of the metabolic syndrome to BMD and microstructure. Hypertriglyceridemia and waist circumference have been identified as indicators of the presence and progression of AAC, as well as cardiovascular mortality in several studies,(84,85) and fasting glucose levels have been found to be related significantly to aortic, but not coronary, calcium scores in postmenopausal women.(86) The relationship of these additional factors to bone structure and microstructure was not examined because of the constraints of our study design. Future studies may explore these metabolic components as potential explanations for the sex- and age-specific relationships between AAC and trabecular structure that we report in this study.
In our cohort, we plan to analyze longitudinal data from QCT and HRpQCT scans at 3 and 6 yr and gather data regarding cardiovascular and fracture endpoints, which should alleviate some of the limitations inherent in this cross-sectional study. Other limitations of this study include the homogenous white population of the Rochester, MN, community. The absence of racial groups with traditionally higher risk for cardiovascular disease will make future studies necessary to determine whether our findings are consistent across all ethnic backgrounds. Also, although AAC is a marker of subclinical atherosclerosis, the pathologic origin and physiologic significance of abdominal AAC may differ from calcification occurring in other anatomic sites such as the coronary arteries, as well as other types of vascular calcification. Finally, we did not examine specific novel markers of cardiovascular risk [homocysteine and lipoprotein (a)], which could be mechanistically linked to changes in vBMD and bone microstructure and AAC. These are areas for future study.
In conclusion, we provided further proof of an age-dependent relationship between vascular calcification and BMD. The finding of a link between abdominal AAC and bone microstructure in older men even after multivariable adjustment is novel and should be further studied to elucidate a possible common pathogenic mechanism between vascular calcification and bone structure.
ACKNOWLEDGMENTS
The authors thank Margaret Holets for making the pQCT measurements, Lisa McDaniel, RN, and Louise McCready, RN, for assistance in recruitment and management of the study subjects, Sara Achenbach, MS, for performing the statistical analyses, and James Peterson for assistance in graphical design. This work was supported by National Institutes of Health Grants AR-027065 and RR-024150.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Marum GJ. Roentgenographic observations in age: Atrophy and osteoporosis of the spine. Radiology. 1946;37:220–226. doi: 10.1148/46.3.220. [DOI] [PubMed] [Google Scholar]
- 2.Elkeles A. A comparative radiological study of calcified atheroma in males and females over 50 years of age. Lancet. 1957;273:714–715. doi: 10.1016/s0140-6736(57)92256-0. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JB, Barnett E, Nordin BE. The Relation between Osteoporosis and Aortic Calcification. Br J Radiol. 1964;37:910–912. doi: 10.1259/0007-1285-37-444-910. [DOI] [PubMed] [Google Scholar]
- 4.Smith RW, Jr, Rizek J. Epidemiologic studies of osteoporosis in Women of Puerto Rico and southeastern Michigan with special reference to age, race, national origin and to other related or associated findings. Clin Orthop Relat Res. 1966;45:31–48. [PubMed] [Google Scholar]
- 5.Reid IR, Evans MC, Ames R, Wattie DJ. The influence of osteophytes and aortic calcification on spinal mineral density in postmenopausal women. J Clin Endocrinol Metab. 1991;72:1372–1374. doi: 10.1210/jcem-72-6-1372. [DOI] [PubMed] [Google Scholar]
- 6.Banks LM, Lees B, MacSweeney JE, Stevenson JC. Effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: Links between osteoporosis and cardiovascular disease. Eur J Clin Invest. 1994;24:813–817. doi: 10.1111/j.1365-2362.1994.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 7.Vogt MT, San Valentin R, Forrest KY, Nevitt MC, Cauley JA. Bone mineral density and aortic calcification: The Study of Osteoporotic Fractures. J Am Geriatr Soc. 1997;45:140–145. doi: 10.1111/j.1532-5415.1997.tb04498.x. [DOI] [PubMed] [Google Scholar]
- 8.Aoyagi K, Ross PD, Orloff J, Davis JW, Katagiri H, Wasnich RD. Low bone density is not associated with aortic calcification. Calcif Tissue Int. 2001;69:20–24. doi: 10.1007/s002230020003. [DOI] [PubMed] [Google Scholar]
- 9.Boukhris R, Becker KL. Calcification of the aorta and osteoporosis. A roentgenographic study. JAMA. 1972;219:1307–1311. [PubMed] [Google Scholar]
- 10.Ouchi Y, Akishita M, de Souza AC, Nakamura T, Orimo H. Age-related loss of bone mass and aortic/aortic valve calcification–reevaluation of recommended dietary allowance of calcium in the elderly. Ann N Y Acad Sci. 1993;676:297–307. doi: 10.1111/j.1749-6632.1993.tb38743.x. [DOI] [PubMed] [Google Scholar]
- 11.Jensen G, Boesen J, Transbol I. Spinal osteoporosis: A local vascular disease. Calcif Tissue Int. 1986;39(Suppl):A62. [Google Scholar]
- 12.Fitzpatrick LA. Gender-related differences in the development of atherosclerosis: Studies at the cellular level. Clin Exp Pharmacol Physiol. 1996;23:267–269. doi: 10.1111/j.1440-1681.1996.tb02609.x. [DOI] [PubMed] [Google Scholar]
- 13.Jie KG, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K status and bone mass in women with and without aortic atherosclerosis: A population-based study. Calcif Tissue Int. 1996;59:352–356. doi: 10.1007/s002239900139. [DOI] [PubMed] [Google Scholar]
- 14.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 15.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 16.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 17.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 18.Uyama O, Yoshimoto Y, Yamamoto Y, Kawai A. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke. 1997;28:1730–1732. doi: 10.1161/01.str.28.9.1730. [DOI] [PubMed] [Google Scholar]
- 19.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 20.Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–213. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- 21.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- 23.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: A population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 24.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: The Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 26.Rajzbaum G, Roger VL, Bezie Y, Chauffert M, Breville P, Roux F, Safar ME, Blacher J. French women, fractures and aortic calcifications. J Intern Med. 2005;257:117–119. doi: 10.1111/j.1365-2796.2004.01430.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanko LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- 28.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 29.Bakhireva LN, Barrett-Connor EL, Laughlin GA, Kritz-Silverstein D. Differences in association of bone mineral density with coronary artery calcification in men and women: The Rancho Bernardo Study. Menopause. 2005;12:691–698. doi: 10.1097/01.gme.0000184422.50696.ef. [DOI] [PubMed] [Google Scholar]
- 30.Marcovitz PA, Tran HH, Franklin BA, O'Neill WW, Yerkey M, Boura J, Kleerekoper M, Dickinson CZ. Usefulness of bone mineral density to predict significant coronary artery disease. Am J Cardiol. 2005;96:1059–1063. doi: 10.1016/j.amjcard.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Ness J, Aronow WS. Comparison of prevalence of atherosclerotic vascular disease in postmenopausal women with osteoporosis or osteopenia versus without osteoporosis or osteopenia. Am J Cardiol. 2006;97:1427–1428. doi: 10.1016/j.amjcard.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Sinnott B, Syed I, Sevrukov A, Barengolts E. Coronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcif Tissue Int. 2006;78:195–202. doi: 10.1007/s00223-005-0244-z. [DOI] [PubMed] [Google Scholar]
- 33.Farhat GN, Newman AB, Sutton-Tyrrell K, Matthews KA, Boudreau R, Schwartz AV, Harris T, Tylavsky F, Visser M, Cauley JA. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int. 2007;18:999–1008. doi: 10.1007/s00198-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 34.Varosy PD, Shlipak MG, Vittinghoff E, Black DM, Herrington D, Hulley SB, Browner WS. Fracture and the risk of coronary events in women with heart disease. Am J Med. 2003;115:196–202. doi: 10.1016/s0002-9343(03)00330-9. [DOI] [PubMed] [Google Scholar]
- 35.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259:598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 36.Frye MA, Melton LJ, III, Bryant SC, Fitzpatrick LA, Wahner HW, Schwartz RS, Riggs BL. Osteoporosis and calcification of the aorta. Bone Miner. 1992;19:185–194. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- 37.Laroche M, Pouilles JM, Ribot C, Bendayan P, Bernard J, Boccalon H, Mazieres B. Comparison of the bone mineral content of the lower limbs in men with ischaemic atherosclerotic disease. Clin Rheumatol. 1994;13:611–614. doi: 10.1007/BF02243003. [DOI] [PubMed] [Google Scholar]
- 38.van der Klift M, Pols HA, Hak AE, Witteman JC, Hofman A, de Laet CE. Bone mineral density and the risk of peripheral arterial disease: The Rotterdam Study. Calcif Tissue Int. 2002;70:443–449. doi: 10.1007/s00223-001-2076-9. [DOI] [PubMed] [Google Scholar]
- 39.Samelson EJ, Kiel DP, Broe KE, Zhang Y, Cupples LA, Hannan MT, Wilson PW, Levy D, Williams SA, Vaccarino V. Metacarpal cortical area and risk of coronary heart disease: The Framingham Study. Am J Epidemiol. 2004;159:589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- 40.Wong SY, Kwok T, Woo J, Lynn H, Griffith JF, Leung J, Tang YY, Leung PC. Bone mineral density and the risk of peripheral arterial disease in men and women: Results from Mr. and Ms Os, Hong Kong. Osteoporos Int. 2005;16:1933–1938. doi: 10.1007/s00198-005-1968-3. [DOI] [PubMed] [Google Scholar]
- 41.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 42.Witteman JC, Kannel WB, Wolf PA, Grobbee DE, Hofman A, D'Agostino RB, Cobb JC. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 43.Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 44.Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985;37:594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich D, van Rietbergen B, Laib A, Ruegsegger P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone. 1999;25:55–60. doi: 10.1016/s8756-3282(99)00098-8. [DOI] [PubMed] [Google Scholar]
- 46.Muller R, Hildebrand T, Ruegsegger P. Non-invasive bone biopsy: A new method to analyse and display the three-dimensional structure of trabecular bone. Phys Med Biol. 1994;39:145–164. doi: 10.1088/0031-9155/39/1/009. [DOI] [PubMed] [Google Scholar]
- 47.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ., III Effects of sex and age on bone microstructure at the ultradistal radius: A population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21:124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 49.Riggs BL, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 50.Melton LJ, III, Riggs BL, Achenbach SJ, Amin S, Camp JJ, Rouleau PA, Robb RA, Oberg AL, Khosla S. Does reduced skeletal loading account for age-related bone loss. J Bone Miner Res. 2006;21:1847–1855. doi: 10.1359/jbmr.060908. [DOI] [PubMed] [Google Scholar]
- 51.Bouxsein ML, Melton LJ, III, Riggs BL, Muller J, Atkinson EJ, Oberg AL, Robb RA, Camp JJ, Rouleau PA, McCollough CH, Khosla S. Age- and sex-specific differences in the factor of risk for vertebral fracture: A population-based study using QCT. J Bone Miner Res. 2006;21:1475–1482. doi: 10.1359/jbmr.060606. [DOI] [PubMed] [Google Scholar]
- 52.Riggs BL, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, Oberg AL, Rouleau PA, McCollough CH, Khosla S, Bouxsein ML. Population-based analysis of the relationship of whole bone strength indices and fall-related loads to age- and sex-specific patterns of hip and wrist fractures. J Bone Miner Res. 2006;21:315–323. doi: 10.1359/JBMR.051022. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. Geneva, Switzerland: World Health Organization; 1998. Report of a WHO Consultation on Obesity. Obesity: Preventing and Managing the Global Epidemic. [PubMed] [Google Scholar]
- 54.Camp JJ, Karwoski RA, Stacy MC, Atkinson EJ, Khosla S, Melton LJ, III, Riggs BL, Robb RA. A system for the analysis of whole-bone strength from helical CT images. Proc SPIE. 2004;5369:74–88. [Google Scholar]
- 55.Cann CE. Quantitative CT for determination of bone mineral density: A review. Radiology. 1988;166:509–522. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 56.Kalender WA, Felsenberg D, Genant HK, Fischer M, Dequeker J, Reeve J. The European Spine Phantom–a tool for standardization and quality control in spinal bone mineral measurements by DXA and QCT. Eur J Radiol. 1995;20:83–92. doi: 10.1016/0720-048x(95)00631-y. [DOI] [PubMed] [Google Scholar]
- 57.Muller R, Hildebrand T, Hauselmann HJ, Ruegsegger P. In vivo reproducibility of three-dimensional structural properties of noninvasive bone biopsies using 3D-pQCT. J Bone Miner Res. 1996;11:1745–1750. doi: 10.1002/jbmr.5650111118. [DOI] [PubMed] [Google Scholar]
- 58.Laib A, Hildebrand T, Hauselmann HJ, Ruegsegger P. Ridge number density: A new parameter for in vivo bone structure analysis. Bone. 1997;21:541–546. doi: 10.1016/s8756-3282(97)00205-6. [DOI] [PubMed] [Google Scholar]
- 59.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6:329–337. [PubMed] [Google Scholar]
- 60.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24:35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 61.Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 63.Goldin JG, Yoon HC, Greaser LE, III, Heinze SB, McNitt-Gray MM, Brown MS, Sayre JW, Emerick AM, Aberle DR. Spiral versus electron-beam CT for coronary artery calcium scoring. Radiology. 2001;221:213–221. doi: 10.1148/radiol.2211001038. [DOI] [PubMed] [Google Scholar]
- 64.Kaufmann RB, Sheedy PF, II, Breen JF, Kelzenberg JR, Kruger BL, Schwartz RS, Moll PP. Detection of heart calcification with electron beam CT: Interobserver and intraobserver reliability for scoring quantification. Radiology. 1994;190:347–352. doi: 10.1148/radiology.190.2.8284380. [DOI] [PubMed] [Google Scholar]
- 65.O'Connor S, Baker HW, Dulmanis A, Hudson B. The measurement of sex steroid binding globulin by differential ammonium sulphate precipitation. J Steroid Biochem. 1973;4:331–339. doi: 10.1016/0022-4731(73)90002-2. [DOI] [PubMed] [Google Scholar]
- 66.Tremblay RR, Dube JY. Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives. Contraception. 1974;10:599–605. doi: 10.1016/0010-7824(74)90099-7. [DOI] [PubMed] [Google Scholar]
- 67.Khosla S, Melton LJ, III, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 68.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: The Study of Women's Health Across the Nation. J Bone Miner Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 69.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis–from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 70.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 71.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 72.Schoppet M, Preissner KT, Hofbauer LC. RANK ligand and osteoprotegerin: Paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22:549–553. doi: 10.1161/01.atv.0000012303.37971.da. [DOI] [PubMed] [Google Scholar]
- 73.Jono S, Nishizawa Y, Shioi A, Morii H. Parathyroid hormone-related peptide as a local regulator of vascular calcification. Its inhibitory action on in vitro calcification by bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:1135–1142. doi: 10.1161/01.atv.17.6.1135. [DOI] [PubMed] [Google Scholar]
- 74.Jono S, Nishizawa Y, Shioi A, Morii H. 1,25-Dihydroxy vitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation. 1998;98:1302–1306. doi: 10.1161/01.cir.98.13.1302. [DOI] [PubMed] [Google Scholar]
- 75.Henley C, Colloton M, Cattley RC, Shatzen E, Towler DA, Lacey D, Martin D. 1,25-Dihydroxyvitamin D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol Dial Transplant. 2005;20:1370–1377. doi: 10.1093/ndt/gfh834. [DOI] [PubMed] [Google Scholar]
- 76.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–1951. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 77.Samelson EJ, Cupples LA, Broe KE, Hannan MT, O'Donnell CJ, Kiel DP. Vascular calcification in middle-age and long term risk of hip fracture: The Framingham Study. J Bone Miner Res. 2007;22:1449–1454. doi: 10.1359/jbmr.070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trivedi DP, Khaw KT. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos Int. 2001;12:259–265. doi: 10.1007/s001980170114. [DOI] [PubMed] [Google Scholar]
- 79.von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 80.Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR. Rate of bone loss is associated with mortality in older women: A prospective study. J Bone Miner Res. 2000;15:1974–1980. doi: 10.1359/jbmr.2000.15.10.1974. [DOI] [PubMed] [Google Scholar]
- 81.Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR. Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke. 1993;24:940–946. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- 82.Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991;338:355–358. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 83.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: An observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 84.Tanko LB, Bagger YZ, Qin G, Alexandersen P, Larsen PJ, Christiansen C. Enlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal women. Circulation. 2005;111:1883–1890. doi: 10.1161/01.CIR.0000161801.65408.8D. [DOI] [PubMed] [Google Scholar]
- 85.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 86.Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: The healthy women study. Arterioscler Thromb Vasc Biol. 1999;19:2189–2198. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]