Abstract
Samples were collected every 2–4 weeks from a set of 37 monitoring wells over a period of 2–3 years in Araihazar, Bangladesh, to evaluate the temporal variability of groundwater composition for As and other constituents. The monitoring wells are grouped in 6 nests and span the 5–91 m depth range. Concentrations of As, Ca, Fe, K, Mg, Mn, Na, P, and S were measured by high-resolution ICPMS with a precision of 5% or better; concentrations of Cl were measured by ion chromatography. In shallow wells <30 m deep, As and P concentrations generally varied by <30%, whereas concentrations of the major ions (Na, K, Mg, Ca and Cl) and the redox-sensitive elements (Fe, Mn, and S) varied over time by up to ± 90%. In wells tapping the deeper aquifers > 30 m often below clay layers concentrations of groundwater As were much lower and varied by <10%. The concentrations of major cations also varied by <10% in these deep aquifers. In contrast, the concentration of redox-sensitive constituents Fe, S, and Mn in deep aquifers varied by up to 97% over time. Thus, strong decoupling between variations in As and Fe concentrations is evident in groundwaters from shallow and deep aquifers.
Comparison of the time series data with groundwater ages determined by 3H/3He and 14C dating shows that large seasonal or inter-annual variations in major cation and chloride concentrations are restricted to shallow aquifers and groundwater recharged < 5 years ago. There is no corresponding change in As concentrations despite having significant variations of redox sensitive constituents in these very young waters. This is attributed to chemical buffering due to rapid equilibrium between solute and solid As. At two sites where the As content of groundwater in existing shallow wells averages 102 µg/L (range: < 5 to 648 µg/L; n=118) and 272 µg/L (range: 10 to 485 µg/L; n=65), respectively, a systematic long-term decline in As concentrations lends support to the notion that flushing may slowly deplete an aquifer of As. Shallow aquifer water with > 5 yr 3H/3He age show a constant As:P molar ratio of 9.6 over time, suggesting common mechanism of mobilization.
Keywords: arsenic, phosphorus, redox, residence time, variability, seasonality, groundwater, composition, Bangladesh, time series
1. INTRODUCTION
The spatial variability of groundwater As concentrations at scales of 101 to 104 m has been well documented for Holocene (<10 kyr old) fluvial-deltaic aquifers of the Bengal Basin (BGS & DPHE, 2001; van Geen et al., 2003; Yu et al., 2003). There is growing evidence that at least part of this heterogeneity can be attributed to variations in local geology and its effect on shallow groundwater flow (van Geen et al., 2006; Stute et al., 2007; Aziz et al., in revision; Weinman et al., in press.). Such spatial variability naturally leads to the concern that shallow groundwater As concentration may also change over time, especially because subsurface flow is likely to be affected by large water withdrawals for irrigation in certain areas of Bangladesh (Harvey et al., 2002; Klump et al., 2006). The persisting gaps in our knowledge of the mechanisms that lead to As mobilization (Horneman et al., 2004; Zheng et al., 2004), combined with pronounced seasonal fluctuations in water levels in shallow and deep aquifers linked to the monsoon, make it particularly difficult to predict variations of groundwater As concentration over space or time. Yet, this understanding is urgently needed because a significant proportion of those shallow wells that presently meet the Bangladesh drinking water standard of 50 µg/L are, at least temporarily, shared by villagers of Bangladesh to reduce their exposure to As and therefore reduce the likelihood of contracting a series of debilitating diseases (van Geen et al., 2002; Opar et al., 2007).
There are few high-quality time series data of groundwater As concentration from the Bengal Basin. The available data with the As concentrations ranging from 0.4 µg/L to 64 µg/L generally indicate little seasonality or long-term trends once very shallow (<10 m) are excluded (BGS & DPHE, 2001; Cheng et al., 2005; 2006; van Geen et al., 2005; 2007). On a different continent, little change in As concentration over a period of 1–20 years was also reported for 759 wells from western Nevada, USA, where concentrations range from <5 to 6200 µg/L in a wide depth range (28 m ± 46 m, median 16 m) (Steinmaus et al., 2005).
Besides a few very shallow (<10 m) wells monitored over several years (Cheng et al., 2005), there are other credible reports of significant changes in As concentrations in groundwater over time. A striking example was the case of a highly-contaminated private well of unreported depth at Ramnagar in West Bengal, India, that was monitored biweekly between July 1992 and June 1993 and showed occasional variations of ~30% around an average of ~2700 µg/L (Chatterjee et al., 1995). The same group observed a long term rise in groundwater As concentration in a number of private wells in 23 villages out of 100 villages of West Bengal where initially water with low As (<50 µg/L) exceeded 50 µg/L over time, although the data were not reported (Chakraborti et al., 2002; Chakraborti et al., 2004). Large seasonal variations of groundwater As levels were also reported in 5 monitoring wells at depths of 3–60 m in Samta village of Western Bangladesh (AAN, 1999), although the measurements of As were few and made by a less reliable method (silver dithiodicarbomate spectrometry) in a local laboratory. There is more convincing evidence that As concentration declined between September-December, 1999, and May 2000 in many of the 68 wells sampled twice in four districts of the Red River delta (Berg et al., 2001). Naturally occurring As in groundwater of Granite Falls, Washington, ranging in concentration from <10 µg/L to 14,000 µg/L also showed substantial temporal variability of 12–79% for 15 out of 25 private drinking water wells monitored over 12 months (Frost et al., 1993).
With the present study, we contribute to the body of groundwater monitoring data by presenting up to 3 years of bi-weekly to monthly measurements of As, P, Fe, Mg, Ca, K, Na, Mn, S, and Cl in groundwater at 6 well nests comprised of a total of 37 monitoring wells installed in Araihazar, Bangladesh. These observations not only fill a gap in our understanding of temporal variability in groundwater As concentrations, they also shed new light on the mechanisms of As mobilization and may help us anticipate future trends in affected areas. The monitoring wells tap aquifers from 5–91 m with a wide range of As concentrations from <5 to 600 µg/L in an area where previous studies have documented a spectrum of hydrogeological conditions that is representative of much of the Bengal Basin (Horneman et al., 2004; Zheng et al., 2005; van Geen et al., 2006). We describe the main temporal patterns in groundwater chemistry, including long-term trends, short-term excursions, and seasonal variations in both shallow and deep aquifers. The variations in groundwater major ion composition, or mostly lack thereof, are then discussed in the context of groundwater ages.
2. METHODS
2.1 Monitoring wells
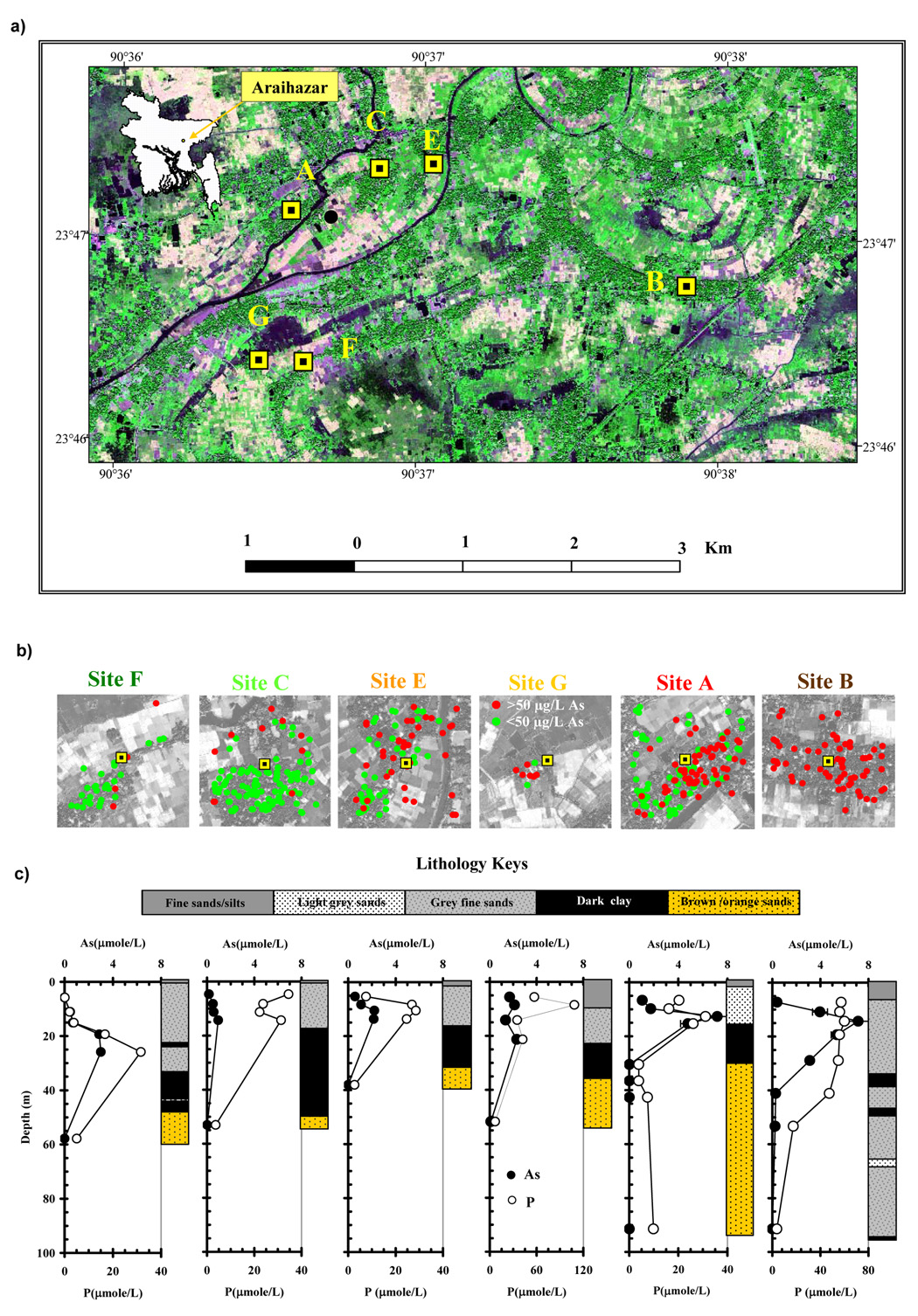
The locations of the well nests were chosen to cover the spatial patterns of groundwater As concentrations established by a previous survey of 6000 wells distributed over a ~ 25 km2 area of Araihazar in central Bangladesh (van Geen et al., 2003). A total of 37 monitoring wells from 5–91 m in depth were installed at 6 sites (Fig.1a, Table 1). At each of the sites, 4 or 5 monitoring wells tapped shallow aquifers composed of Holocene alluvial deposits ranging from 15–30 m in thickness (Fig. 1c). The sites were arranged in most figures according to increasing average As concentrations of existing shallow (<30 m) wells within the 0.16 km2 area centered by each of the nest of wells: 18 µg/L (< 5 to 112 µg/L ) at Site F, 19 µg/L (< 5 to 143 µg/L ) at Site C, 65 µg/L (< 5 to 254 µg/L ) at Site E, 94 µg/L (8 to 352 µg/L ) at Site G, 102 µg/L (< 5 to 648 µg/L) at Site A, and 272 µg/L (10 to 485 µg/L) at Site B (Fig. 1b). At all locations except for Site B, at least one monitoring well > 30 m deep reached distinctly orange/brown colored sands of Pleistocene age (Fig. 1c). At Site B, grey Holocene sediments were observed even to a depth of 91 m (Zheng et al., 2005). At each site, one or several layers of fine-grained sediment separate the shallow aquifers that are elevated in As from the deep aquifers that are low in As concentration (Fig. 1c).
Figure 1.
(a) Locations of six nests of wells were plotted on an IKONOS image of Araihazar study area in central part of Bangladesh(Inset).
(b) The 400 m × 400 m squares represent an enlarged view of the spatial distribution of As in existing wells surrounding the 6 well nests. Green and red solid circles indicate the As level < 50 µg/L, and greater or equal to 50 µg/L, respectively. Sites F, C, E, G, A, and B are arranged from left to right and color coded with increasing average As concentration in the surrounding wells located in the 400 m × 400 m squares.
(c) The depth profiles of average groundwater As and P concentration for all sites. The scales for P concentration were different for Sites G and B. Error bars on As profiles showed the fluctuations over the entire monitoring period. A lithology sketch is placed next to the vertical profile at each site.
Table-1.
Composition of groundwater in aquifers from six well nests of 37 monitoring wells in Araihazar, Bangladesh.
| Depth | Age | Elevation | Sample | pH1 | Eh1(mV) | Cond.1(mS/m) | As (µg/L) | P(µmole/L) | SMC(meq/L) | Na (meq/L) | Cl (meq/L) | Fe (µmole/L) | Mn (µmole/L) | S (µmole/L) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (m) | years2 | (m) | No | Avg | Stdev | Avg | Stdev | Avg | Stdev | Avg | Stdev | Avg | Stdev | Avg | Stdev | Avg | Stdev | Avg | Stdev | Avg | Stdev | Avg | Stdev | Avg | Stdev | |||||||||||
| Site A (23.785°N, 90.603°E) - Village: Dari Satyabandi, all multlevel wells were installed in Jan. 2001 | ||||||||||||||||||||||||||||||||||||
| 7 | 3.5 | 7.47 | 43 | 6.46 | ± | 0.06 | −76 | ± | 12 | 54 | ± | 13 | 80 | ± | 8 | 20 | ± | 5 | 5.6 | ± | 1.0 | 1.0 | ± | 0.16 | 1.2 | ± | 0.13 | 413 | ± | 86 | 47 | ± | 13 | 374 | ± | 106 |
| 10 | 10.8 | 7.47 | 43 | 6.66 | ± | 0.04 | −79 | ± | 13 | 39 | ± | 10 | 132 | ± | 8 | 16 | ± | 1 | 4.2 | ± | 0.6 | 0.7 | ± | 0.11 | 0.7 | ± | 0.08 | 159 | ± | 22 | 51 | ± | 7 | 135 | ± | 60 |
| 13 | 20.3 | 7.43 | 42 | 6.92 | ± | 0.05 | −150 | ± | 17 | 51 | ± | 18 | 538 | ± | 64 | 31 | ± | 8 | 5.4 | ± | 0.4 | 0.9 | ± | 0.20 | 1.3 | ± | 0.12 | 262 | ± | 37 | 96 | ± | 6 | 25 | ± | 16 |
| 15 | 31.7 | 7.40 | 38 | 7.00 | ± | 0.04 | −158 | ± | 14 | 63 | ± | 21 | 359 | ± | 45 | 26 | ± | 5 | 7.0 | ± | 0.3 | 2.0 | ± | 0.16 | 1.0 | ± | 0.07 | 334 | ± | 51 | 18 | ± | 2 | 6 | ± | 1 |
| 30 | - | 7.53 | 41 | 6.60 | ± | 0.05 | −58 | ± | 17 | 34 | ± | 9 | 0.5 | ± | 0.03 | 4 | ± | 0.3 | 3.5 | ± | 0.2 | 2.5 | ± | 0.14 | 0.8 | ± | 0.04 | 10 | ± | 5 | 2 | ± | 1 | 14 | ± | 12 |
| 37 | - | 7.51 | 41 | 6.52 | ± | 0.06 | −45 | ± | 24 | 28 | ± | 7 | 0.5 | ± | 0.02 | 4 | ± | 0.3 | 3.7 | ± | 0.2 | 2.6 | ± | 0.24 | 0.8 | ± | 0.02 | 10 | ± | 5 | 2 | ± | 1 | 15 | ± | 14 |
| 43 | - | 7.48 | 41 | 6.52 | ± | 0.05 | −57 | ± | 25 | 28 | ± | 7 | 1.0 | ± | 0.03 | 7 | ± | 1 | 3.0 | ± | 0.2 | 1.8 | ± | 0.13 | 0.9 | ± | 0.06 | 52 | ± | 30 | 6 | ± | 3 | 18 | ± | 16 |
| 91 | - | 7.50 | 21 | 6.53 | ± | 0.09 | −56 | ± | 27 | 32 | ± | 9 | 1.3 | ± | 0.04 | 10 | ± | 1 | 3.8 | ± | 0.3 | 1.8 | ± | 0.27 | 1.4 | ± | 0.05 | 224 | ± | 85 | 2 | ± | 1 | 29 | ± | 9 |
| Site B (23.780°N, 90.640°E) - Village: Baylakandi, multlevel wells were installed in Jan. and May 2001 | ||||||||||||||||||||||||||||||||||||
| 7 | 1.6 | 7.42 | 31 | 6.62 | ± | 0.07 | −100 | ± | 26 | 101 | ± | 31 | 30 | ± | 6 | 57 | ± | 18 | 11.8 | ± | 1.3 | 2.3 | ± | 0.48 | 4.3 | ± | 0.44 | 430 | ± | 95 | 17 | ± | 4 | 677 | ± | 189 |
| 11 | 19.2 | 7.37 | 29 | 6.64 | ± | 0.05 | −120 | ± | 17 | 92 | ± | 31 | 298 | ± | 47 | 56 | ± | 8 | 12.0 | ± | 1.2 | 2.3 | ± | 0.53 | 3.5 | ± | 0.35 | 431 | ± | 180 | 40 | ± | 6 | 350 | ± | 117 |
| 14 | 19.3 | 7.52 | 38 | 6.84 | ± | 0.08 | −142 | ± | 24 | 57 | ± | 15 | 536 | ± | 65 | 60 | ± | 8 | 9.7 | ± | 1.1 | 1.7 | ± | 0.25 | 4.0 | ± | 0.46 | 380 | ± | 58 | 20 | ± | 6 | 161 | ± | 104 |
| 19 | 23.3 | 7.49 | 32 | 6.93 | ± | 0.07 | −155 | ± | 16 | 58 | ± | 18 | 403 | ± | 36 | 56 | ± | 6 | 8.2 | ± | 0.5 | 1.2 | ± | 0.26 | 1.9 | ± | 0.16 | 417 | ± | 104 | 24 | ± | 3 | 9 | ± | 6 |
| 29 | - | 7.49 | 25 | 6.71 | ± | 0.07 | −126 | ± | 5 | 94 | ± | 1 | 234 | ± | 21 | 55 | ± | 5 | 8.2 | ± | 0.2 | 1.3 | ± | 0.16 | 2.5 | ± | 0.21 | 363 | ± | 52 | 6 | ± | 1 | 3 | ± | 3 |
| 41 | - | 7.46 | 40 | 6.94 | ± | 0.07 | −143 | ± | 16 | 58 | ± | 14 | 21 | ± | 2 | 45 | ± | 4 | 6.0 | ± | 0.3 | 0.7 | ± | 0.06 | 0.3 | ± | 0.02 | 193 | ± | 39 | 1 | ± | 0.3 | 5 | ± | 3 |
| 53 | - | 7.47 | 40 | 7.13 | ± | 0.10 | −151 | ± | 23 | 44 | ± | 8 | 17 | ± | 1 | 17 | ± | 1 | 4.4 | ± | 0.2 | 1.8 | ± | 0.14 | 0.3 | ± | 0.01 | 120 | ± | 13 | 3 | ± | 0.3 | 4 | ± | 4 |
| 91 | - | 7.60 | 23 | 6.91 | ± | 0.16 | −113 | ± | 21 | 33 | ± | 5 | 0.3 | ± | 0.01 | 4 | ± | 0.3 | 3.5 | ± | 0.1 | 1.8 | ± | 0.12 | 0.1 | ± | 0.00 | 25 | ± | 2 | 1.6 | ± | 0.1 | <0.03 | ± | 0.0 |
| Site C (23.790°N, 90.611°E) - Village: Bhuyan Para (Satyabhandi), multlevel wells were installed in March 2002 | ||||||||||||||||||||||||||||||||||||
| 5 | 0.0 | 6.93 | 18 | 7.04 | ± | 0.25 | −113 | ± | 27 | 58 | ± | 23 | 12 | ± | 2 | 35 | ± | 7 | 2.7 | ± | 2.3 | 1.5 | ± | 2.25 | 0.6 | ± | 0.72 | 818 | ± | 280 | 24 | ± | 7 | 25 | ± | 18 |
| 8 | 0.8 | 7.01 | 18 | 6.79 | ± | 0.17 | −132 | ± | 8 | 83 | ± | 16 | 38 | ± | 2 | 24 | ± | 4 | 2.6 | ± | 2.4 | 1.4 | ± | 2.04 | 0.7 | ± | 0.77 | 1694 | ± | 704 | 63 | ± | 28 | 26 | ± | 16 |
| 11 | 2.6 | 7.01 | 18 | 6.46 | ± | 0.11 | −111 | ± | 22 | 52 | ± | 9 | 42 | ± | 3 | 22 | ± | 2 | 1.6 | ± | 0.7 | 0.6 | ± | 0.50 | 0.6 | ± | 0.56 | 1311 | ± | 697 | 34 | ± | 14 | 11 | ± | 7 |
| 14 | 2.8 | 7.00 | 18 | 6.58 | ± | 0.15 | −114 | ± | 29 | 36 | ± | 12 | 70 | ± | 9 | 31 | ± | 4 | 2.8 | ± | 0.3 | 0.4 | ± | 0.06 | 0.3 | ± | 0.04 | 773 | ± | 76 | 33 | ± | 3 | 23 | ± | 16 |
| 53 | - | 7.01 | 18 | 6.38 | ± | 0.15 | −33 | ± | 25 | 28 | ± | 3 | 1 | ± | 1 | 4 | ± | 1 | 2.9 | ± | 0.2 | 1.2 | ± | 0.10 | 0.6 | ± | 0.04 | 117 | ± | 92 | 13 | ± | 2 | 10 | ± | 8 |
| Site E (23.790°N, 90.616°E) - Village: Hatkhola Para, multlevel wells were installed in March 2002 | ||||||||||||||||||||||||||||||||||||
| 5 | 9.6 | 6.39 | 16 | 6.65 | ± | 0.11 | −40 | ± | 29 | 34 | ± | 7 | 43 | ± | 2 | 8 | ± | 1 | 4.9 | ± | 0.6 | 0.4 | ± | 0.04 | 0.2 | ± | 0.02 | 48 | ± | 11 | 17 | ± | 2 | 232 | ± | 74 |
| 8 | - | 6.42 | 16 | 6.73 | ± | 0.12 | −118 | ± | 38 | 34 | ± | 10 | 82 | ± | 4 | 27 | ± | 2 | 3.8 | ± | 0.5 | 0.4 | ± | 0.04 | 0.1 | ± | 0.01 | 424 | ± | 48 | 35 | ± | 4 | 80 | ± | 42 |
| 11 | 18.1 | 6.44 | 16 | 6.90 | ± | 0.09 | −140 | ± | 48 | 35 | ± | 10 | 166 | ± | 12 | 29 | ± | 2 | 4.2 | ± | 0.3 | 0.5 | ± | 0.08 | 0.1 | ± | 0.01 | 392 | ± | 123 | 33 | ± | 3 | 2 | ± | 1 |
| 14 | - | 6.42 | 16 | 6.82 | ± | 0.13 | −148 | ± | 1 | 42 | ± | 23 | 162 | ± | 15 | 25 | ± | 2 | 5.2 | ± | 0.5 | 1.2 | ± | 0.17 | 0.8 | ± | 0.03 | 498 | ± | 143 | 17 | ± | 5 | 1 | ± | 1 |
| 38 | - | 6.45 | 16 | 6.55 | ± | 0.10 | −60 | ± | 39 | 52 | ± | 19 | 0.3 | ± | 0.02 | 3 | ± | 0.3 | 5.7 | ± | 0.3 | 2.5 | ± | 0.30 | 1.2 | ± | 0.03 | 56 | ± | 19 | 5 | ± | 2 | <0.03 | ± | 0.0 |
| Site F (23.774°N, 90.605°E) - Village: Lashkardi (Mosque), multlevel wells were installed in March and November 2002 | ||||||||||||||||||||||||||||||||||||
| 6 | 0.8 | 7.90 | 21 | 6.18 | ± | 0.12 | 95 | ± | 92 | 18 | ± | 2 | 0.2 | ± | 0.02 | 0.1 | ± | 0.1 | 1.6 | ± | 0.3 | 0.2 | ± | 0.06 | 0.2 | ± | 0.06 | 2.4 | ± | 2 | 2.6 | ± | 1 | 37 | ± | 25 |
| 11 | - | 7.87 | 22 | 6.23 | ± | 0.10 | 10 | ± | 50 | 21 | ± | 8 | 35 | ± | 3 | 2 | ± | 1 | 1.8 | ± | 0.3 | 0.1 | ± | 0.02 | 0.2 | ± | 0.04 | 63 | ± | 22 | 31 | ± | 8 | 24 | ± | 15 |
| 15 | 5.3 | 7.81 | 22 | 6.73 | ± | 0.23 | −47 | ± | 60 | 21 | ± | 3 | 51 | ± | 5 | 4 | ± | 1 | 2.4 | ± | 0.1 | 0.1 | ± | 0.01 | 0.1 | ± | 0.01 | 31 | ± | 18 | 27 | ± | 3 | 15 | ± | 9 |
| 19 | 29.1 | 7.76 | 22 | 6.88 | ± | 0.11 | −63 | ± | 55 | 29 | ± | 6 | 215 | ± | 19 | 17 | ± | 3 | 3.7 | ± | 0.3 | 0.3 | ± | 0.02 | 0.04 | ± | 0.00 | 27 | ± | 16 | 42 | ± | 2 | 35 | ± | 4 |
| 26 | - | 7.74 | 14 | 7.00 | ± | 0.00 | −200 | 63 | 225 | ± | 15 | 32 | ± | 4 | 5.2 | ± | 0.4 | 0.5 | ± | 0.03 | 0.2 | ± | 0.01 | 350 | ± | 22 | 35 | ± | 3 | 0.3 | ± | 0.02 | ||||
| 58 | - | 7.91 | 22 | 6.95 | ± | 0.08 | −63 | ± | 23 | 154 | ± | 8 | 0.3 | ± | 0.02 | 5 | ± | 0.4 | 12.5 | ± | 1.0 | 11.4 | ± | 0.94 | 9.7 | ± | 0.74 | 5.7 | ± | 2 | 9 | ± | 0.4 | 9 | ± | 4 |
| Site G (23.774°N, 90.601°E) - Village: Lashkardi (Bilbari), multlevel wells were installed in March 2002 | ||||||||||||||||||||||||||||||||||||
| 6 | 21.4 | 20 | 6.75 | ± | 0.07 | −127 | ± | 24 | 41 | ± | 8 | 127 | ± | 16 | 57 | ± | 13 | 4.6 | ± | 0.2 | 0.4 | ± | 0.02 | 0.1 | ± | 0.01 | 610 | ± | 189 | 25 | ± | 4 | 22 | ± | 1 | |
| 9 | 13.1 | 20 | 6.67 | ± | 0.10 | −111 | ± | 38 | 45 | ± | 11 | 159 | ± | 13 | 108 | ± | 23 | 5.2 | ± | 0.3 | 0.4 | ± | 0.02 | 0.1 | ± | 0.01 | 501 | ± | 66 | 8 | ± | 2 | 6 | ± | 4 | |
| 14 | 26.0 | 20 | 6.93 | ± | 0.08 | −144 | ± | 23 | 36 | ± | 10 | 102 | ± | 5 | 35 | ± | 3 | 4.0 | ± | 0.3 | 0.4 | ± | 0.06 | 0.2 | ± | 0.02 | 294 | ± | 50 | 30 | ± | 3 | 1 | ± | 0.4 | |
| 21 | - | 20 | 7.05 | ± | 0.11 | −163 | ± | 31 | 42 | ± | 7 | 173 | ± | 15 | 42 | ± | 4 | 4.2 | ± | 0.3 | 0.4 | ± | 0.09 | 0.6 | ± | 0.06 | 194 | ± | 46 | 28 | ± | 2 | 2 | ± | 2 | |
| 52 | - | 20 | 6.85 | ± | 0.09 | −88 | ± | 34 | 145 | ± | 19 | 7 | ± | 1 | 7 | ± | 1 | 10.9 | ± | 1 | 9.9 | ± | 0.56 | 7.2 | ± | 0.60 | 13 | ± | 8 | 12 | ± | 1 | 31 | ± | 2 | |
data based on monthly measurements from March 2004 to February 2005
3H/3He age reported by Stute et al., 2006
2.2 Sampling and field measurements
Bi-weekly to monthly groundwater samples from the monitoring wells were collected from Jan. 2001 to Feb. 2004 at Sites A and B, and from March 2002 to Feb. 2004 at Sites C, E, F and G. Each well was pumped for at least 15 minutes by a battery-driven submersible pump (Whale SuperPurger) at a rate of ~2 L/min. The 15 minutes of pumping allowed conductivity and temperature readings to stabilize before sampling. Samples for As, other trace elements, and major cations were collected in 30-ml or 60-ml acid-cleaned HDPE bottles and acidified to 1% HCl (Fisher Optima) immediately after collection and without filtration. We, and others before us, have shown that the standard monitoring well screens in Bangladesh are typically sufficient to exclude particles that might dissolve upon acidification, whereas filtration can produce artifacts unless carried out under nitrogen in-line (Zheng et al., 2004). Samples for anions were collected in nanopure-washed 30 ml HDPE bottles without filtration. Starting in April, 2004, pH, ORP, temperature, and electrical conductivity of the groundwater was measured using a pH/Eh meter (Orion 210A) and a conductivity/temperature meter (Orion 105A+) with waterproof probes that were calibrated on the day of sampling. The groundwater level in each well was monitored every 1–2 weeks over the same time period using an electric water-level meter (Solinst model 101).
2.3 Laboratory measurements
Concentrations of As, P, Fe, Mn, S, Ca, Mg, K, Na and 33 other trace elements in acidified groundwater were measured at Lamont-Doherty Earth Observatory with a reproducibility typically < 5% by high-resolution inductively-coupled plasma mass spectrometry (HR ICP-MS) using an Axiom single-collector instrument (Thermo Elemental, Germany) (Cheng et al., 2004). Protocols that were followed to ensure the accuracy and precision of the data included: (1) Two NIST standard reference materials (1640 & 1643E, Trace element in natural water), and an internal laboratory consistency standard (LDEO tap water spiked with analyte elements) were included with each run. Results for these standards were always within 5% of the certified values after calibration of the instrument with separate standards at the beginning and end of each run (Cheng et al., 2004); (2) Whenever possible, time-series samples from the same well were analyzed within the same run of 30 samples, which usually improved the reproducibility to < 3%; (3) At least 2 samples were re-analyzed between two consecutive runs for the same well to ensure consistency between runs. Concentrations obtained for these replicates usually did not differ from each other by more than 3%.
Dissolved Cl− and SO42− concentrations in un-acidified groundwater samples were measured at Queens College by ion chromatography (IC) using a DIONEX-500 IC system, following the standard protocol of EPA method 300. Comparison of SO4 data obtained by IC with total S concentrations in acidified samples obtained by HR ICP-MS showed that S quantified by HR ICP-MS was essentially all in the form of sulfate at all sites (slope of S by ICPMS versus SO4 by IC is 1.0027, R2: 0.9812, n=181).
In addition to measurement by HR ICP-MS, phosphate present in groundwater was also quantified as dissolved reactive phosphate (DRP) using molybdate-blue colorimetry, modified to determine also dissolved As (Dhar et al., 2004). A comparison of colorimetric and HR ICP-MS data indicates that not all P present in groundwater reacts with molybdate. At Sites A and B, total P concentrations in shallow (< 30 m) groundwater measured by HR ICP-MS were consistently higher by 30% (R2 = 0.99, n=11) than DRP concentrations for samples collected in January 2003, suggesting that a fraction of the P could be in a non-reactive organic form (Stauffer, 1980). In contrast, total P concentrations measured by HR ICP-MS were only ~ 10% and 5% higher than DRP concentrations in shallow groundwater from Site C and Site F, respectively, for 2 sets of samples collected in Jan. 2003 and Oct. 2003 (Site C: R2 = 0.99, n = 4; Site F: R2 = 0.99, n = 6).
2.4 Statistical Analysis
The rate of increase or decrease per year in 37 shallow and deep wells was examined by performing a regression analysis of As, P and other ions concentration versus time for each well over the entire period except for C where the post summer 2003 flood period was excluded (Nov. 2003 to Feb. 2004, Fig. 2 and 3) due to very large change in Na and Cl. Trends of different constituents including As, P, sum of major cations (SMC), Cl, Fe, Mn and S were considered to be statistically significant if p values were <0.05. For most wells, the residuals were randomly and normally distributed around the linear trend line. The uncertainties in the rate of increase or decrease (e.g. the slope of regression) were expressed as 95% confidence intervals.
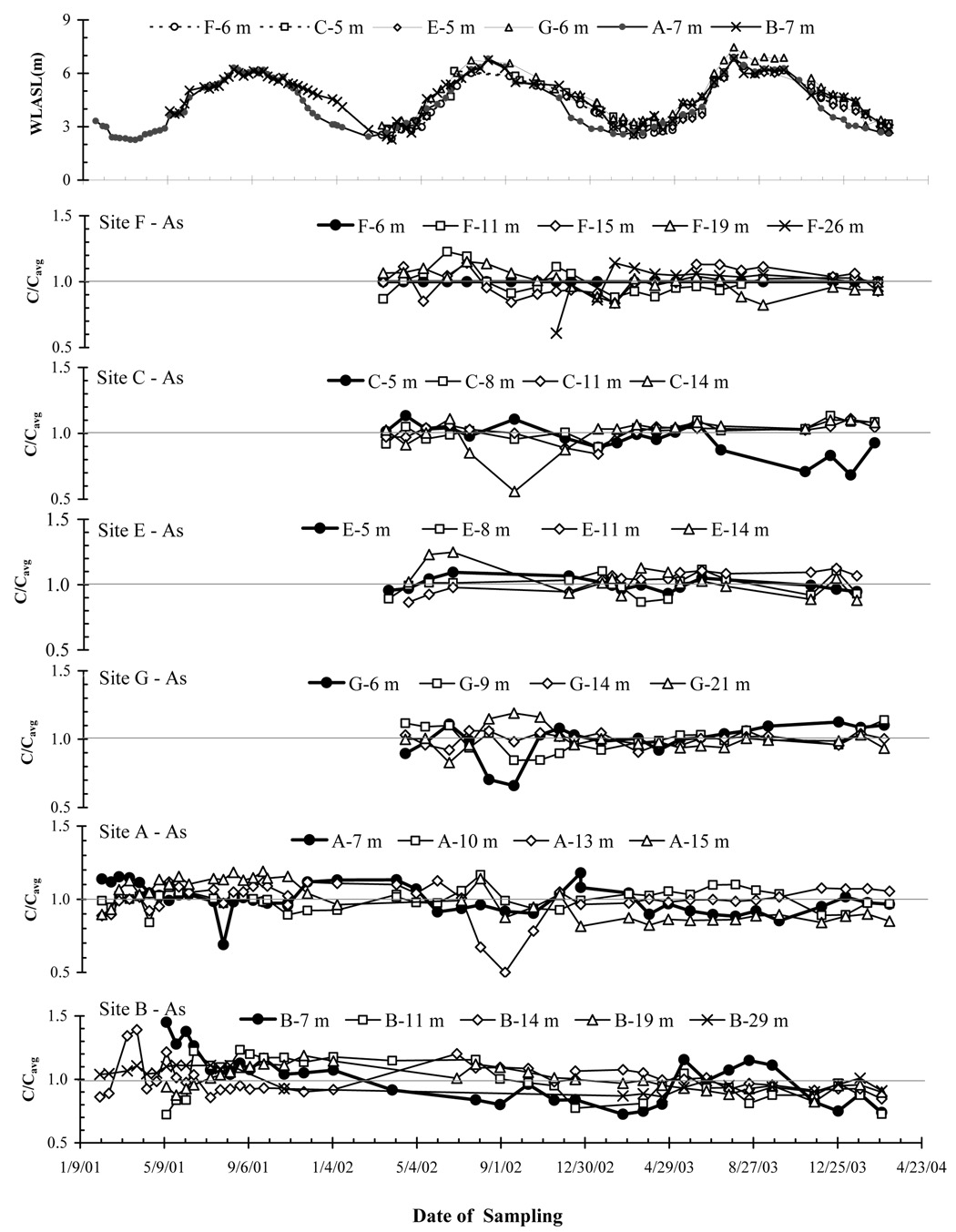
Figure 2.
Water level above sea level (WLASL) spanning the entire monitoring periods from the shallowest well of each site is shown in the upper most panel. The rest of the panels from top to bottom show in sequence, the temporal variability of As concentration in shallow aquifers for Sites F, C, E, G, A, and B. Dissolved As concentrations are plotted as the ratio (C/Cavg) of the concentration at the time of the sampling (C) versus the average concentration of the entire time series (Cavg). In each panel, the bold line always represents the shallowest well of the site.
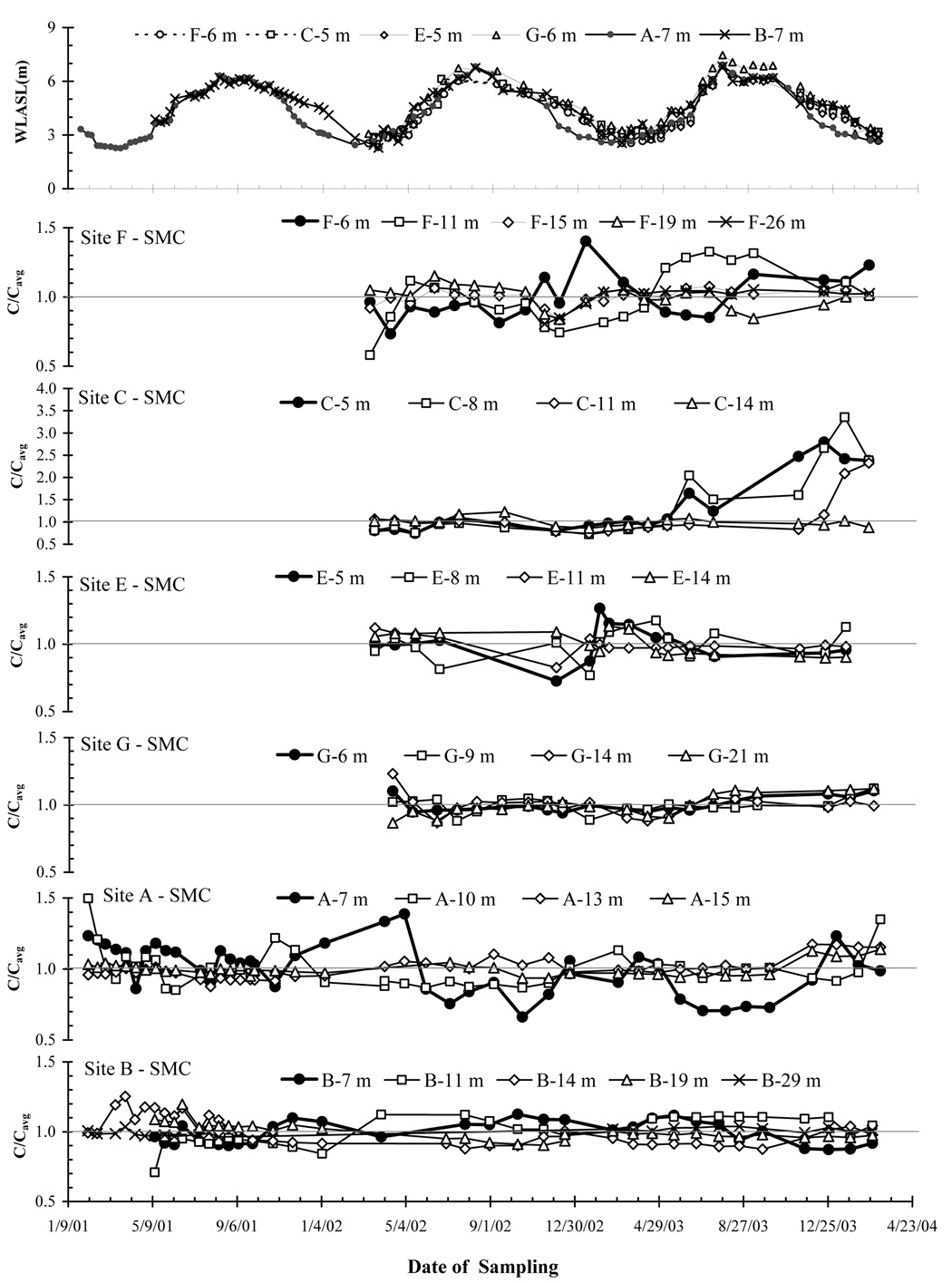
Figure 3.
Temporal variability of the sum of major cations (SMC) in shallow aquifer was shown with the groundwater level fluctuation of the shallowest well (same as in Fig. 2 panel) from top to bottom in sequence for Sites F, C, E, G, A and B. Concentrations of SMC are plotted as the ratio (C/Cavg) of the concentration at the time of the sampling (C) versus the average concentration of the entire time series (Cavg). Similar to Fig. 2, the bold line always represents the shallowest well of the site.
3. RESULTS
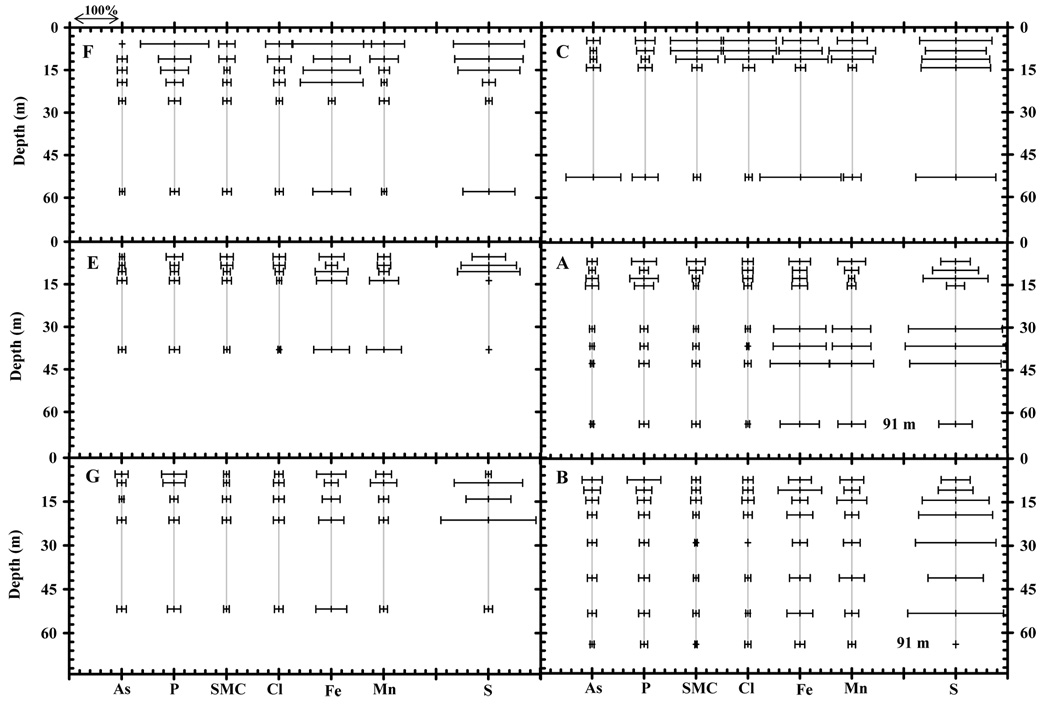
The temporal data of both shallow (< 30 m) and deep (> 30 m) groundwater As and SMC (2[Ca]+2[Mg]+[Na]+[K]) are plotted as the ratio (C/Cavg) of the concentration at the time of the sampling (C) vs. the average concentration of the entire time series (Cavg) for Sites F, C, E, G, A to B (Fig. 2, Fig. 3 and Fig. 4). Similar plots for other constituents are included as supplemental material. The depth profiles of variation in concentration of groundwater constituents including As, P, SMC, Cl, Fe, Mn and SO4 are shown for both shallow and deep aquifers of all 6 sites, with the variation expressed as percent relative standard deviation (%RSD) (Fig. 5). A statistically significant temporal trend of As concentration is observed for 11 out of 37 wells in both the shallow and deep Holocene aquifer (Table 2). A decrease in As concentration over time is observed at 9 wells; an increase is recorded at only 2 wells. Correlation coefficients (R) for As trends in these wells varied from 0.5 to 0.85. The 26 wells that did not show statistically significant trends include most of the shallow and deep wells with a very low As (<10 µg/L) content (Table 1 and Table 2).
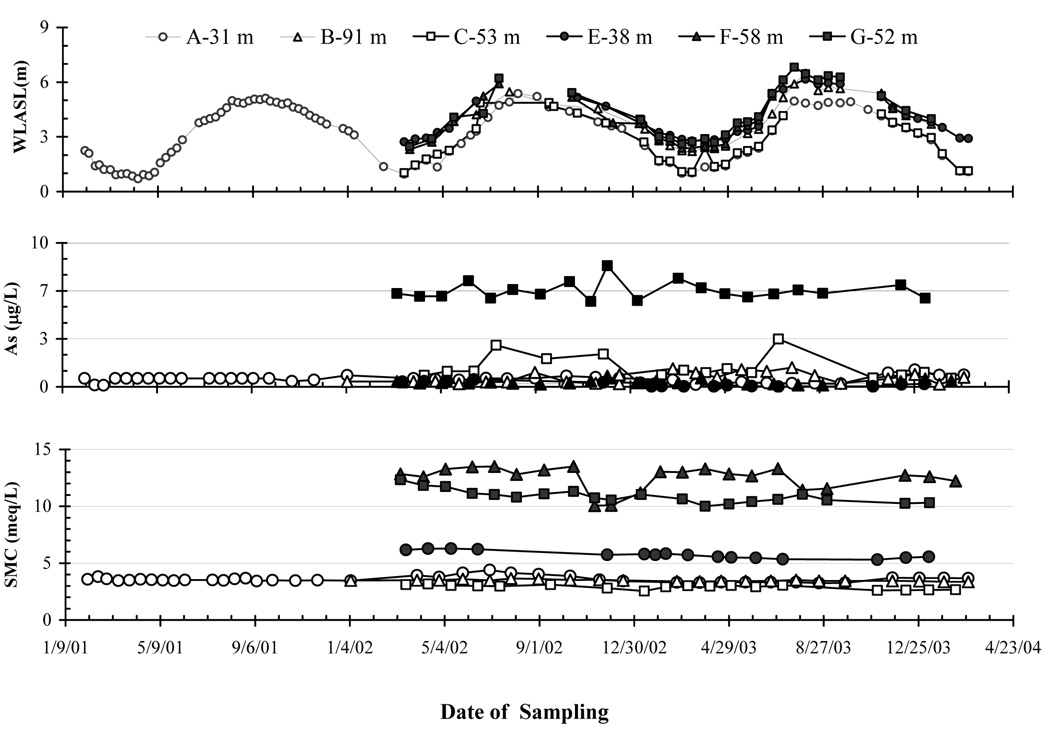
Figure 4.
The temporal variability of As and SMC in deep aquifers spanning the entire monitoring periods are shown with the water level fluctuations in deep aquifers. The upper most panel shows fluctuation of water level above sea level (WLASL) at the deep aquifer wells in six sites. Other two panels displayed the variability of As in µg/L and SMC in meq/L at the deep aquifer wells in all sites.
Figure 5.
Variation of As, P, SMC, Cl, Fe, Mn, S in shallow and deep aquifers of six sites are shown as %RSD. The variations of the two 91m deep wells at Sites A and B are shown right below the 60 m depth and do not represent the actual depths of the wells.
Table-2.
Trends in groundwater chemistry from six well nests of 37 monitoring wells in Araihazar, Bangladesh.
| Depth | 1As | P | SMC | Cl | Fe | Mn | S |
|---|---|---|---|---|---|---|---|
| (m) | (µgL−1 y−1) | (µmoleL−1y−1) | (meqL−1 y−1) | (µmoleL−1 y−1) | |||
| Site A | |||||||
| 27 | −3 ± 2 | −2 | −0.5 | 35 | −9 | −62 | |
| 10 | −1 | −3 | 27 | ||||
| 13 | −5 | 0.3 | 0.1 | 15 | |||
| 215 | −34 ± 10 | −3 | −1 | ||||
| 30 | −3 | −1 | 10 | ||||
| 37 | −0.7 | −8 | 10 | ||||
| 43 | −1.3 | 4 | |||||
| 91 | 11 | ||||||
| Site B | |||||||
| 7 | −4 ± 1.8 | −0.2 | −2 | ||||
| 211 | −41 ± 11 | −5 | 0.9 | 0.3 | 171 | −4 | 65 |
| 14 | - | 7 | −1.0 | −0.5 | −5 | −86 | |
| 19 | −19 ± 12 | −0.4 | −0.2 | 94 | −3 | ||
| 29 | −23 ± 8.3 | 0.1 | |||||
| 241 | −2 ± 0.6 | −6 | 0.2 | 27 | 2 | ||
| 53 | −1 ± 0.5 | −0.2 | 26 | −3 | |||
| 91 | 2 | ||||||
| Site C | |||||||
| 5 | 270 | ||||||
| 8 | |||||||
| 11 | −0.3 | −0.2 | 251 | ||||
| 14 | |||||||
| 53 | −0.2 | −0.1 | |||||
| Site E | |||||||
| 5 | −0.1 | −2 | |||||
| 8 | |||||||
| 11 | 19 ± 7 | −0.3 | 0.0 | 169 | |||
| 14 | −8 | −0.6 | −7 | ||||
| 38 | −1.2 | −3 | |||||
| Site F | |||||||
| 6 | 0.2 | −21 | |||||
| 11 | −1 | 0.3 | |||||
| 15 | −15 | 2 | −8 | ||||
| 19 | −21 ± 12 | −0.2 | −17 | ||||
| 26 | 0.6 | 0.0 | |||||
| 58 | 0.9 | ||||||
| Site G | |||||||
| 36 | 14 ± 12 | 0.2 | −161 | −4 | |||
| 9 | |||||||
| 14 | −3 | 0.0 | −42 | ||||
| 21 | −4 | 0.5 | 0.1 | 3 | |||
| 52 | −0.8 | 0.9 | −1 | −3 | |||
Uncertainties was shown as 95% confidence intervals
Wells that show the decreasing trend for [As] and [P]
Well that does not fall in normal probability plot blank space indicates that trend was not statistically significant (p value is >0.05)
3.1. Water levels
Water levels in shallow and deep monitoring wells varied seasonally from 2–3 m above sea-level (asl) during the dry season to 6–7 m asl during the wet season in both shallow and deep monitoring wells (Fig. 2, Fig. 3 and Fig. 4). Water level data for the period July-October 2003 in both shallow (Fig. 2 and Fig. 3) and deep aquifers (Fig. 4) at low-lying Sites C, E, and G were not recorded because the monitoring wells were not accessible due to pronounced flooding. The fluctuations in water levels tracked each other within ~ 0.3 m in all shallow (< 30 m) monitoring wells over a period of 3 years. In contrast, the deep wells from the Pleistocene deep aquifer could be grouped in two categories, with water levels at fresh water Sites A and C remaining ~ 2 m below water levels at more saline water Sites E, F, and G throughout the seasonal cycle (Fig. 4).
3.2. Chemistry of Shallow Aquifers
3.2.1. Arsenic
Concentrations of As in groundwater sampled from the 26 shallow (< 30 m) monitoring wells spanned three orders of magnitude, from < 1 µg/L at F-6 m to 600 µg/L at A-13 m and B-14 m. Groundwater As concentration generally increased with depth starting from the shallowest monitoring well, peaks at ~ 15 m at Sites A, B, C and E, and at ~ 20 m at Sites F and G, and then declined again towards the deeper part of the shallow aquifer (Fig. 1c).
3.2.1.1. Trends
Overall, the temporal variability of As concentration observed in the 26 shallow monitoring wells was limited (Fig. 5), with a RSD < 19% over a monitoring period of up to 3 years (Table 1). However, linear regression of As concentration as a function of time indicated that there were statistically significant long-term trends (p < 0.05) for 11 wells out of a total of 26 shallow monitoring wells (Table 2). The largest decreases of −19 to −41 µg/L yr−1 were observed at Sites A, B and F for 5 wells containing > 200 µg/L of As. In contrast, monitoring data for one well at Site E and one well at Site G, both containing ~ 150 µg/L of As, showed average increases of +19 and +14 g/L yr−1, respectively.
3.2.1.2. Excursions
In addition to these long-term trends, there were noteworthy reductions of ~ 50% of As in groundwater at three different Sites: C-14 m, G-6 m and A-13 m for shorter duration starting July-August 2002 (Fig. 2). By November 2002, however, As concentration in all three wells had returned to within 10 µg/L of their respective long-term averages. A different situation was observed during the particularly severe flooding season of 2003 when, at well C-5 m and only in this well, a drop from 14 to ~ 9 µg/L in As concentration was sustained over several months but returned to 12 µg/L by Feb. 2004 (Fig. 2).
3.2.1.3. Seasonality
Only a single well A-7 m, the shallowest at Site A, exhibited seasonal variations in groundwater As concentration that were consistent from year to year (Fig. 2). The amplitude of the fluctuations was on the order of ~ 10% around a mean As concentration of 80 µg/L, with lower concentrations corresponding to the wet season (May and October). There may also be a connection between As concentration and water level at B-7 m, but it was limited to 2003 and in this case As concentration was high during the wet season.
3.2.2. Phosphorus
Similar to As, concentrations of phosphorus (P) in shallow groundwater spanned three orders of magnitude: from 0.11 ± 0.07 µmole/L at F-6 m to 108 ± 23 µmole/L at G-9 m (Table 1). The contrast between these two monitoring wells was particularly striking because they are only 400 m apart and their depths are comparable (Fig. 1a). Depth profiles of groundwater P concentration at Sites A, E, and F was broadly similar in shape to their corresponding As concentration profiles, at an average atomic P:As ratio of 11 ± 6 (Fig. 1c). For at least one shallow monitoring well at Sites B, C, and G, groundwater P concentration exceeded 30 µmole/L even though As concentration in the same well water was no higher than ~ 100 µg/L, corresponding to a P:As molar ratio of 105 ± 92.
3.2.2.1. Trends
The fluctuations (%RSD) in groundwater P concentration over time remained < 32 % (Fig. 5), excluding F-6 m where P concentration were particularly low (Table 1). Statistical analyses for 9 shallow wells indicated a small long-term decrease between −1 to −8 µmole/L yr−1. For only 4 wells (A-7 m, A-15 m, B-11 m and B-41 m), however, the decrease in P concentration was accompanied by a detectable decline in As concentration. An increase in P concentration over time of +7 µmole/L yr−1 was recorded at a single well B-14 m (Table 2).
3.2.2.2. Excursions
Along with the As concentration decreases of ~ 50% observed in A-13 m, C-14 m, and G-6 m in July-August 2002, P concentration in the same shallow wells declined by up to 80% in September 2002 before returning to previous levels by November 2002. During the summer flood of 2003 that coincided with a decrease in As concentration in well C-5 m, there was instead a P concentration increase from 27 to 46 µmole/L that extended to the end of the monitoring period.
3.2.2.3. Seasonality
Seasonal fluctuations in P concentration were found in a few shallow wells and also observed to be in phase with As concentration in two shallow wells at Sites A and B. For well A-7 m, P concentrations varied between 10 and 30 µmole/L during the wet and dry season, respectively. There was also a measurable increase in P concentration during the wet season of 2003 in well B-7 m, is consistent with As fluctuation in the same year. Well F-6 m displayed seasonality in P concentration with higher values in the wet seasons, albeit around a low average of 0.1 ± 0.1 µmole/L.
3.2.3. Major Cations and Chloride
Expressed in equivalents, the sum of major cations (SMC), including Na+, K+, Mg+2, and Ca+2, in shallow (< 30 m) monitoring wells spanned an order of magnitude, from 1.6 ± 0.7 meq/L at C-11 m to 12 ± 1.3 meq/L at B-7 m (Table 1). Chloride concentrations in shallow aquifers also spanned about an order of magnitude, from 0.040 ± 0.004 meq/L at F-19 m to 4.3 ± 0.4 meq/L at B-7 m (Table 1).
3.2.3.1. Trends
The temporal variability of the major ion composition of shallow well water was comparable to that of As and P concentration, with %RSDs remaining below 20%, 30% and 30% for SMC, Na and chloride concentrations, respectively (Table 1; Fig. 5). These measures of variability exclude, however, the nearly 3-fold increases in SMC and Cl concentrations in monitoring wells C-5 m, C-8 m, and C-11 m observed at the end of the wet season in 2003 (Fig. 3). Of the total of 26 shallows wells that were monitored, the SMC times-series indicated an statistically significant (p < 0.05) decrease of −0.24 to −1.03 meq/L yr−1 at 7 wells and an increase of +0.11 to +0.88 meq/L yr−1 at 8 other wells (Table 2).
3.2.3.2. Excursions
SMC concentrations did not vary appreciably in the 3 shallow wells where both As and P concentration declined markedly for the period of several months centered on September 2002 (Fig. 2 and Fig. 3). On the other hand, a major salt pulse was observed after the summer flood of 2003 in three shallow wells at Site C, with up to 3 times higher concentrations of SMC and Cl compared to the previous year (Fig. 3). Concentrations of SMC actually already started to rise in wells C-5 m and C-8 m in May 2003 and reached their highest level in November 2003 and January 2004, respectively. Concentrations of SMC also eventually rose in well C-11 m, but only later in January 2004, whereas no marked change in SMC was observed throughout the period at well C-14 m. The increases in SMC reflected largely a rise in dissolved Na in groundwater, from ~ 0.3 meq/L in April 2003 to maxima ranging from 2 to 7 meq/L in subsequent months. The progression of Cl concentrations over the same period in all shallow wells at site C was similar to that of Na, although there was a gap in the Cl time series that extended from February 2002 to October 2003.
3.2.3.3. Seasonality
The concentrations of SMC in well A-7 m, where the clearest seasonal variations in As and P concentration were also detected, were low during the wet season and high during the dry season (Fig. 3). In contrast to some of the other shallow wells, these changes reflected primarily changes in Ca and Mg, with the sum of their concentrations fluctuating between 1.2 and 2.2 meq/L. Although the duration of sampling was relatively short at F-6 m, SMC and Cl both appeared to be systematically elevated in this well during the dry season (Fig. 3), when P concentration was particularly low (As concentration remained < 0.5 µg/L).
3.2.4 Iron, Manganese and Sulfur
Of all the constituents of groundwater that were quantified, the concentrations of the redox-sensitive elements Fe, Mn and S varied the most spatially and temporally (Fig. 5 and Table 1). Dissolved Fe concentrations spanned nearly three orders of magnitude, from 2.4 ± 2 µmole/L at F-6 m to 1694 ± 704 µmole/L at C-8 m (Table 1). Dissolved S concentrations also ranged over three orders of magnitude, from 0.3 ± 0.02 µmole/L at F-26 m to 677 ± 189 µmole/L at B-7 m. Mn concentrations were not quite as variable and ranged from 2.6 ± 1 µmole/L at F-6 m to 96 ± 6 µmole/L at A-13 m. There was no consistent relationship between depth profiles of Fe or Mn at each site with the corresponding profiles of As or P concentration. Groundwater SO4 concentration, however, generally decreased with depth at all sites.
3.2.4.1. Trends
There is no systematic relationship between trends in Fe, Mn, S, and As (Table 2).
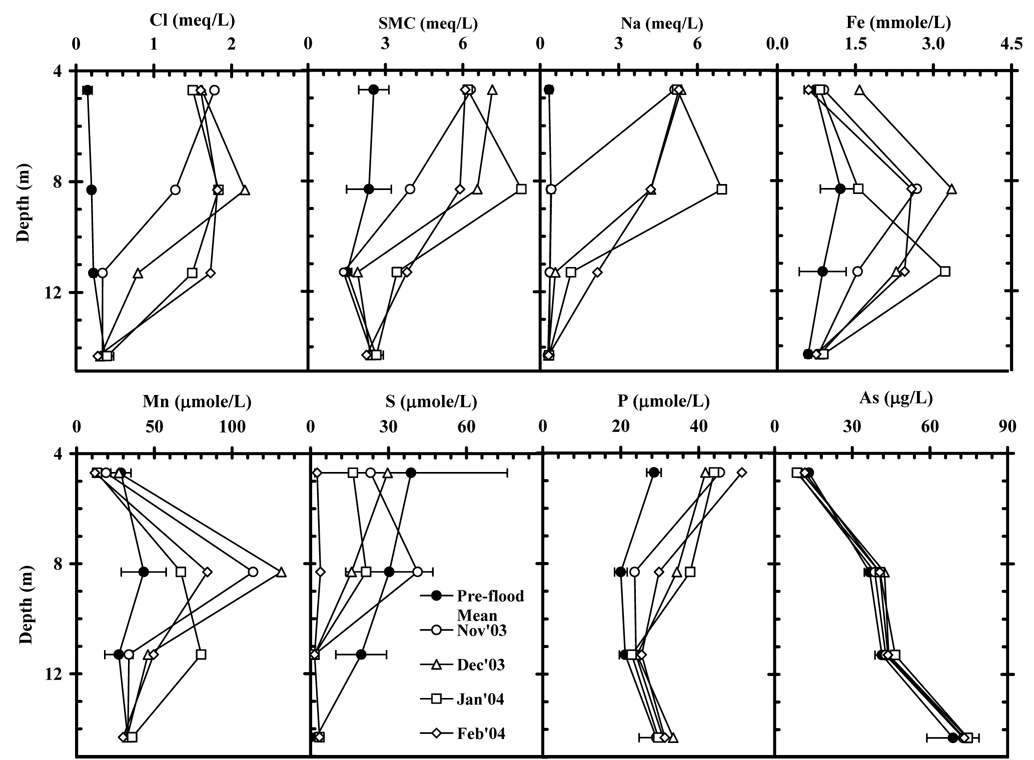
3.2.4.2. Excursions
At Site C, three shallow wells that were impacted by the salt pulse during the summer 2003 flood, showed an increase of Fe concentration and Mn concentration but a decrease of SO4 concentration after the summer (Fig. 6). Fe concentration increased by a factor of 2 to 4; Mn concentration increased by a factor of 3 (Fig. 6). Groundwater SO4 concentration decreased from 129, 72 and 16 µmole/L to very low values of 3, 4 and 2 µmole/L in Feb., 2004 (Fig. 6).
Figure 6.
Depth profiles of Cl, SMC, Na, Fe, Mn, S, P, and As of 4 shallow wells at Site C. Average pre-flood profiles were compared with 4 consecutive post-flood profiles after the flood in the summer of 2003. The black solid circles indicate the average concentration prior to the 2003 flood (March 2002 to July 2003) and error bar represents the standard deviation from the average in pre-flooding period.
3.2.4.3. Seasonality
The seasonal fluctuations in groundwater composition observed at well A-7 m over a 3 year period were systematic for As, P, SMC, Fe and Mn. In parallel with changes in concentration of SMC, As and P, concentrations of Fe and Mn rose during the dry season and dropped during the wet season. These variations were not accompanied by a consistent seasonal pattern for S in well A-7 m. In well F-6 m, a pronounced seasonality in groundwater constituents other than As was observed for Fe, Mn and P with low values found in the wet seasons.
3.3. Deep Aquifers
In contrast to shallow aquifers of Araihazar, the overall composition of deep (> 30 m) aquifers was remarkably stable over time except for Fe and SO4 concentration (Fig. 5). In a sense, the time series data for the deep wells provide an independent measure of the quality of the data because all samples were collected, preserved, and analyzed in exactly the same fashion. At all but Site B (Fig. 1c), these deep wells tapped sandy aquifers with a characteristic orange-brown color that is typically associated with low sediment As concentrations (Horneman et al., 2004; Zheng et al., 2005). At Sites A, C, E, F, and G, the redox potential (ORP) of groundwater was less negative than in shallow aquifers (Table 1). Conductivity measurements and SMC data indicated that deep groundwater was fresher than in shallow aquifers at Sites A, B and C, but saltier at Sites E, F and G (Table 1). The elevated conductivity of deep groundwater at Sites E, F and G reflected primarily high concentrations of Na and Cl (Table 1).
The SMC concentration in the deepest well at all 6 sites was the most stable property measured over the monitoring period, with RSDs usually < 9% (Table 1 and Fig. 5). Concentrations of As in the deepest wells at Sites A, B, C, E, and F, were stable and low at 1 ± 0.7 µg/L. The levels of As concentration were somewhat higher at B-41 m (21 ± 2 µg/L As), B-53 m (17 ± 1 µg/L As); and G-52 m (7 ± 0.6 µg/L). There was a statistically significant decline in As concentration over time of −2 and −1 µg/L yr−1 at B-41 m and B-53 m, respectively (Table 2). Concentrations of P ranged from 4 to 10 µmole/L in the deepest well from all 6 sites and varied within 30% (Fig. 5). Little systematic variation over time was observed in the deep aquifers, with the exception of a large increase in Fe and SO4 concentration in C-53 m that lasted from May 2002 to July 2003. In addition, there was steady rise in SO4 concentration from <0.003 to 37 µmole/L in the deep aquifer at site A over the entire monitoring period (Table 2).
4. DISCUSSION
4.1. Fluctuations in Major Ion Compositions and Groundwater Age
Groundwater ages in deep aquifers at sites A and B are 10–1000 times higher than in the corresponding shallow aquifers of Araihazar (Zheng et al., 2005; Stute et al., 2007). Radiocarbon ages of dissolved inorganic carbon from wells C-53 m, F-58 m, and G-52 m were 10700 ± 55, 6240 ± 30, and 3620 ± 35 years, respectively (Dhar, 2006). These observations show that the deep aquifers in Araihazar contain groundwater that was recharged centuries to thousands of years ago.
Not surprisingly, the variability of groundwater composition for all major ion constituents that were quantified was lower (i.e., generally < 10 % for SMC and Cl; Table 1) than in any of the shallow aquifers. The age of deep groundwater is higher and, therefore, flow lines are likely to be longer, and dispersive mixing may have smoothed out any initial temporal fluctuations.
The shallow aquifers at the 6 sites that were monitored over a period of 2–3 years were divided into 2 groups on the basis of 3H/3He ages of groundwater collected from the same set of wells (Stute et al., 2007). In one group comprised of Sites C and F, groundwater 3H/3He ages remain <5 years down to a depth of ~14 m, whereas 3H/3He ages already exceed 20 years at the same depth at Sites A, B, E, and G. Sites C and F are also the only 2 sites where the 3H/3He age of groundwater from the shallowest well was < 1 year. The rapid recharge of shallow aquifers at Sites C and F indicated by the 3H-3He data is probably linked to the upward extension, almost to the surface, of sandy deposits at these two sites (van Geen et al., 2006; Aziz et al., in revision; Weinman et al., in press). At Sites A, B, E, and G, instead, recharge appears to be limited by the presence of a thicker layer of silt or clay that capped the local sandy aquifers. These hydrogeological constraints are used next to interpret the behavior of relatively conservative constituents of groundwater, i.e., SMC, Na, and Cl.
It is probably not a coincidence that variations in the composition of groundwater e.g. SMC, Na, and Cl, are particularly pronounced (up to 40%) and go beyond in the shallow monitoring wells at the Site C where sandy deposits extend to the surface (Fig. 3 and Table 1). What is less clear is to what extent these variations reflect vertical advection of recently recharged water, lateral motion of groundwater that is heterogeneous in composition, or a combination of both. At Site C, a progressive deepening of a front containing elevated Na and Cl levels during and following the 2003 flood is consistent with at least some vertical penetration to ~ 11 m (Fig. 6). At present, we cannot rule out the possibility that the changes in NaCl were due to leakage of flood water along the well casings, although that seemed unlikely given the systematic pattern of the multi-elements depth profiles obtained at different time post-flood (Fig. 6). Elevated concentrations of NaCl have previously been linked to human waste because large quantities of salt are mobilized and added to flood water in densely populated areas where sanitation is limited to pit latrines (Ahmed et al., 2004). A sizable trench (~ 10 m wide) that presumably could collect latrine runoff is located next to Site C and separates a vegetable field from the village.
At site F, despite similar depositional settings, the seasonal cycle of variations in Na (as SMC) and Cl concentrations is considerably muted compared to Site C and lacks the response to the 2003 flood (Fig. 3). Site F is located in a village that is built up to higher elevation than the surrounding area and has no such trench or pond next to it. This may be why Na and Cl concentrations do not vary as much as Site C.
Some fluctuations in major ion concentrations were also observed at clay/silt covered Sites A and B. The most notable variation linked to seasonal water level fluctuation was observed only in shallowest wells (Fig. 3, Fig. 5 and Table 1). The 3H/3He ages of the groundwaters from shallowest wells at these sites are also <5 years, although the groundwater age rapidly increases beyond the depth of these wells (Stute et al., 2007). Given the higher ages, and the capping of shallow aquifers around Sites A and B by relatively impermeable surface sediment, it seemed more plausible to attribute the variations in groundwater composition to lateral motion of groundwater of heterogeneous composition. In a 3–dimensional groundwater flow model developed for Araihazar, reversal of lateral flow directions between wet-dry seasons are found, in addition to flow path oscillations driven by the seasonal variation of the groundwater table (Horneman, 2006). The oscillation and reversal of flow implies that older groundwater with different compositions can mix on seasonal time scale without influencing the residence time. Seasonal patterns in major ion concentrations similar to the one observed in the shallowest well at Site A, though not necessarily in phase, were previously reported for two shallow existing wells in entirely different sampling locations in Araihazar (Cheng et al., 2005). Whereas maxima and minima in major ion concentrations for shallow wells in the area often do not coincide, the seasonal pacing of the fluctuations might still be linked to variations in water level of the nearby stream (Fig. 2, Fig. 3 and Fig. 4) that in turn modulate an oscillating lateral flow field.
In contrast to the shallowest wells at Sites A, B, C, and F, fluctuations in groundwater composition were limited at G-6 m (Fig. 3). At this location, the 3H/3He age of groundwater in even the shallowest wells is ≥10 years (Stute et al., 2007). Variations at E-5 m, also with 3H/3He age of ~ 10 yrs, on the other hand, were comparable to Sites A and B but clearly not as stable as at G-6 m (Fig. 3).
In summary, the major ion compositions of deep aquifer groundwater is much less variable than the shallow aquifer groundwater due to a much longer residence time. Within the shallow aquifer, flooding can sometimes but not always alter major ion compositions at locations where sandy sediment extended to the surface. More importantly, fluctuation of major ion occurred on shorter time scale, and sometimes seasonal time scale, for shallow groundwater with ages > 10 years, consistent with a flow regime with oscillation of horizontal flow driven by fluctuating seasonal hydraulic gradient.
4.2. Decoupling Between Variations in Redox-Sensitive Constituents and As
Perhaps the greatest surprise that resulted from this study is that variations in groundwater As concentrations were considerably muted in comparison with other redox-sensitive constituents, such as Fe, Mn and SO4 in many wells from multiple sites, including both the low-As deep aquifers and high-As shallow aquifers (Fig. 5). Considerable variations of Fe and SO4, and to a lesser extent Mn, were observed in the deep aquifer where major ions and As remained stable (Fig. 5). In the case of deep aquifers, such decoupling is consistent with low concentrations of mobilizable As in deep aquifer sediment (Zheng et al., 2005). In such conditions, even if microbially-mediated reduction of Fe oxyhydroxides occurred, the release of Fe could be decoupled from As due to either re-adsorption or the lack of a pool of mobilizable As in the sediment (van Geen et al., 2004).
Like the deep aquifer, the temporal patterns of concentrations of As, vs. Fe, Mn, and SO4 can also be decoupled in shallow aquifers. One example of such decoupling is the three shallow monitoring wells (C-5 m, C-8 m and C-11 m) at sandy Site C where major ion concentrations and redox-sensitive elements were strongly affected by the 2003 flood whereas the depth profiles of As concentrations did not change (Fig. 6). Only in the shallowest well C-5 m was the concentration of As affected by the flood evident when the relative change was examined (Fig. 2), although the As level returned to pre-flood level approximately 6-months after flooding both in relative change (Fig. 2) and absolute level (Fig. 6). Whereas slight decrease in As concentration is consistent with dilution from flood water, it is not what might have been expected from the dissolution of mineral oxides suggested by the rise in Fe and Mn concentrations (Fig. 6). The ability of the shallow aquifer at Site C to maintain a relative constant concentrations of groundwater As is not surprising in light of recent finding that partitioning between solute and solid As in shallow aquifer has a fairly constant coefficient based on a regional study (van Geen et al., in press). In this scenario, groundwater is rapidly equilibrated with sediment such that it is the sediment As level that controls the groundwater As concentration. Such decoupling was also evident at F-6 m (Fig. 2, Table 1) where consistently low As concentrations were observed over the monitoring period even though both major ions and redox-sensitive elements exhibited a strong seasonal pattern (data shown in supplementary figure). What is the implication of limited temporal variation of As despite the significant variation of redox sensitive ions such as Fe and Mn? Considered as a whole, not only it reinforces the notion that there is significant decoupling between the mobilization of As and the redox state of an aquifer (Horneman et al., 2004; van Geen et al., 2004; Polizzotto et al., 2005; van Geen et al., 2006), but also it supports a rapid equilibrium between solute and solid As in the aquifer (van Geen et al., in press). Finally, strong temporal decoupling between As and Fe, Mn and S in the shallowest wells (5–7 m) from our sites imply that the chemical compositions of recharge water could be highly heterogeneous both spatially and highly variable temporally.
4.3. Trends in groundwater As in the shallow aquifer
Stute et al. (2007) have pointed out on the basis of paired measurement in groundwater from the same set of shallow monitoring wells (< 20 m) that there is a surprisingly linear relationship between As concentrations and groundwater age across a wide range of settings (Table 1). It is noted that the deepest wells of most sites including A-15 m, B-19 m, E-14 m, F-19 m, G-14 m are affected by mixing, therefore were not included in regression analysis (Stute et al. 2007). The simplest interpretation of this linear relationship is that the release rate of As is relatively constant at ~ 20 µg/L/year in the shallow aquifers of Araihazar. This rate appears to be insensitive to, and therefore decoupled from, the redox state of the aquifer. This steady release of As under a wide range of conditions is consistent with the observation that concentrations of major ions or redox-sensitive constituents are not necessarily linked to variations in dissolved As temporally. Taken together, these observations imply that the spatial heterogeneity of As concentration in shallow aquifers is considerably less than that of major ions or redox-sensitive constituents in groundwater. However, the reasons for the inferred heterogeneity in major ion and redox sensitive constituents relative to As remain unclear.
If groundwater As concentrations remain constant over time, then a steady rate of As release (e.g. source) would require that As is either discharged from the aquifer or immobilized (e.g. sink) to sediment for maintaining a steady state. If groundwater As increases over time, then there must be a surplus of As, or vice versa if groundwater As decreases over time. We recognize that our time series data span only 2 to 3 years and it may therefore be premature to draw any firm conclusions. But, systematic differences of trends are observed at different sites. At Sites F and C where sandy sediment extends to surface, there is little trend in groundwater As (Table 2), suggesting no net loss or gain of As. Although F-19 m showed a decline of 21 ± 12 µg/L yr−1, it was below a silty layer with lower hydraulic conductivity and thus probably more akin to old meander environment at Sites A and B (Fig. 1a). Clay/silt covered Sites A and B located in older meanders >6000 yrs old (Weinman et al., in press) showed a systematic decline over time of groundwater As in 6 out of 9 shallow wells (Table 2). This implies that such settings presently experience a net loss of As. Similarly clay/silt surface cover Sites E and G located in a young flood plain <4000 years old (Weinman et al., in press) showed increase of As over time in 2 out of 8 shallow wells. This implies that such setting has a net gain of As.
Why does some aquifer system gain As, some lose As and yet some with no net change? The reasons for much of this remain unclear, but sites with net loss of As located on older meanders is consistent with the notion that flushing over time may gradually decrease the inventory of As in the Holocene aquifer but that process will take thousands of years if not longer (McArthur et al., 2001; Stute et al., 2007; van Geen et al., in press). The increasing trends observed in Sites E and G suggest that near surface mobilization and subsequent transport (Polizzotto et al. 2005) may have provided a fresh source of As to the aquifer in very young flood plain.
4.4 Coupled and Decoupled Behavior of P and As
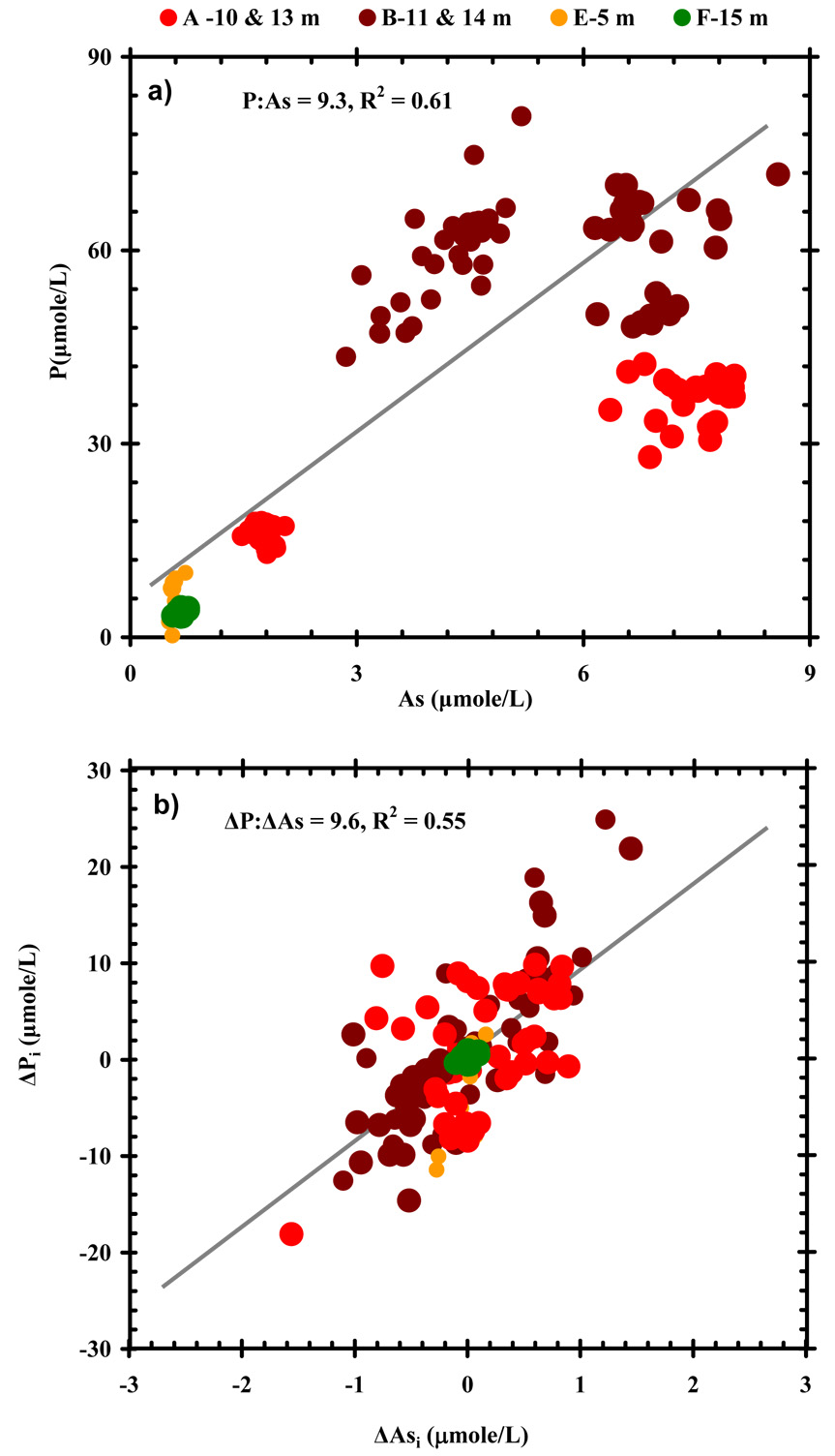
Arsenic in its oxidized form is a chemical analog for phosphate. For this reason, parallel behaviors or interactions between these two constituents are frequently invoked. Arsenic in aquifers of Araihazar, however, is predominantly present in groundwater in the As(III) form (Zheng et al., 2004). In older groundwater (> 5 years), As was observed to be co-variance of P in time series data (Fig. 7). When the deviation of P from the average P concentrations over time (ΔP) was plotted over the deviation of As from the average As concentrations over time (ΔAs), a ΔP: ΔAs atomic ratio of 9.6 was found (R2 = 0.55, n= 181, Fig. 7b), nearly identical to the ratio (9.3) established independently by using P:As ratio in these wells directly (Fig. 7a). Together these suggest a similar release mechanism for As and P in the older (> 5 years) shallow groundwaters.
Figure 7.
a) Concentrations of groundwater P vs. As for the shallow monitoring wells with 3H/3He ages of > 5 years The data are include for the entire time period (2–3 yrs). b) The deviation of P concentration above or below the mean value (ΔPi=Pi−Pavg) vs. the deviation of As concentration (ΔAsi=Asi−Asavg) of the same wells as in panel a).
Why then is there more P relative to As in relatively young (< 5 yr) shallow groundwaters? P concentrations are high not only in young water at sandy Site C (but not F), but also in young water tapped by the shallowest well at Sites A and B (Table 1). One possibility is that P is supplied from surface water recharge whereas As is not. For instance, P concentrations increased by ~ 20 µmole/L at both C-5 m and C-8 m after the 2003 flood (Fig. 6). These observations confirm that As was probably not derived from recharged water but that P was. Alternatively, the increase of P post-flood at both C-5 m and C-8 m result from chemical reactions that liberate P from sediment, but those reactions did not influence As level.
5. CONCLUSIONS
Groundwater age is a key variable influencing the temporal variability of groundwater chemistry in shallow Holocene aquifers and deeper aquifers of Araihazar. The principal findings of the study are:
In shallow and young (<3.5 years) groundwater, the variability of As concentrations over 2–3 years is much more muted when compared to that of major ions and redox sensitive constituents. The decoupling between As and redox sensitive constituents under such conditions reflects the greater availability and mobility of Fe in the shallow sediment compared to As.
The concentration of As in shallow and older groundwater (>3.5 years) as well as deep groundwater in deeper aquifers that is thousands of years old, is stable over time despite having considerable variability of redox sensitive constituents. The reason for this decoupling remains unclear but may result from relatively constant solute to solid As partitioning observed in Bangladesh.
Trends in groundwater As concentration over the entire monitoring period of 2–3 years may be governed by sediment geology and its effect on the groundwater flow regime. Older sedimentary aquifers (6000–10,000 yrs) is experiencing a net loss of As, consistent with gradual flushing of As from the aquifer.
Supplementary Material
Acknowledgments
Funding for this study was provided by the USEPA-NIEHS/Superfund Basic Research Program through grant 1 P42 ES10349. This is LDEO contribution xxxx.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- AAN. Interim Report of the Research at Samta Village. AAN (Asia Arsenic Network, Japan), Research Group of Applied Geology and DOEH-NIPSOM, (Department of Occupational & Environmental Health, National Institute of Preventive & Social Medicine, Bangladesh); 1999. Arsenic contamination of groundwater Bangladesh. [Google Scholar]
- Ahmed KM, Bhattacharya P, Hasan MA, Akhter SH, Mahbub Alam SM, Hossain Bhuyian MA, Imam MB, Khan AA, Sracek O. Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: an overview. Applied Geochemistry. 2004;19:181–200. [Google Scholar]
- Aziz Z, van Geen A, Versteeg R, Horneman A, Zheng Y, Goodbred SL, Jr, Steckler M, Stute M, Weinman B, Gavrieli I, Shamsudduha M, Hoque A, Ahmed KM. Impact of local recharge in arsenic concentrations in shallow aquifers inferred from electromagnetic conductivity of soils in Araihazar, Bangladesh. Water Resources Research. in revision. [Google Scholar]
- Berg M, Tran HC, Nguyen TC, Pham HV, Schertenleib R, Giger W. Arsenic contamination of ground and drinking water in Vietnam: a human health threat. Environmental Science and Technology. 2001;35:2621–2626. doi: 10.1021/es010027y. [DOI] [PubMed] [Google Scholar]
- BGS & DPHE. Arsenic contamination of groundwater in Bangladesh. In: Kinniburgh DG, Smedley PL, editors. British Geological survey Technical Report WC/00/19. British Geological Survey; 2001. [Google Scholar]
- Chakraborti D, Mukherjee A, Sengupta MK, Hossian MA, Ahamed S, Lodh D, Das B, Nayek B, Rahman MM, Saha KC, Mukherjee SC, Pati S, Dutta RN, Chatterjee G. Chronic arsenic poisoning in the Asian countries: technical issues to be addressed to combat the situation. Taiyuan, China: UNICEF Inter-regional Conference on Water Quality-Arsenic mitigation; 2004. [Google Scholar]
- Chakraborti D, Rahman MM, Paul K, Chowdhury UK, Sengupta MK, Lodh D, Chanda CR, Saha KC, Mukherjee SC. Arsenic calamity in the Indian subcontinent What lessons have been learned? Talanta. 2002;58:3–22. doi: 10.1016/s0039-9140(02)00270-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Das D, Mandal BK, Chowdhury TR, Samanta G, Chakraborti D. Arsenic in ground-water in 6 districts of West Bengal, India-The biggest arsenic calamity in the world. A. Arsenic species in drinking water and urine of the affected people. Analyst. 1995;120:643–650. doi: 10.1039/an9952000917. [DOI] [PubMed] [Google Scholar]
- Cheng Z, van Geen A, Ahmed KM, Seddique AA. Response to comment on “Limited temporal variability of tube-well arsenic in Bangladesh: results from a 3-year monitoring effort”. Environmental Science and Technology. 2006;39(14):5503–5504. [Google Scholar]
- Cheng Z, van Geen A, Seddique AA, Ahmed KM. Limited temporal variability of arsenic concentrations in 20 wells monitored for 3 years in Araihazar, Bangladesh. Environmental Science and Technology. 2005;39:4759–4766. doi: 10.1021/es048065f. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Zheng Y, Mortlock R, van Geen A. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Analytical and Bioanalytical Chemistry. 2004;379(3):512–518. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- Dhar RK. Ph.D. thesis. New York: City University of New York; 2006. Arsenic and groundwater properties of Araihazar, Bangladesh. [Google Scholar]
- Dhar RK, Zheng Y, Rubenstone P, van Geen A. A rapid colorimetric method for measuring arsenic concentration in groundwater. Analytica Chimica Acta. 2004;526:203–209. [Google Scholar]
- Frost F, Frank D, Pierson K, Woodruff L, Raasina B, Davis R, Davies J. A seasonal study of arsenic in groundwater, Snohomish County, Washington, USA. Environmental Geochemistry and Health. 1993;15(4):209–214. doi: 10.1007/BF00146744. [DOI] [PubMed] [Google Scholar]
- Harvey CF, Swartz CH, Badruzzaman ABM. Arsenic mobility and groundwater extraction in Bangladesh. Science. 2002;2998:1602. doi: 10.1126/science.1076978. [DOI] [PubMed] [Google Scholar]
- Horneman A. Ph.D. dissertation. New York: Columbia University; 2006. Geochemical and hydrological studies of arsenic in Bangladesh groundwater. [Google Scholar]
- Horneman A, van Geen A, Kent D, Mathe PE, Zheng Y, Dhar RK, O'Connell S, Hoque MA, Aziz Z, Shamsudduha M, Seddique A, Ahmed KM. Decoupling of arsenic and iron release to Bangladesh groundwater under reducing conditions. Part I: Evidence from sediment profiles. Geochimica Cosmochimica Acta. 2004;68(17):3459–3473. [Google Scholar]
- Klump S, Kipfer R, Cirpka OA, Harvey CF, Brennwald MS, Ashfaque KN, Badruzzaman ABM, Hug SJ, Imboden DM. Groundwater dynamics and arsenic mobilization in Bangladesh assessed using noble gases and tritium. Environmental Science and Technology. 2006;40:243–250. doi: 10.1021/es051284w. [DOI] [PubMed] [Google Scholar]
- McArthur JM, Ravenscroft P, Safiullah S, Thirlwall MF. Arsenic in groundwater: testing pollution mechanism for sedimentary aquifer in Bangladesh. Water Resources Research. 2001;37(1):109–117. [Google Scholar]
- Opar A, Pfaff A, Seddique AA, Ahmed KM, Graziano JH, van Geen A. Responses of 6500 house-holds to arsenic mitigation in Araihazar, Bangladesh. Health and Place. 2007;13:164–172. doi: 10.1016/j.healthplace.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Polizzotto ML, Harvey CF, Sutton SR, Fendorf S. Processes conducive to the release and transport of arsenic into aquifers of Bangladesh. PNAS. 2005;102:18819–18823. doi: 10.1073/pnas.0509539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer RE. Molybdenum blue applied to arsenic and phosphorus determination in fluoride- and silica-rich geothermal waters. Environmental Science and Technology. 1980;14(12):1475–1481. doi: 10.1021/es60172a003. [DOI] [PubMed] [Google Scholar]
- Steinmaus CM, Yuan Y, Smith AH. The temporal stability of arsenic concentrations in well water in western Nevada. Environmental Research. 2005;99:164–168. doi: 10.1016/j.envres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Stute M, Zheng Y, Schlosser P, Horneman A, Dhar RK, Hoque MA, Seddique AA, Shamsudduha M, Ahmed KM, van Geen A. Hydrological Control of As Concentrations in Bangladesh Groundwater. Water Resources Research 43, W09417. 2007 [Google Scholar]
- van Geen A, Zheng Y, Goodbred SL, Jr, Horneman A, Aziz Z, Cheng Z, Stute M, Mailloux B, Weinman B, Hoque MA, Seddique, Hossain MS, Chowdhury SH, Ahmed KM. Flushing history as a hydrogeological control on the regional distribution of arsenic in shallow groundwater of the Bengal basin. Environmental Science and Technology. 2008 doi: 10.1021/es702316k. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geen A, Cheng Z, Jia Q, Seddique AA, Rahman MW, Rahman MM, Ahmed KM. Monitoring 51 deep community wells in Araihazar, Bangladesh, for up to 5 years: Implications for arsenic mitigation. Journal of Environmental Science and Health. 2007;42:1729–1740. doi: 10.1080/10934520701564236. [DOI] [PubMed] [Google Scholar]
- van Geen A, Zheng Y, Cheng Z, Aziz Z, Horneman A, Dhar RK, Mailloux B, Stute M, Weinman B, Goodbred S, Seddique AA, Hoque A, Ahmed KM. A transect of groundwater and sediment properties in Araihazar, Bangladesh: Further evidence of decoupling between As and Fe mobilization. Chemical Geology. 2006;228(1–3):85–96. [Google Scholar]
- van Geen A, Cheng Z, Seddique AA, Hoque A, Gelman A, Graziano JH, Ahsan H, Parvez F, Ahmed KM. Reliability of a commercial kit to test groundwater arsenic in Bangladesh. Environmental Science and Technology. 2005;39:299–303. [PubMed] [Google Scholar]
- van Geen A, Rose J, Thoral S, Garnier JM, Zheng Y, Bottero JY. Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions. Part II: Evidence from sediment incubations. Geochimica Cosmochimica Acta. 2004;68:3475–3486. [Google Scholar]
- van Geen A, Zheng Y, Versteeg V, Stute M, Horneman A, Dhar R, Steckler M, Gelman A, Small A, Ahsan H, Graziano J, Hussain I, Ahmed KM. Spatial variability of arsenic in 6000 contiguous tube wells of Araihazar, Bangladesh. Water Resources Research. 2003;39(6):1140. [Google Scholar]
- van Geen A, Ahsan H, Horneman A, Dhar RK, Zheng Y, Hussain I, Ahmed KM, Gelman A, Stute M, Simpson HJ, Wallace S, Small C, Parvez F, Slavkovich V, LoIacono NJ, Becker M, Cheng Z, Momotaj H, Shahnewaz M, Seddique AA, Graziano JH. Promotion of well-switching to mitigate the current arsenic crisis in Bangladesh. Bulletin of the World Health Organization. 2002;80:732–737. [PMC free article] [PubMed] [Google Scholar]
- Weinman B, Goodbred SL, Jr, Zheng Y, Aziz Z, Singhvi AK, Nagar YC, Steckler M, van Geen A. Contributions of floodplain stratigraphy and evolution to the spatial patterns of groundwater arsenic in Araihazar, Bangladesh. Geological Society of America Bulletin. 2008 In press. [Google Scholar]
- Yu W, Harvey CM, Harvey CF. Arsenic in groundwater in Bangladesh: A geostatistical and epidemiological framework for evaluating health effects and potential remedies. Water Resources Research. 2003;39(6):1146. [Google Scholar]
- Zheng Y, van Geen A, Stute M, Dhar R, Mo Z, Cheng Z, Horneman A, Gavrieli I, Simpson HJ, Versteeg R. Geochemical and hydrogeological contrasts between shallow and deeper aquifers in two villages of Araihazar, Bangladesh: Implications for deeper aquifers as drinking water sources. Geochimica Cosmochimica Acta. 2005;69(22):5203. [Google Scholar]
- Zheng Y, Stute M, van Geen A, Gavrieli I, Dhar R, Simpson HJ, Schlosser P, Ahmed KM. Redox control of arsenic mobilization in Bangladesh groundwater. Applied Geochemistry. 2004;19:201–214. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.