Abstract
The methods of the Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III) are described in detail. PIOPED III is a multicenter collaborative investigation sponsored by the National Heart, Lung and Blood Institute. The purpose is to determine the accuracy of gadolinium-enhanced magnetic resonance angiography (Gd-MRA) in combination with venous phase magnetic resonance venography (Gd-MRV) for the diagnosis for acute pulmonary embolism (PE). A composite reference standard based on usual diagnostic methods for pulmonary embolism is used. All images will be read by two blinded and study-certified central readers. Patients with no PE according to the composite reference test will be randomized to undergo Gd-MRA/MRV. This will reduce the proportion of patients with negative tests at no loss in evaluation of sensitivity and specificity.
The purpose of the Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III) is to estimate the diagnostic accuracy of gadolinium-enhanced magnetic resonance pulmonary angiography (Gd-MRA) and venous phase venography (Gd-MRV) for the diagnosis of acute pulmonary embolism (PE). If shown to be a valid test, it may eliminate the need for iodinated contrast material in patients with a relative contraindication, and it would eliminate exposure to ionizing radiation. Magnetic resonance angiography is technically demanding and may be more prone to artifacts than is CT angiography. The Evidence Report/Technology Assessment of the Agency for Healthcare Research and Quality has identified a need to study the feasibility of Gd-MRA in PE patients with tachypnea and tachycardia (1).
The diagnostic accuracy of Gd-MRA alone or the combination of Gd-MRA/MRV will be expressed as the sensitivity, specificity, likelihood ratio for a positive test and likelihood ratio for a negative test. The sensitivity and specificity of Gd-MRA/MRV also will be evaluated in combination with prior clinical assessment by the Wells criteria (2). Prior investigations of Gd-MRA showed a sensitivity that ranged from 77% to 100% in studies of 8 to 35 patients with PE and specificity ranged from 95% to 98% among 22 to 85 patients in whom PE was excluded (3-5) (Table 1). More recently one study reported estimated sensitivities that differed considerably between reader 1 and reader 2 (6). Sensitivities were 31% with reader 1 and 71% with reader 2 were 85% and 92% (6).
Table 1.
Sensitivity and Specificity of Gd-MRA for PE
Research Plan
PIOPED III is a multicenter prospective study of consecutive patients incorporating standardized inclusion/exclusion criteria, ascertainment of patient characteristics and outcomes, uniform diagnostic criteria, and unbiased central interpretation of imaging studies. The study will include a broad spectrum of patients with and without PE and a variety of patients with comorbid conditions that are commonly associated with PE. All patients will undergo a reference test and a pretest objective clinical assessment based on the Wells criteria (7). All patients with PE and a random sample of patients without PE will undergo Gd-MRA/MRV. Expert readers will interpret the results of Gd-MRA/MRV and the results of the reference tests independently without knowledge of the results of clinical or ancillary tests.
Included Patients
Patients will be 18 years of age or older in whom acute PE is of diagnostic concern. An attempt will be made to recruit all patients with suspected PE. Patients with suspected acute PE will be identified and recruited in the emergency department, outpatient clinic, consultation service, hospital beds, or radiology department. As soon as an eligible patient with suspected PE is identified, the patient will be recruited and written informed consent will be obtained (Figure 1). The patient will undergo any diagnostic tests for PE that are required according to the judgment of the attending physician.
Figure 1.
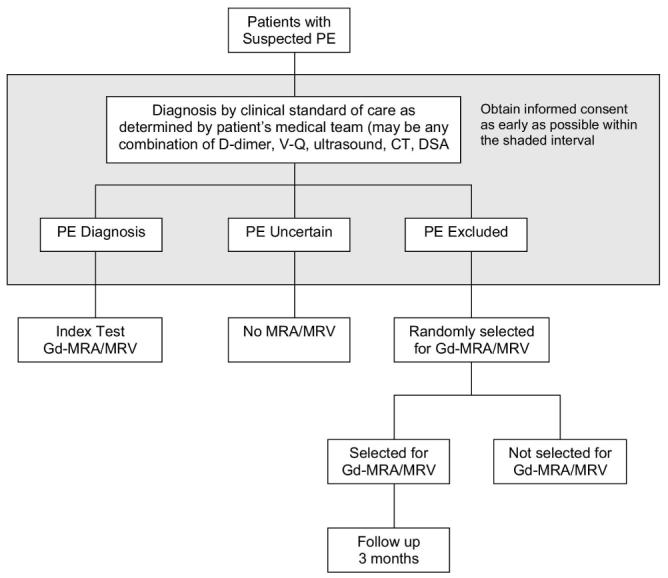
Excluded Patients
Contraindications for Gd-MRA/MRV include any implanted ferromagnetic foreign body (8). Contraindications also include dependency on a continuous connection to an external electrical device or pump. Additional contraindications include severe claustrophobia, severe shaking, an inability to lie still for 30 min, and body size too large for scanner. Pregnant women and nursing mothers will be excluded because it is not known if there may be adverse effects of gadolinium-based contrast material on the fetus or to what extent such contrast material is excreted in human milk (gadopentetate dimeglumine package insert). Additional exclusion criteria are shown in Table 2.
Table 2.
Exclusion Criteria
| • Critically ill |
| • Currently suffer from shock or hypotension (systolic pressure < 80 mm Hg) or hemodynamically unstable |
| • On ventilatory support |
| • Documented episodes of ventricular fibrillation or sustained ventricular tachycardia within the past 24 hours |
| • Myocardial infarction within the past month |
| • Sickle cell disease, other hemoglobinopathies, and other hemolytic anemias |
| • Estimated creatinine clearance < 60 mL/min/1.73 m2 |
| • Acute renal insufficiency or likely causes or findings with acute renal insufficiency including: |
| • Severely dehydrated |
| • Severe hemorrhage |
| • Oliguria |
| • Anuria |
| • Severe trauma, within 7 days |
| • Renal transplant, ever |
| • Liver transplant, ever |
| • Rhabdomyolysis |
| • End-stage malignancy |
| • Third degree burns, within 7 days |
| • Poisoned (methanol, ethylene glycol), within 7 days |
| • Increase in creatinine of >1.5 times initial level during hospitalization or in past 2 weeks |
| • On renal dialysis |
| • Gd-enhanced MRA within the previous 3 months |
| • Gd-enhanced MRA is planned or likely within the next 3 months. |
| • History of allergy to gadolinium-containing contrast agents or to iodinated contrast media (because patients allergic to iodinated contrast material are sometimes allergic to gadolinium containing contrast media) |
| • Currently symptomatic asthma |
| • Pregnant |
| • Nursing mother |
| • Previously enrolled in PIOPED III |
| • Institutionalized or mentally handicapped |
| • Prisoners |
| • Unable to personally give informed consent |
There is concern about nephrogenic systemic fibrosis/ nephrogenic fibrosing dermopathy (NSF/NFD), which occurs rarely in patients with poor renal function who receive gadolinium-containing contrast material (9,10). Recommended exclusions, based on levels of serum creatinine or estimated glomerular filtration rate (GFR), underwent several iterations following the US Food and Drug Administration (FDA) alert in June, 2006 indicating that NSF/NFD may occur following exposure to a gadolinium-containing contrast agent (11). As more information about the risks of NSF/NFD was obtained, the protocol of PIOPED III was modified accordingly. The June 2006 FDA alert reported 25 cases of NSF/NFD in patients who received the gadodiamide (OmniscanR) for MRA (11). All had advanced renal failure requiring dialysis or with a glomerular filtration rate < 15 ml/min/1.73 m2 (11). On December 22, 2006, the FDA issued a Public Health Advisory indicating that they received reports of 90 patients with NSF/NFD (12). According to the International Center for Nephrogenic Fibrosing Dermopathy Research Registry, “over 215 cases have been identified in the Registry so far” (13). In December 2006, the FDA indicated that NSF/NFD has occurred in patients with moderate renal disease (GFR 30 - 59 ml/min/1.73 m2 BSA) as well as severe (GFR 15-29 mL/min/1.73 m2) and end-stage renal disease following exposure to Gd-containing contrast agents (14). Two patients, in addition, had a mild decrease in renal function (GFR 30-59 mL/min/1.73 m2), but both also had acute renal failure (15). The literature described 1 or 2 cases in other patients with a GFR>60 mL/min/1.73m2, but these cases have been refuted due to improper calculation of the GFR in patients experiencing acute kidney injury (16).
Since Gd-containing contrast agents were introduced in the 1980′s, 200 million patients have received it (17). Among 100 cases reviewed in detail, 95% received a gadolinium chelate within 2 - 3 months of exposure (18). Gadopentetate dimeglumine (MagnevistR), gadodiamide (OmniscanR), and gadoversetamide (OptimarkR) had been used in some of these patients (19). There is a potential for NSF/NFD to occur with any of the 5 approved Gd-containing contrast agents (12), especially at high doses (20,21). Differences in the structure of gadolinium complexes may affect their propensity to trigger NSF/NFD (19). No cases have been reported with gadobenate dimeglumine (MultiHanceR) alone (22). One case was reported in a patient who received OmniscanR a few days prior to MultiHanceR (22).
Prior to May 2006, PIOPED III excluded patients with serum creatinine levels > 2.5 mg/dl. On May 23, 2006, the protocol was revised to exclude patients with an abnormal serum creatinine (>1.5 mg/dL for men and >1.4 mg/dL for women). In July, 2006, the protocol was revised again to exclude patients on renal dialysis or with an estimated glomerular filtration rate (GFR) < 95 ml/min for men or < 75 ml/min for women based on the Cockcroft-Gault equations (23) as follows:
Lean body weight:
On September 29, 2006 the protocol was changed to exclude patients with an abnormal serum creatinine based on local institutional values. On January 25, 2007, based on new information, exclusions were based on an abnormal estimated GFR (≤90 mL/min/1.73 m2) as measured by the Modification of Diet in Renal Disease (24). Finally, on May 1, 2007 the protocol was modified to require a GFR ≥ 60 mL/min/1.73 m2 based on this equation. MultiHanceR became the only contrast agent allowed. The recommended dose, 0.1 mmol/kg, could not be exceeded. Patients who had a prior administration of a Gd-contrast agent within 3 months or in whom a repeat MRA was expected in the next 3 months were excluded. The estimated GFR will be calculated no more than 24-hours prior to the MRA. All patients who undergo MRA will receive a follow-up call at 3-months. Those with an estimated GFR < 90 mL/min/1.73 m2 will be contacted at least twice at 3-month intervals after enrollment.
The Modification of Diet in Renal Disease equation (24) is calculated as follows:
Until the end of March 2007, the MDRD.com website used 175 as the factor in the equation to calculate estimated GFR. The appropriate equation for computing estimated GFR depends upon the creatinine method. On approximately March 27, 2007, the protocol required that the user identify the creatinine method used in the local laboratory. Using a method that is isotope dilution mass spectrometry traceable (the gold standard) the factor is 175 (25). For the method that is not isotope dilution mass spectrometry traceable (the standard method), the factor in the Modification of Diet in Renal Disease Equation is 186 (25).
Diagnostic Reference Standard for PE
The diagnosis of PE will be based on any one of the following:
Positive contrast enhanced spiral CT angiogram showing PE in a main or lobar pulmonary artery irrespective of clinical assessment.
Positive contrast enhanced spiral CT angiogram or venous phase imaging of the lower extremities (CT venogram) in a patient with a high or intermediate clinical probability by the Wells criteria. If the CT venogram is positive, it will be a surrogate for the diagnosis of PE.
High probability ventilation-perfusion (V-Q) lung scan in a patient with no prior PE and high or intermediate probability clinical assessment by the Wells criteria.
Positive venous ultrasound in a patient with no prior deep venous thrombosis (DVT) at the same site in a patient with a nondiagnostic V-Q scan and high or intermediate clinical probability by Wells criteria. This is a surrogate for the diagnosis of PE.
Positive conventional digital subtraction pulmonary angiogram (DSA).
The diagnosis of PE will be excluded on the basis of on any one of the following:
Normal D-dimer by quantitative rapid ELISA (D-dimer < 500 ng/mL), in a patient with a low or intermediate probability objective clinical assessment based on the Wells criteria. If whole blood or latex D-dimer is used, clinical assessment must be low probability.
Negative contrast enhanced spiral CT angiogram in a patient with a low probability clinical assessment by the Wells criteria.
Negative CT angiogram and negative venous phase CT venogram in a patient with a low or intermediate clinical probability by the Wells criteria. After February 20, 2008, the protocol was revised to permit a negative ultrasound of the legs if CT venography is not obtained.
Normal V-Q lung scan.
Low probability V-Q lung scan, low clinical probability by Wells criteria, and negative venous ultrasound. Some of these patients had a very low probability V-Q scan interpretation (26,27).
Negative conventional DSA.
The study excludes patients from further investigation if a definitive diagnosis or exclusion of PE cannot be achieved. All patients undergoing CT angiography will also undergo venous phase CT venography in combination. During the course of PIOPED III, data analyzed from PIOPED II showed that venous phase CT venography and venous ultrasound were diagnostically equivalent (28). Therefore, after nearly 2 years of recruitment, some centers substituted venous ultrasound for CT venography. Untreated patients in whom PE was excluded by the composite reference test will be followed 3 months. Deaths, new studies for PE, and new studies for DVT will be reviewed by an Outcome Committee. A positive clinical outcome may reverse a negative reference test.
The composite criteria for the diagnosis or exclusion of PE eliminates the ethical problem of asking patients to volunteer for a conventional DSA, which carries some risk, although the risk is small (29).
Random Sampling to Reduce Number of Negative Tests
A random sample of patients in whom PE is excluded by the composite reference test will undergo Gd-MRA/MRV. Random sampling of PE negative patients for Gd-MRA/MRV is a design feature that makes the investigation feasible and ethical and provides valid, informative and relevant answers with optimal precision and optimal utilization of resources. If all patients with PE excluded by the composite reference test were required to undergo Gd-MRA/MRV, many more patients without PE than with PE would be evaluated, because the prevalence of PE in the previous PIOPED studies was 23% and 28% (30,31). This would give an unnecessarily narrow confidence interval for specificity at great financial cost, diminished feasibility, and burden on a large number of patients. It would cause overloading of heavily scheduled magnetic resonance imaging systems and perhaps raise a question on the ethics of investigating so many normal subjects.
The Data and Coordinating Center will manage the computer-based random sampling system to achieve approximately equal numbers of patients positive and negative by the reference standard. Characteristics of PE negative patients by the composite reference test who were selected by random sampling for Gd-MRA/MRV will be compared to PE negative patients who were not elected for Gd-MRA/MRV. The proportion of patients randomized for Gd-MRA/MRV may change during the period of recruitment, depending on the prevalence of PE at each clinical center. The goal is to achieve an approximately equal number of subjects with and without PE.
Time of Recruitment
Informed consent may be obtained any time after the patient with suspected PE is identified and before Gd-MRA is obtained (or before randomization for Gd-MRA if PE is excluded by the reference test.
Central Readings of Imaging Studies
Investigators from each clinical center may serve as central readers of images from other centers. For each patient undergoing Gd-MRA/MRV, two study-certified central readers will interpret the V-Q scan, CT angiogram / CT venogram and DSA used for the definitive reference test. Central readers also will interpret Gd-MRA/MRVs and assess quality. Central readers will not re-interpret venous compression ultrasounds. Central readers will not have clinical information, nor will they have the results of other imaging studies except chest roentgenograms for V-Q readers. All reference tests will be obtained prior to Gd-MRA/MRV. The reference tests of PE-negative patients not randomized for Gd-MRA/MRV will not be read centrally because such patients will be excluded from analysis of the data.
Contrast enhanced CT angiograms, pulmonary DSA, and Gd-MRA will require adjudication if there is disagreement on which lobes contain acute PE. Both CT venograms and MRVs will require adjudication if there is disagreement on which vein shows acute DVT. Adjudication for V-Q scans will be required if there is disagreement on the diagnostic probability (high probability, intermediate probability, low probability or normal). Adjudicated readings will be performed blindly by a third reader without knowledge of the other two readings.
Central Reader Training, Certification and Variability
Each central reader will be certified as having participated in training prior to reading any images for the study. Reader variability will be compared in central readers on the basis of detailed per-vessel readings. Differences between central and local readers on whether PE is present or absent also will be determined.
Differences Between Local and Central Readers
Potential differences between local and central interpretations of diagnostic images will not result in many lost cases. If the local reading is positive and the central reading is negative, the patient would receive a Gd-MRA/MRV and all necessary data would be obtained. Final interpretation would be based on the central reading (except venous ultrasound). If the local reading is negative and the central reading is positive and if the patient is randomized for a Gd-MRA, all of the necessary data would be obtained. Only if the patient is randomized for no Gd-MRA/MRV, would the case be lost. Loss of a case would, therefore, require the combination of a misread local reading as negative plus randomization to no Gd-MRA/MRV.
Standard for Reporting Diagnostic Accuracy
The design of PIOPED III conforms to the design requirement of studies of diagnostic accuracy (32-34), and to the Standards for Reporting of Diagnostic Accuracy (STARD) statements (32,33). An important design requirement for a study of diagnostic accuracy is that the study should include a wide spectrum of patients having the condition to be diagnosed (32,33).
Time Intervals for Imaging
Gadolinium enhanced Gd-MRA will be obtained within 72 hours of the definitive imaging study.
GADOLINIUM ENHANCED MRA/MRV
Gd-MRA for PE: Methods
Gadolinium enhanced MRA/MRV will be performed on commercially available 1.5T systems with fast gradient-echo capability (30-40 mT/m max gradient field strength) and slew rate 130-200 mT/msec (Table 3). At one center (University of Michigan) a 3.0 T unit (max gradient strength of 40 mT/m and 200 mT/msec slew rate) will be used in some patients. The protocol is otherwise analogous to that designed for the 1.5 T scanner.
Table 3.
MRI Equipment and Characteristics
| Site | MRI | Field Strength (T) |
Max Gradient Field Strength (mT/m) |
Slew Rate mT/msec |
|---|---|---|---|---|
| Calgary | Siemens Sonata | 1.5 | 30 | 200 |
| Emory | GE Signa Horizon LX High Speed |
1.5 | 40 | 150 |
| MGH | Siemens Sonata | 1.5 | 30 | 200 |
| Michigan | GE Signa Horizon LX Echo Speed Philips Achieva |
1.5 3.0 |
30 40 |
150 200 |
| NYU | Siemens Sonata | 1.5 | 30 | 200 |
| St. Joseph | Toshiba XGV Vantage |
1.5 | 30 | 130 |
| Washington | Siemens Sonata | 1.5 | 30 | 200 |
At all centers, parallel imaging will be used. Thus, a specialized multichannel (6-12 channel) phased array coil will be used for reception of the pulmonary MRA data. The body coil will be used for signal transmission.
MR Imaging protocol
All scout imaging of both the chest and thighs will be obtained at the beginning of the examination with the table prescribed to move to the thigh station automatically. A torso multichannel phased array coil will be used for the pulmonary Gd-MRA. Magnetic resonance venography of the thigh will be performed with the body coil. A single bolus of gadolinium-containing contrast material timed to the main pulmonary artery will be used for all imaging, with multiple measures (data acquisitions) obtained immediately and consecutively in the thighs to avoid missing the maximal venous opacification.
Patient Preparation
A 20-gauge catheter will be placed in an antecubital vein and connected to a power injector by an intravenous tubing extension. The patient will be positioned supine within the bore of the magnet. A torso/body multichannel phased array coil will be placed around the chest as well as a respiratory bellow sensor, if the MR system requires it. The patient will receive 2L/min of oxygen through a nasal cannula throughout the study.
Scout Imaging
Sagittal and transverse locators will be performed at the thorax, centered at the midline in the left-to-right direction and at the nipple line in the superior-to-inferior direction. A second coronal locator will be acquired mid thighs for graphical thigh MRV prescription.
Gd-MRA of Pulmonary Arteries
Scan parameters
Pulmonary Gd-MRA imaging will be performed using a 3D gradient recalled echo (GRE) sequence in the coronal plane using the following parameters: “MinTR” (TR≤ 6.6ms), and “MinTE” (≤2.3ms), flip angle=20°-35°, T1-weighting approximately 384 by 288 matrix, 40 cm field of view, bandwidth 380-1500 Hz/pixel, single acquisition/number of excitations (NEX), 3 mm slice thickness (interpolated to 1.5) and at least 44 (88 interpolated) slices or sufficient to cover the anatomy. In large or obese patients, phase wrap artifact can be a significant problem with parallel imaging. For studies in which phase wrap is likely to occur, parallel imaging will not be used. With obese patients the breath-hold length (no more than 22 sec) and field of view will be kept constant (40 cm) and the matrix will be reduced, thus decreasing the in-plane resolution in the phase-encoding direction.
Scan location
The coronal volume will be prescribed from the midline sagittal or transverse locator slice. The posterior aspect of the imaging volume will be placed in the thorax at or near the posterior border of the vertebral body at the level of the heart. The position of the imaging volume will be set to ensure that the descending pulmonary arteries, segmental and proximal subsegmental branches are included in the imaging region.
Scan timing
Imaging time (breath hold) will be approximately 14-22 seconds. The MR technologists will record breath hold duration relative to the scan duration (breath-hold duration 0-25% to100% of scan). The scan delay will be determined using 1-2 mL of contrast agent as a test bolus during a gradient recalled echo sequence performed at 1 image/sec of one slice positioned sagittally through the main pulmonary artery. The scan delay will be calculated to place the peak of the infusion enhancement at the center of “k-space” of the pulmonary MRA since the reconstructed image contrast depicts the imaged object contrast at the time the center of k-space was acquired.
Contrast injection
Immediately before the administration of gadolinium-containing contrast material, the patient will hyperventilate for 30 seconds, and then suspend breathing in full inspiration during the pulmonary Gd-MRA scan (<22 sec). Prior to recognition of the dangers of NSF/NFD, pulmonary Gd-MRA was performed during an intravenous infusion of 0.2 mmol/Kg (approximately 40 mL) of conventional gadolinium-based MR contrast agent (gadodiamide or gadopentetate dimeglumine). In response to the increased awareness of the risk of NSF/NFD, the protocol was changed to require MultiHanceR and the maximum dose was 0.1mmol/kg body weight (approximately 20 mL). The contrast agent is injected by a commercially available magnetic resonance-compatible power injector at an infusion rate of 2 mL/sec followed by an injection of 15 mL of normal saline.
Gd-MRA: Diagnostic Criteria for PE
The diagnostic criteria for acute PE are:
A partially occlusive intraluminal filling. This may be shown as “railway tracking”, i.e. a small amount of contrast material between the central filling defect and the arterial wall or, in cross sectional images, as a filling defect surrounded by contrast material
Complete arterial occlusion with termination of the column of contrast material in a meniscus that outlines the trailing edge of the embolus (35).
Examination time
Total examination time for Gd-MRA of the pulmonary arteries will be 20 minutes. This includes scout scanning for position of the images and the test bolus.
State-of-the-Art Technology
With newer 1.5 T scanners that allow whole body multichannel potential (for instance the Siemens Avanto), the same sequences planned can be employed, with the added advantage that whole body, multichannel, phased array imaging is present. This permits an improved signal-to-noise ratio in the legs.
MR Perfusion Imaging
Some have used Gd-MRA to show pulmonary perfusion based on filling of small vessels (36). This is analogous to the perfusion phase of a pulmonary angiogram, which has been shown to be useful, but nonspecific (37). It is also analogous to a perfusion lung scan. Most perfusion studies have been physiological investigations (38-40). Those that have focused on detection of perfusion defects from PE have been preliminary and showed inconclusive results (36,41,42). Newer time-resolved three-dimensional contrast enhanced MR angiography sequences, such as Time-Resolved Imaging of Contrast Kinetics (TRICKS), that can provide both pulmonary angiography and perfusion in a single breath-hold sequence, have been developed (43,44). Although these methods are now commercially available on most systems, they were not widely available at the start of PIOPED III. In addition, they typically provide lower spatial resolution MRA images in comparison to non-time resolved methods. These methods, therefore, will not be used in PIOPED III and perfusion will not be evaluated.
Delayed-enhanced MRV of the veins of the thighs
Upon completion of the pulmonary MRA, MR venography of the thighs will be performed with the table prescribed to immediately move to the region of scout of the thighs performed at the beginning of the examination. The same imaging sequence performed for pulmonary Gd-MR angiography will be performed in the thighs with minor modifications of the parameters.
Scan parameters
Delayed MR venography will be performed using a transmit and receive body coil and 3D gradient-recalled echo sequence. Dynamic thigh Gd-MRV imaging will be performed in the coronal plane using “MinTR” (TR≤ 6.6 ms), and “MinTE” (≤2.3 ms), flip angle= 20°-35°, 384 by 288 matrix, 40 cm field of view, bandwidth 380-1500 Hz/pixel, 3 - 4 mm slice thickness (interpolated to 1.5 - 2.0 mm) and 44 slices (88 interpolated slices). Parallel imaging will not be used, as breath holding is not an issue. Six measurements will be performed in rapid succession prior to image construction in order to ensure that the bolus is not missed. Each measurement will take approximately 30 seconds.
Scan location
Imaging will be prescribed in the coronal plane to cover the thighs from the acetabulum to the tibial plateaus. The posterior aspect of the imaging volume will be set at the sacrum and the anterior aspect set anterior to the pubic symphysis to encompass femoral to popliteal veins. Veins of the calf will not be studied.
Scan timing
Scanning will begin immediately upon completion of the pulmonary MRA station. Six measurements over approximately 3 minutes will be performed to ensure that the bolus will not be missed.
Contrast Injection
No additional gadolinium-based contrast material is necessary for the Gd-MRV following MR angiography. The protocol does not acquire baseline (i.e. precontrast) images for subsequent subtraction from Gd-MRA/MRV images. Coronal source images of the pulmonary Gd-MRA will be submitted for interpretation. Transverse images of the thigh MRV data set obtained at greatest venous opacification will be constructed at 5 mm intervals contiguously and submitted for interpretation. A single projection image created by subtracting the first (arterial phase) of thigh imaging from the most opacified venous phase images will also be created to be used at the discretion of the central MR reader.
Gd-MRV: Diagnostic Criteria for Acute DVT
The diagnostic criteria for acute DVT on Gd-MRV are:
Occlusive = complete filling defect, i.e., failure to opacify the entire lumen due to a central filling defect (the vessel may enlarge compared to the opposite vein);
Nonocclusive = partial filling defect surrounded by opacification.
Comprehensive Examination
The use of MRV of the veins of the lower extremities in combination with Gd-MRA of the pulmonary arteries creates a comprehensive study for thromboembolism comparable to the combination of contrast enhanced pulmonary spiral CT of the pulmonary arteries in combination with venous phase CT of the veins of the lower extremities (CTV) (30,45,46). The pelvic veins will not be investigated in order to decrease the duration of the examination. In PIOPED II it was shown that most patients with pelvic vein DVT also had DVT of the veins of the thighs (30).
MRI Workstations
Workstations with a minimum resolution of 1 k pixel × 1 k pixel will be used for readings of all Gd-MRA and Gd-MRV images. All source images will be reviewed systematically using dynamic paging at appropriate gray-scale windows, followed by review of thin slab reformations at least in the coronal and sagittal planes for the pulmonary arteries and coronal and transverse planes for the veins of the lower extremities. Source images will be loaded into 3D reformat software routines for interactive display of full volume maximum intensity projection and limited volume maximum intensity views. Reformations including maximum intensity projection images will be made at the discretion of the reader. Principal central reader interpretation will be made from the original, unsubtracted source images. Multiple window/level settings, including ‘custom’ settings will be used (47). In selected cases the readers may reformat images parallel or perpendicular to the long axis of the vessel, using sliding thin slab reformations. Since most of the pulmonary arteries have an oblique course (particularly the middle lobe and lingular arteries), this can help eliminate the possibility of volume averaging (48). Although the range of tissue signal intensity that must be accommodated in Gd-MRV data sets is less, and the anatomy is more conducive to straightforward cross-sectional image review than the pulmonary Gd-MRA, dynamic visualization of the veins of the thighs is still advantageous.
Incomplete visualization of the popliteal veins may result in patients over 6 feet tall, as the plan is to avoid moving the patient and move only the table. The distal thighs in tall patients, therefore, may be outside of the field of view. A correction filter will be used to insure homogeneous linearity at the periphery of the image.
MRI Equipment Quality Control
System performance tests will be performed using the American College of Radiology MRI phantom. The relevant phantom components for this study include uniform signal and background noise regions, geometric accuracy, and a high-contrast resolution insert. Tests will be performed on a semi-annual basis. All images will be submitted on compact disc to the University of Michigan for analysis. The standard American College of Radiology MRI head coil test protocol is performed to assess overall system status. In addition, the high-contrast resolution performance of each site will be measured using a protocol close to the local pulmonary MRA settings to scan the high-contrast resolution insert of the MRI phantom reoriented for a coronal acquisition. Comparable performance of all scanners will be confirmed and monitored for signal-to-noise ratio. The value of the signal-to-noise ratio will also reflect system performance for the thigh MRV scan that utilizes the body coil.
Clinical Assessment: Methods
The Wells scoring system for a pretest clinical probability of PE will be used (2). It was described previously (30).
D-DIMER
D-dimer: Methods
D-dimer, measured by the quantitative rapid ELISA, will be considered normal if < 500 ng/ml (49).
VENOUS COMPRESSION ULTRASOUND
Compression Ultrasound: Methods
Bilateral venous duplex imaging of the lower extremities will use B-mode real time venous compression in combination with color Doppler. Ideally the patient will be supine, with the hip externally rotated and the knee slightly flexed. Knee flexion is used for popliteal evaluation. B-mode real time venous compression will be performed in the transverse orientation. The common femoral, femoral, popliteal, and proximal greater saphenous veins will be evaluated with sequential compression throughout with identification of flow characteristics. The calf veins will not be evaluated. Acute DVT will be diagnosed if there is noncompressibility of the vein in combination with: a) vein enlarged in size, b) hypoechoic vein lumen, and c) lack of significant collaterals.
CHEST RADIOGRAPH
An upright 6-foot posterior-anterior chest and lateral radiograph will be obtained within 2 hours of the V-Q lung scan if possible and within 12 hours in all cases. If the patient is unable to sit or stand, an anterior-posterior supine chest radiograph will be obtained.
Ventilation Lung Scan and Perfusion Lung Scan Methods
Ventilation lung scan and perfusion lung scan methods and criteria for interpretation were described in detail in the methods of PIOPED II (50). The criteria for interpretation of V-Q scans are based on the revised PIOPED criteria (51).
Contrast Enhanced Spiral CT
All clinical centers are encouraged to use scanners with 16 or more detectors. Newer scanners installed during PIOPED III will be used. In general, the techniques described for PIOPED II will be used (30,50), but local centers may modify the techniques. For scanners with 16 or more detectors, the scan may start at the diaphragm or start at the top of the apex of lung and proceed to 2 cm below the lowest hemidiaphragm. The diagnostic criteria for acute PE are as described in PIOPED II (30,50).
Venous Phase Spiral CT of the Veins of the Lower Extremities
Venous phase contrast enhanced spiral CT of the veins of the thighs (CTV) will be performed and interpreted as in PIOPED II (50), except scans will commence at the acetabulum rather than the iliac crest.
DIGITAL SUBTRACTION PULMONARY ANGIOGRAPHY (DSA)
Pulmonary DSA’s typically will use 1024 × 1024 pixels when possible to allow 6 frames/sec. Maximum magnification, which allows visualization of the entire lung on both views, will be used. Pulmonary DSA’s will be obtained during the intrapulmonary artery injection of 25 to 50 mL of a low or iso-osmolar nonionic contrast agent injected at 20-25 mL/sec by power injector through a 5.0-8.0 French side hole catheter. The criteria for interpretation will be as in PIOPED II (50).
Design Similarities and Differences Compared with PIOPED II
The methods of PIOPED III will parallel the methods of PIOPED II (50) to the extent that a composite reference standard will be used for the diagnosis and exclusion of PE and all images will be read by two blinded and study certified readers at centers other than where the images were obtained (30). An important difference is that patients with no PE according to the composite reference test will be randomized to undergo Gd-MRA/MRV. This will reduce the proportion of patients with negative tests at no loss in evaluation of sensitivity and specificity, and reduce the cost of the trial.
Acknowledgments
This study was supported by Grants from the U.S. Department of Health and Human Services, Public Health Services, National Heart, Lung, and Blood Institute, Bethesda, Maryland. HL081593, HL177150, HL077149, HL077151, HL077154, HL081594, HL077358, HL077155, HL077153
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evidence Report/Technology Assessment No. 68, Diagnosis and treatment of deep venous thrombosis and pulmonary embolism. Agency for Healthcare Research and Quality. Ann Intern Med. 2001;135:98–107. www.ahrq.gov D-dimer
- 2.Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 3.Meaney JFM, Weg JG, Chenevert TL, et al. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med. 1997;336:1422–1427. doi: 10.1056/NEJM199705153362004. [DOI] [PubMed] [Google Scholar]
- 4.Oudkerk M, Van Beek EJR, Weilopolski P, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359:1643–1647. doi: 10.1016/S0140-6736(02)08596-3. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Frazer CK, Ferguson JM, et al. Acute pulmonary embolism: Diagnosis with MR angiography. Radiol. 1999;210:353–359. doi: 10.1148/radiology.210.2.r99fe53353. [DOI] [PubMed] [Google Scholar]
- 6.Blum A, Bellou A, Guillemin F, et al. GENEPI study group Performance of magnetic resonance angiography in suspected acute pulmonary embolism. Thromb Haemost. 2005;93:503–511. doi: 10.1160/TH04-08-0495. [DOI] [PubMed] [Google Scholar]
- 7.Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997–1005. doi: 10.7326/0003-4819-129-12-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 8.Shellock FG.Manual for Magnetic Resonance Safety Implants and Devices 2004. Edition. [Google Scholar]
- 9.U.S. Food and Drug Administration, Healthcare professional sheet Gadolinium-containing contrast agents for magnetic resonance imaging (MRI) (marketed as Omniscan, OptiMARK, Magnevist, ProHance and Multihance) http://www.fda.gov/cder/drug/InfoSheets/HCP/gccaHCP.htm 6/23/06
- 10.Cowper SE, International Center for Nephrogenic Fibrosing Dermopathy Research (ICNFDR) http://www.pathmax.com/dermweb/ last updated March 27, 2007.
- 11.FDA Alert 6/2006 http://www.fda.gov/cder/drug/InfoSheets/HCP/gccaHCP.htm
- 12.Public Health Advisory http://www.fda.gov/cder/drug/advisory/gadolinium_agents_20061222.htm
- 13.International Center for Nephrogenic Fibrosing Dermopathy Research http:/www.pathmax.com/dermweb/
- 14.FDA Information for Healthcare Professionals Updated 12/2006.http://www.fda.gov/medwatch/report/hcp.htm
- 15.Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148–57. doi: 10.1148/radiol.2431062144. [DOI] [PubMed] [Google Scholar]
- 16.Cowper S, International Center for Nephrogenic Fibrosing Dermopathy Research (ICNFDR) Personal communication. 2007.
- 17.Thomsen HS. Nephrogenic systemic fibrosis: A serious late adverse reaction to gadodiamide. Eur Radiol. 2006;16:2619–21. doi: 10.1007/s00330-006-0495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo PH, Kanal E, Abu-Alfa AK, et al. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–9. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 19.Medicine and Healthcare Products Regulatory Agency http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON2030229
- 20.Waknine Y. http://www.medscape.com/viewarticle/535842
- 21.Kanal E, Barkovich JA, Bell C, et al. ACR Guidance Document for Safe MR Practices: 2007. AJR. 2007;188:1–27. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- 22.Kanal E. Personal communication. 2007.
- 23.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals Intern Med. 2006;147:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al. Expressing the MDRD Study equation for estimating GFR with IDMS traceable (gold standard) serum creatinine values. [Abstract] J Am Soc Nephrol. 2005;16:69A. [Google Scholar]
- 26.Stein PD, Gottschalk A. Review of criteria appropriate for a very low probability of pulmonary embolism on ventilation-perfusion lung scans: a position paper. Radiographics. 2000;20:99–105. doi: 10.1148/radiographics.20.1.g00ja1399. [DOI] [PubMed] [Google Scholar]
- 27.Gottschalk A, Stein PD, Sostman HD, et al. Very low probability interpretation of ventilation-perfusion lung scans in combination with low probability clinical assessment reliably excludes pulmonary embolism: Data from PIOPED II. J Nucl Med. 2007;48:1411–1415. doi: 10.2967/jnumed.107.040998. [DOI] [PubMed] [Google Scholar]
- 28.Goodman LR, Stein PD, Matta F, et al. CT Venography and compression ultrasound are diagnostically equivalent: Data from PIOPED II. Am J Roentgen. 2007;189:1071–1076. doi: 10.2214/AJR.07.2388. [DOI] [PubMed] [Google Scholar]
- 29.Stein PD, Athanasoulis C, Alavi A, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation. 1992;85:462–469. doi: 10.1161/01.cir.85.2.462. [DOI] [PubMed] [Google Scholar]
- 30.Stein PD, Fowler SE, Goodman LR, et al. PIOPED II Investigators Multidetector computed tomography for acute pulmonary embolism. N Eng J Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 31.The PIOPED Investigators Value of the ventilation/ perfusion scan in acute pulmonary embolism: results of the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) J Am Med Assoc. 1990;263:2753–2759. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 32.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;238:W1–W12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 33.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD initiative. Ann Intern Med. 2003;238:40–44. doi: 10.7326/0003-4819-138-1-200301070-00010. [DOI] [PubMed] [Google Scholar]
- 34.Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design related bias in studies of diagnostic tests. J Am Med Assoc. 1999;282:1061–1066. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]
- 35.Dotter CT. Acquired abnormalities of the pulmonary arteries. In: Abrams HL, editor. Angiography. 3rd Little Brown; Boston: 1983. pp. 743–761. [Google Scholar]
- 36.Berthezene Y, Croisille P, Wiart M, et al. Prospective Comparison of MR Lung Perfusion and Lung Scintigraphy. J Magnetic Resonance Imaging. 1999;9:61–68. doi: 10.1002/(sici)1522-2586(199901)9:1<61::aid-jmri8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Stein PD, O’Connor JF, Dalen JE, et al. The angiographic diagnosis of acute pulmonary embolism: evaluation of criteria. Am Heart J. 1967;73:730–741. doi: 10.1016/0002-8703(67)90224-4. [DOI] [PubMed] [Google Scholar]
- 38.Roberts DA, Rizi RR, Lipson DA, et al. Dynamic observation of pulmonary perfusion using continuous arterial spin labeling in a pig model. JMRI. 2001;14:175–180. doi: 10.1002/jmri.1169. [DOI] [PubMed] [Google Scholar]
- 39.Levin DL, Chen Q, Edelman RR, et al. Evaluation of regional pulmonary perfusion using ultrafast magnetic resonance imaging. Magn Reson Med. 2001;46:166–171. doi: 10.1002/mrm.1172. [DOI] [PubMed] [Google Scholar]
- 40.Mai VM, Chen Q, Bankier AA, et al. Effect of lung inflation on arterial spin labeling signal in MR perfusion imaging of human lung. JMRI. 2001;13:954–959. doi: 10.1002/jmri.1137. [DOI] [PubMed] [Google Scholar]
- 41.Amundsen T, Kvaerness J, Jones RA, et al. Pulmonary embolism: detection with MR perfusion imaging of lung: a feasibility study. Radiol. 1997;203:181–185. doi: 10.1148/radiology.203.1.9122390. [DOI] [PubMed] [Google Scholar]
- 42.Zheng J, Leawoods JC, Nolte M, et al. Combined MR proton lung perfusion/angiography and helium ventilation: potential for detecting pulmonary emboli and ventilation defects. Magn Reson Med. 2002;47:433–438. doi: 10.1002/mrm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swan JS, Carroll TJ, Kennell TW, et al. Time-resolved three-dimensional contrast-enhanced MR angiography of the peripheral vessels. Radiol. 2002;225:43–52. doi: 10.1148/radiol.2251011292. [DOI] [PubMed] [Google Scholar]
- 44.Carr JC, Laub G, Zheng J, et al. Time-resolved three-dimensional pulmonary MR angiography and perfusion imaging with ultrashort repetition time. Acad Radiol. 2002;9:1407–1418. doi: 10.1016/s1076-6332(03)80668-2. [DOI] [PubMed] [Google Scholar]
- 45.Loud PA, Katz DS, Klippenstein DL, et al. Combined CT venography and pulmonary angiography in suspected thromboembolic disease: diagnostic accuracy for deep venous evaluation. AJR. 2001;174:61–65. doi: 10.2214/ajr.174.1.1740061. [DOI] [PubMed] [Google Scholar]
- 46.Cham MD, Yankelevitz DF, Shaham D, et al. for the Pulmonary CT Angiography-Indirect CT Venography Cooperative Group Deep venous thrombosis: detection by using indirect CT venography. Radiology. 2002;216:744–751. doi: 10.1148/radiology.216.3.r00se44744. [DOI] [PubMed] [Google Scholar]
- 47.Brink JA, Woodard PK, Horesh L, et al. Depiction of pulmonary emboli with spiral CT: optimization of display window settings in a porcine model. Radiology. 1997;204:703–708. doi: 10.1148/radiology.204.3.9280246. [DOI] [PubMed] [Google Scholar]
- 48.Remy-Jardin M, Remy J, Cauvain O, et al. Diagnosis of central PE with helical CT: role of two-dimensional multiplanar reformations. AJR. 1995;165:1131–1138. doi: 10.2214/ajr.165.5.7572490. [DOI] [PubMed] [Google Scholar]
- 49.Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of deep venous thrombosis and acute pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 50.Gottschalk A, Stein PD, Goodman LR, et al. Overview of Prospective Investigation of Pulmonary Embolism Diagnosis II. J. Semin Nucl Med. 2002;32:173–182. doi: 10.1053/snuc.2002.124177. [DOI] [PubMed] [Google Scholar]
- 51.Gottschalk A, Sostman HD, Coleman RE, et al. Ventilation-perfusion scintigraphy in the PIOPED study. Part II. Evaluation of the scintigraphic criteria and interpretations. J Nucl Med. 1993;34:1119–1126. [PubMed] [Google Scholar]


