Summary
Podosomes are self-organized dynamic actin-containing structures that adhere to the extracellular matrix via integrins [1–5]. Yet it is not clear what regulates podosome dynamics and whether podosomes can function as direct mechanosensors like focal adhesions [6–9]. We show here that myosin IIs form circular structures outside and at the podosome actin ring to regulate podosome dynamics. Inhibiting myosin II-dependent tension dissipated podosome actin rings before dissipating the myosin ring structure. As podosome rings changed size or shape, tractions underneath the podosomes were exerted onto the substrate, which were abolished when myosin light chain activity was inhibited. The magnitudes of tractions were comparable to those generated underneath focal adhesions and increased with substrate stiffness. The dynamics of podosomes and of focal adhesions were different. Torsional tractions underneath the podosome rings were generated with rotations of podosome rings in a non-motile and non-rotating cell, suggesting a unique feature of these circular structures. Stresses applied via integrins at the apical surface directly displaced podosomes near the basal surface. Stress-induced podosome displacements increased nonlinearly with applied stresses. Our results suggest that podosomes are dynamic mechanosensors in which interactions of myosin tension and actin dynamics are crucial in regulating these self-organized structures in living cells.
Keywords: integrins, myosin, prestress, actin, mechanotransduction, traction
Results and Discussion
It is well established that actins play important roles of forming the core of the podosomes [3]. However, the role of myosin motor proteins is less clear. To determine the role of myosins, we treated the cells with blebbistatin, a specific myosin inhibitor. Within 8 min of blebbistatin treatment, podosome rings (a superstructure consisting of numerous individual podosomes, very dynamic (life span of 2–12 minutes) and small dot-like adhesions (~0.5–1 µm)) disappeared (Figure S1A). Similarly, ML7, an inhibitor of myosin light chain kinase of myosin II, inhibited podosome large bands within 8 min treatment (Figure S1B). Furthermore, inhibiting Rho-associated kinase (ROCK) with Y27632 abolished podosome rings within 20 min (Figure S1C). Importantly, no de novo formation of podosome rings was observed once they were inhibited by one of these drugs. These results suggest that active myosin IIs are necessary for the appropriate ring-like or band-like structures of podosomes.
When the cell was treated with Jasplakinolide (0.5 µM), an actin polymerizer and stabilizer, the dynamic feature of the podosome rings was abolished (Figure S1D). No apparent changes in the podosome rings were observed up to 40 min after the drug treatment, although there was a slight decrease in cell projected area (Figure S1D). In contrast, podosome rings in control conditions undergo rapid dynamic shape changes within the 40-min period [5]. This result suggests that podosome ring dynamics is tightly controlled by the dynamics of the actins, consistent with previous published results [5].
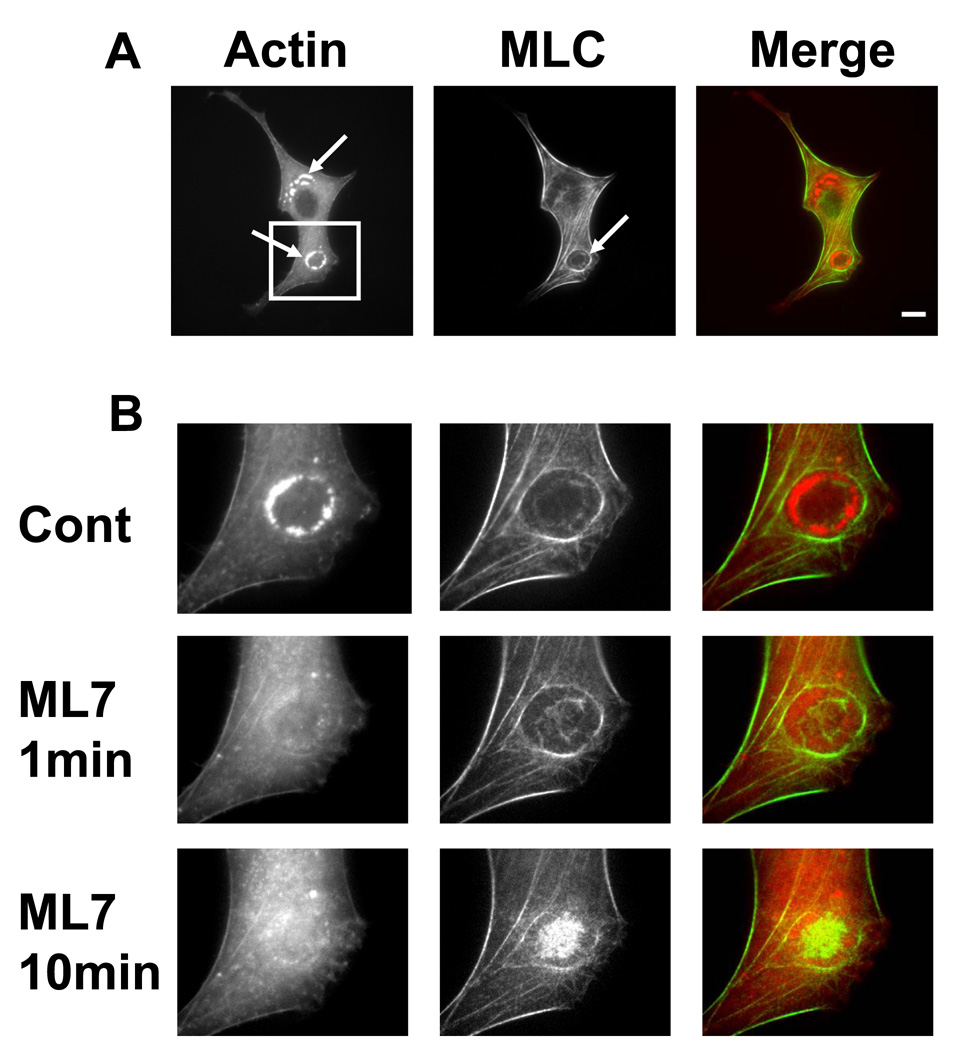
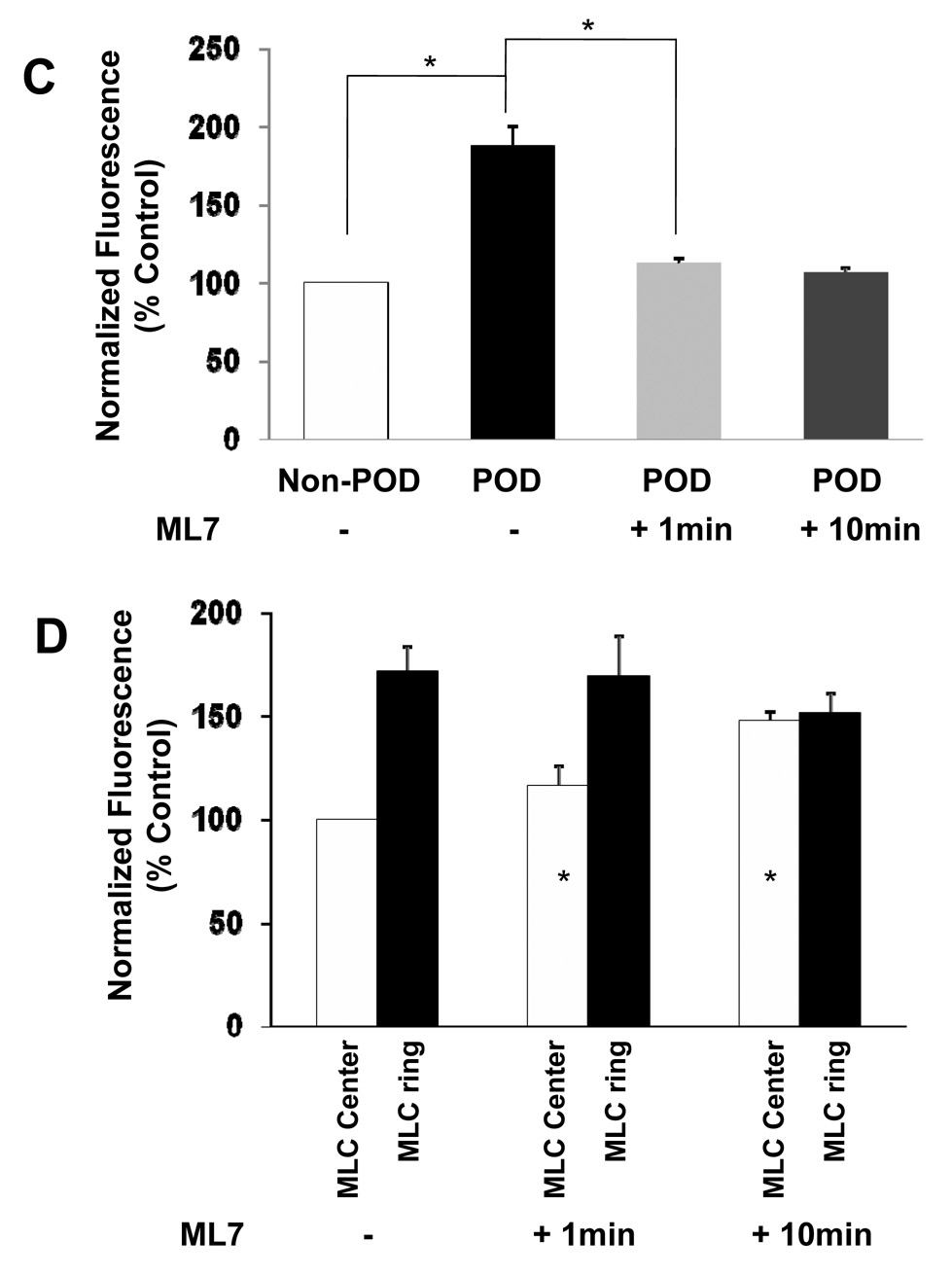
To better understand the structural basis of podosome ring regulation, we co-transfected mCherry-actin and GPF-myosin light chain (MLC) into the same cell. Just outside the podosome actin ring (Actin, Figure 1A, arrow), there was a thick myosin ring that formed bundles (MLC, Figure 1A, arrow). At the site of the podosome actin ring, there was a small faint myosin ring (Figure 1B). When the two images were superimposed, the actin ring colocalized largely with the thin myosin ring (overlay, Figure 1B), consistent with a recent report that myosin-IIA colocalizes with podosomes [4]. Interestingly, when myosin IIs were inhibited with ML7 (25 µM), the podosome actin ring disappeared within 1 min of ML7 treatment, although the two myosin ring-like structures were still present (Figure 1B). Moreover, the inner myosin ring appeared to be smaller and denser at 1 min of ML7. After 10 min of ML7 treatment, the larger myosin ring started to disassemble somewhat and the smaller myosin ring turned into a dense myosin aggregate at the center (Figure. 1B). Quantitative analysis of fluorescence intensity of actin and of MLC before and after ML7 in 8 different cells confirmed the conclusion from the above qualitative images (Figure 1C, 1D). Importantly, the disappearance of the podosome actin ring occurred at the time of the cell relaxation (Movie S1, Movie S2, Movie S3), suggesting that myosin-dependent tension in these myosin ring structures might be essential for the dynamics and relative stability of podosome actin rings.
Figure 1. Myosin tension regulates podosome ring dynamics.
Cells were co-transfected with mCherry actin (red) and GFP-MLC (myosin light chain) (green). (A) BHK cells displayed podosome actin rings (Actin, arrow); myosin networks exhibited a circular fibrillar network (MLC, arrow). The overlay revealed a complex circular network of myosin fibers (green) surrounding the actin ring (red) (scale bar 10 µm). (B) For a better visualization, white square in left image of (A) is cropped and enlarged. In the control cell (Cont), the actin ring (left image) is surrounded by the large circular myosin network, and a small faint myosin ring is located in the inner region (middle image). The overlay (right image) shows that the actin ring colocalizes with the small myosin ring in a majority of locations. ML7 (25 µM) induced a very rapid (1 min) and sustained (10 min) disappearance of the actin ring (left images); however, the circular myosin fibers were still present after 1 min and decreased slightly at 10 minutes (middle images). The small myosin ring shrank after 1 min of ML7 myosin (middle image) and a myosin aggregate appeared at the center after 10 min of ML7 (lower middle image). The overlay shows that the stress fibers at the cell periphery were still present despite a relaxation of the cell (Movie S3) and a concurrent disassembly of the podosome actin ring, suggesting that myosin tension is crucial in regulating podosome ring dynamics. (C) The control level of actin fluorescence is normalized by taking the average fluorescence of the cell outside the ring (Non-POD). The fluorescence of the podosome rings was 187±13% of the control fluorescence in the absence of ML7 (POD, (−) ML7), and rapidly decreased to 112± 3.7% after 1 min of ML7 (25µM) and to 107±3% after 10 min of ML7 (*, p<0.01; n=8 different cells; Mean+/− se). (D) The control level of MLC fluorescence is normalized by taking the average fluorescence in the center of the large myosin ring (MLC Center, -ML7). The fluorescence of the large myosin ring surrounding the actin ring was 172±11.6% of the control fluorescence and remained relatively constant after 1 min of ML7 (25µM), and then decreased somewhat to 152±9.1% after 10 min of ML7. Although the level of fluorescence of the large myosin ring did not significantly decrease with ML7 treatment, the level of myosin in the center rapidly increased to 116.5±9.2% after 1 min and to 148±4.3% after 10 min of ML7 (*, p<0.01; n=8 cells; Mean+/− se), likely largely due to the shrinkage of the small myosin ring.
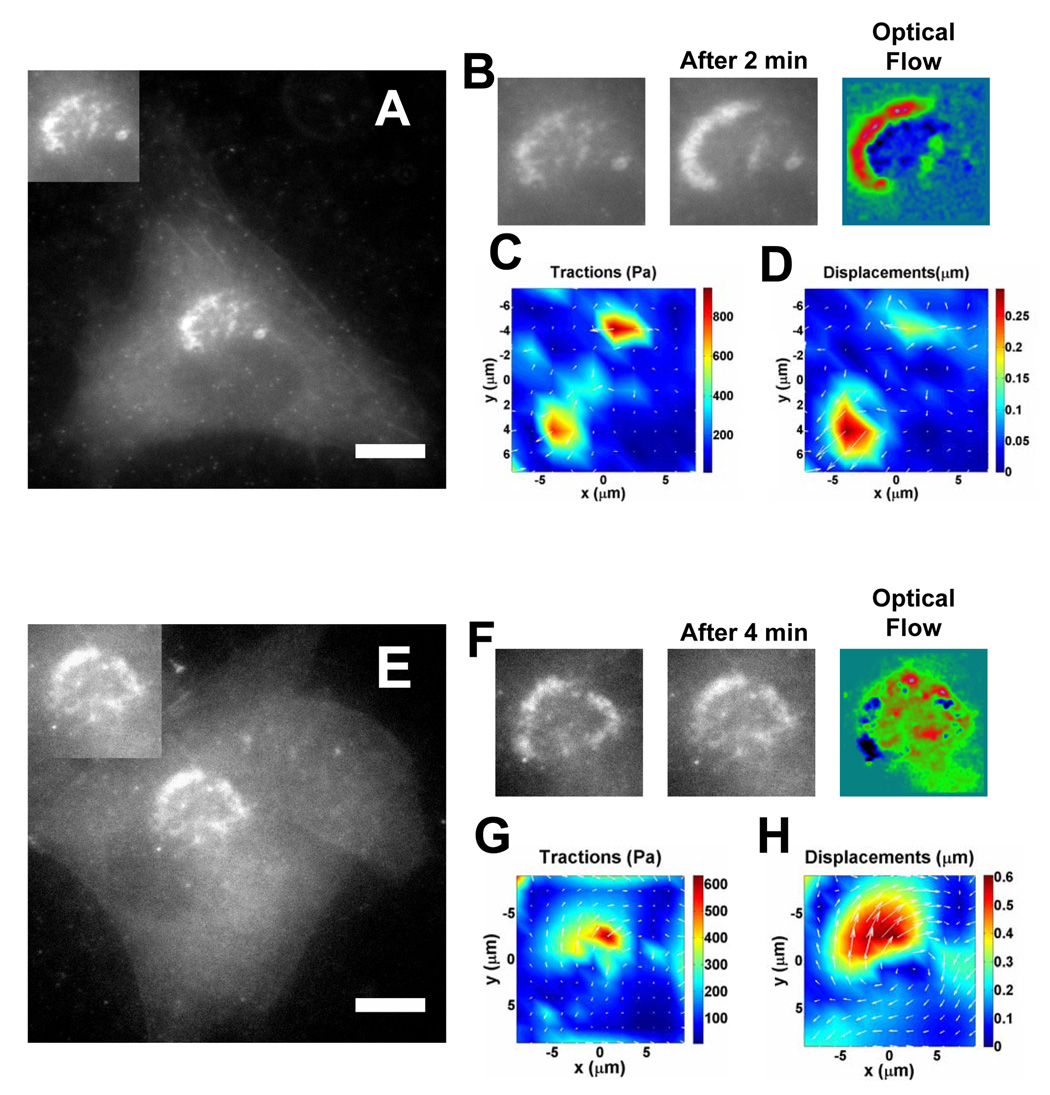
To find out whether a subcellular structure is a direct mechanosensor or not, one needs to determine if the specific structure can directly transmit mechanical forces inside-out and outside-in. While there is active discussion in the field that podosomes are possible candidates for mechanosensors since they share many molecules with focal adhesions, some suggest that podosomes are not a strong candidate for mechanosensors since they are self-organized dynamic structures that move around extensively inside the cytoplasm within minutes. Furthermore, podosome rings can be generated de novo in the cytoplasm in a non-migrating cell with a life span of 2–12 min. Therefore, the conventional wisdom is that these podosome structures are not likely to be a force-sensing structure, which should be stable enough to transmit forces across molecular structures. Surprisingly, when a podosome ring moved inside the cytoplasm within a few minutes and generated an apparent actin “flow” (Figure 2A, B), strong tractions were exerted on the flexible substrate, deforming the substrate just underneath the podosomes (Figure 2C, D). It is interesting that peak tractions were generated just underneath at the two ends of the actin semi-ring-like structure, suggesting that the endogenous forces that move the podosomes around are strongly localized. These data also suggest that not all podosomes in a ring structure are capable of exerting forces to the substrate. It is important to note that these tractions are the increases in stresses associated with podosome movements, not the tractions generated by the focal adhesions, which were the stresses necessary for the adhesion-dependent anchorage and the shape stability of the whole cell. Because local tractions decrease as the reciprocal of distance squared in the substrate, these “actin-flow” associated tractions just underneath the podosomes cannot be due to the dynamic traction changes at focal adhesions that are several µms or tens of µms away from these podosomes (see Fig. 3 below). Some podosomes even underwent rotational movements in the cytoplasm (Figure 2E, F) to generate considerable apparent actin flows in just a few minutes. This rotation of the podosome ring generated significant torsional tractions and deformations on the flexible substrate (Figure 2G, H). Since there was no rotation of the whole cell, these torsional tractions underneath the podosomes are likely to be a unique feature of the podosome ring structures, because it is well known that focal adhesion movements are strictly linear in a non-motile cell [10]. Importantly, tractions generated underneath the podosome ring were almost completely abolished when MLC activities were inhibited with ML7 (Figure S2). All these data demonstrate that podosomes do transmit endogenous forces to the outside ECM proteins.
Figure 2. Podosome rings exert traction stresses on the matrix when they undergo movements in the cytoplasm.
(A) Cells transfected with mCherry actin were seeded on a 6.5 kPa polyacrylamide gel substrate (top left insert: a podosome actin ring, the same as in (B)). (B) The cell displayed an actin ring that expands to the left of the image within two minutes. To better visualize the movement of the ring, timelapse sequences were analyzed with the optical flow method. The dark and blue regions correspond to the location of the podosome ring at time zero and the green and red regions correspond to the location of the podosome ring at 2 min. (C) The podosome ring exerts tractions up to 800 Pa in the direction of the movement of the wave. During the podosome band extension, the peak tractions are exerted at the extremities of the actin band and generated a peak gel deformation up to 0.3 µm (D). (E) A BHK cell, transfected with mCherry actin, was plated on a 3.5 kPa polyacrylamide gel substrate (top left insert: a podosome actin ring, the same as in (F)). Scale bar=20 µm for (A) and (E). (F) Although the size and shape of the podosome ring did not change during a 4-minute period, the optical flow analysis showed that the ring rotated clockwise, with a major actin flow that went from the left of the ring (dark regions) to the upper side of the ring (red regions). (G) The ring rotation exerted torsional tractions on the substrate up to 600 Pa where the actin flow was the highest, resulting a torsional deformation of the substrate up to 0.6 µm (H). (I) The peak tractions during podosome ring movements were computed for cells on different substrate stiffness (n=4 cells each on 2, 3.5, and 6.5 kPa gel respectively). Average peak tractions were 251±26 Pa on 2 kPa gel, 356±109 Pa on 3.5 kPa gel, and 672±100 Pa on 6.5 kPa gel (p<0.01 between 2 kPa and 3.5 kPa gels; p<0.038 between 3.5 kPa and 6.5 kPa gels; Mean+/−se).
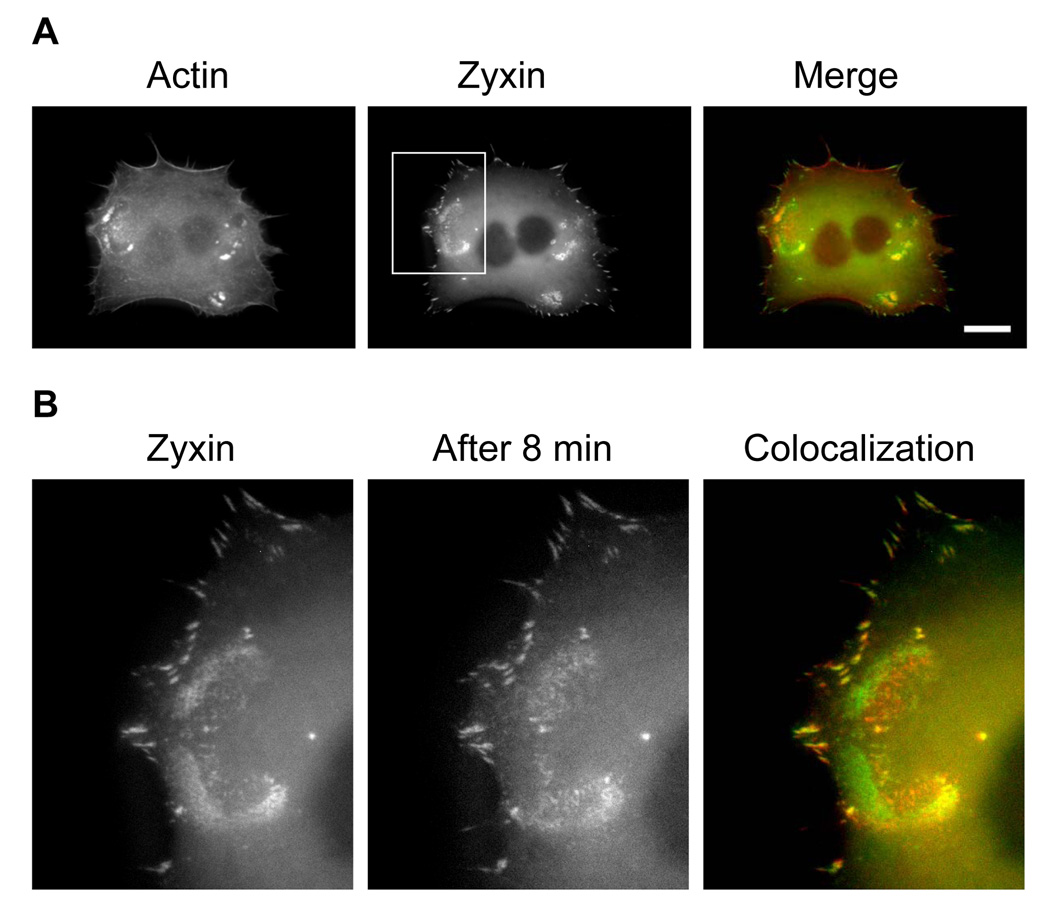
Figure 3. Movements and tractions by podosome rings and focal adhesions.
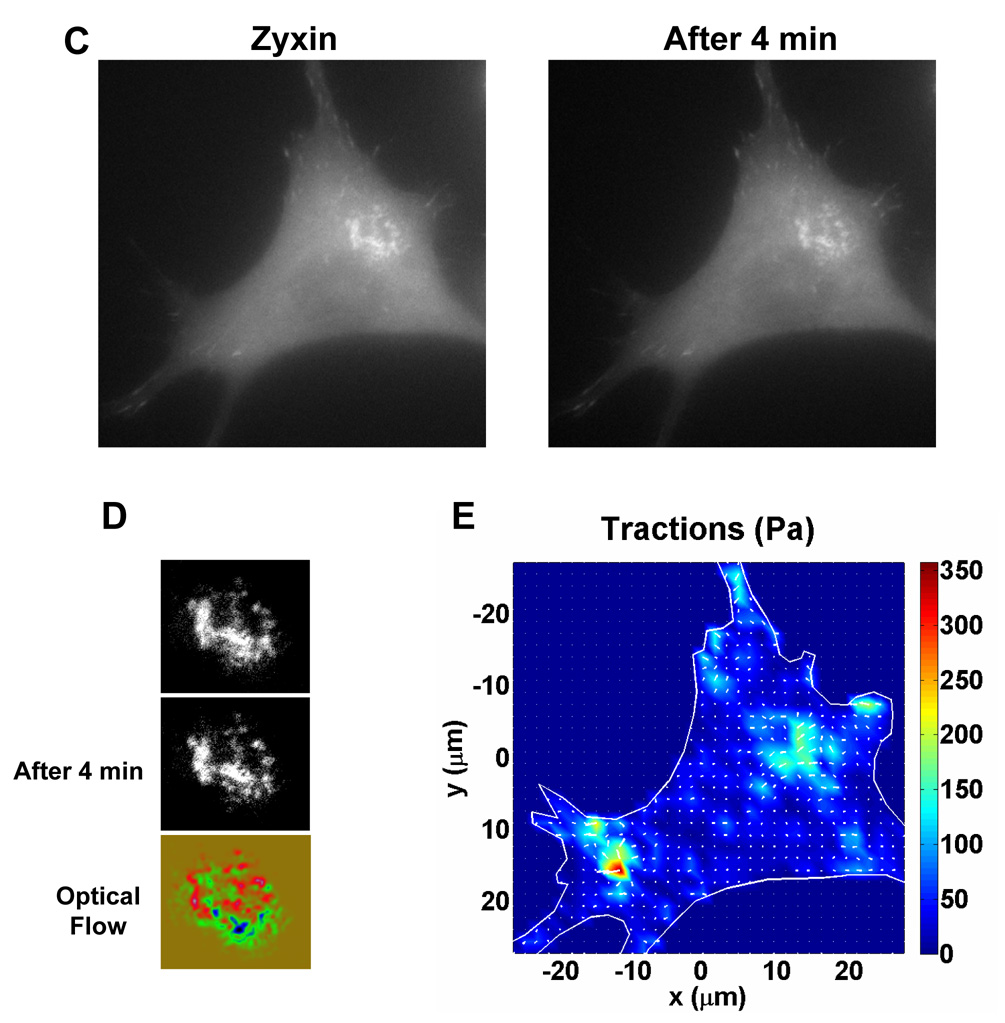
(A) BHK cells are co-transfected with m-Cherry actin (left image) and EGFP zyxin (central image) and seeded on collagen-1 coated rigid glass. The zyxin is located in the focal adhesions at the tip of the actin stress fibers, and also colocalizes with the actin in the podosome rings (left image). (B) The focal adhesions and the podosome ring were visualized in EGFP zyxin at a region of interest (white square in (A)) in a timelapse sequence of 8 minutes (left image and central image). The image at the initial time is artificially colored in green, and the image after 8 minutes is artificially colored in red. The overlay of the two images allows the determination of the movement of the podosomes and the focal adhesions (right image). The zyxin located in the focal adhesions before and after 8 minutes completely colocalizes (yellow dots), thus showing little movement of the focal adhesions during this period. However, during the same period, the podosome zyxin ring moved substantially within 8 min (the red band and the green band). (C) A BHK cell was transfected with EGFP-zyxin and seeded on a substrate of 5 kPa. The dynamics of a podosome ring and of focal adhesions were recorded during a 4-minute period. During this period, the focal adhesions exhibit very small movements, while the podosome ring display expansion. (D) A close visualization of the podosome ring is shown at the beginning of the sequence and after 4 min. Analysis of the ring movement with optical flow shows in blue/green (the position of the ring at the beginning of the sequence) and in red (the position at 4 min). The ring exhibited an expansion to the left on its left part, an expansion to the right in the center of the ring, and a formation or reinforcement of podosomes on the right part of the ring. (E) The tractions exerted by the cell are computed during the timelapse sequence of 4 min. A majority of focal adhesions did not exhibit traction changes at all during this period, although a few focal adhesions exhibited traction changes at the cell periphery ranging from 150 to 350 Pa. In contrast, the podosome ring exerted tractions up to 200 Pa, in the same direction of its movements, at sites far from any focal adhesions. Another cell exhibited similar behavior.
In order for a subcellular structure to be a mechanosensor, it must be sensitive to substrate rigidity [9, 11–13]. To test this possibility for the podosomes, cells were plated on different substrate stiffnesses. When the substrate stiffness varied from 2.0 to 3.5 kPa and to 6.5 kPa, the peak tractions generated beneath the podosomes increased from 251 Pa to 356 Pa (a 42% increase) and 672 Pa (a 168% increase), respectively (Figure 2I). These results demonstrate that the molecular structures in the podosomes and/or in the vicinity of the podosomes are sensitive to changes in substrate rigidity. Our findings are in accordance with those by YL Wang et al. who have demonstrated that when substrate stiffness doubles the tractions increase by ~50% [14], most likely from primary contributions of focal adhesions [9], a major cell-matrix mechanosensor. Considering that podosomes are similar in sensitivity to substrate stiffness as focal adhesions, our result strongly suggests that podosomes are indeed mechanosensors.
It is well known that focal adhesions function to anchor an anchorage-dependent cell, to sense substrate rigidity and to transmit forces, to stabilize its cytoskeleton, and to deform the ECM [6–15]. In contrast, podosomes are dynamic microstructures whose known functions are for local matrix degradation and invasion [1]. To determine whether dynamics of podosomes and dynamics of focal adhesions are different, we examined them in the same cell. In a cell that was double transfected with m-Cherry-actin and EGFP-zyxin and plated on collagen-1 coated rigid glass dish, podosome rings exhibited significant movements within an 8-min period; in contrast, there were little observable movements associated with focal adhesions during the same period (Fig. 3A, B). These data suggest that podosomes and focal adhesions have very different dynamics on rigid substrates.
To further compare changes in tractions under podosomes with those under focal adhesions, we plated EGFP-zyxin transfected cells on a collagen-1 coated substrate of 5 kPa (Fig. 3C). The podosome zyxin ring exhibited significant movements and dynamics during a 4-min period (Fig. 3D). Importantly, tractions up to 200 Pa were exerted on to the substrate just underneath the podosome zyxin ring; the tractions were in the same direction of the podosome movements, and at sites far from any focal adhesions (Fig. 3E). Most focal adhesions did not exhibit traction changes during this period, although a few focal adhesions exhibited tractions changes at the cell periphery ranging from 150–350 Pa (Fig. 3E). Since the magnitudes of tractions underneath the podosomes are comparable to those generated under focal adhesions - the well-established mechanosensors - these results suggest that like focal adhesions, podosomes are mechanosensors. What might be responsible for generating these tractions underneath the podosomes? Myosin motor proteins are found right at or near podosomes [4] (Fig. 1), suggesting that they are strong candidate for generating tractions underneath the podosomes, although we cannot rule out the possibility that forces are generated outside the podosomes and transmitted to the substrate via podosomes through the cytoskeleton.
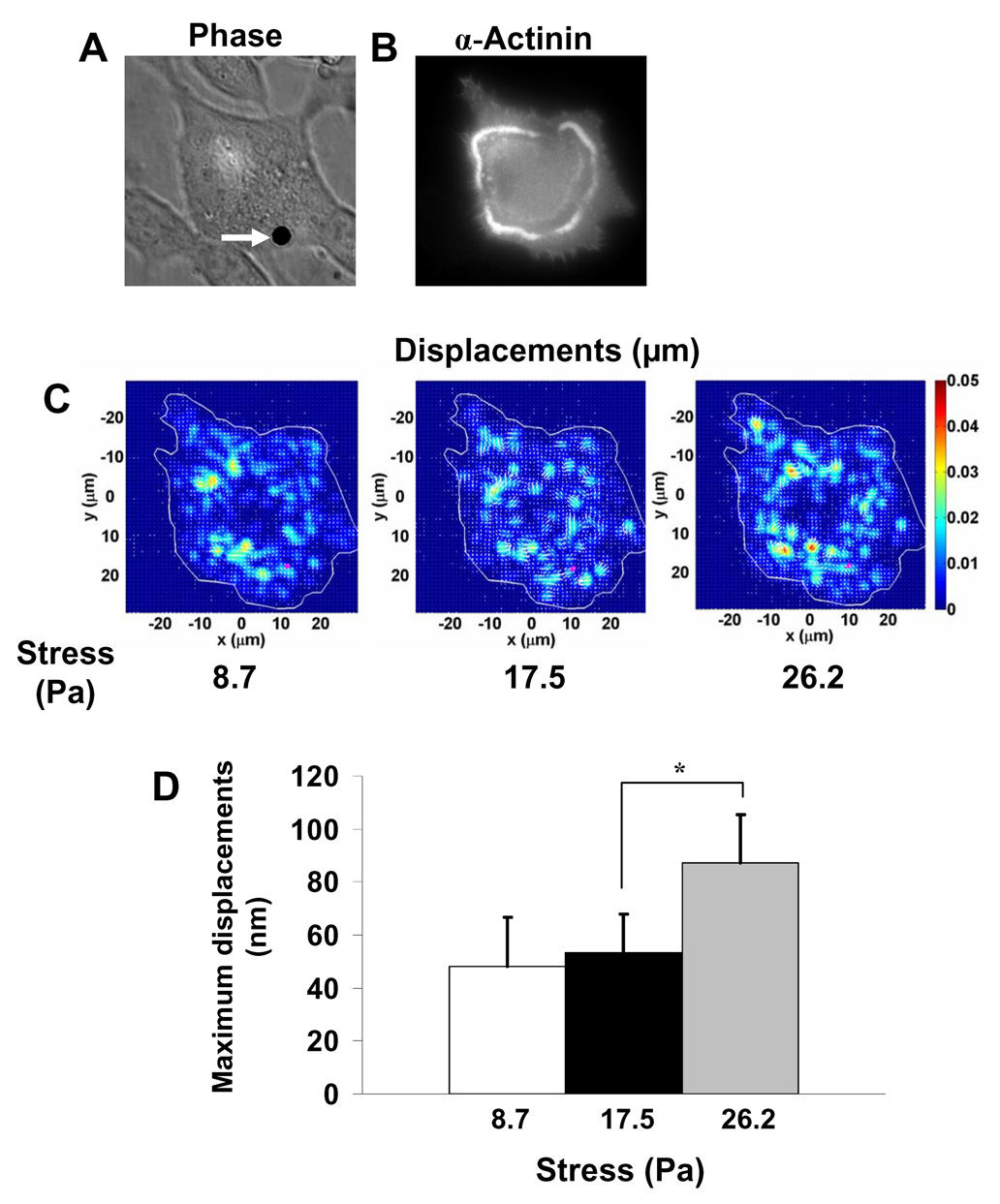
To explore the possibility of outside-in direct force transmission by podosomes, we applied a localized stress to the apical surface of the cell via integrins and quantified the induced deformations in the podosome actin rings (Figure S3A, B). It is clear that stresses applied at cell apex resulted in appreciable deformations in the podosomes (Figure S3A, B). A mechanosensor that senses direct mechanical stresses should be sensitive to the magnitudes of the applied stresses. To test this possibility in podosomes, we transfected EGFP-α-actinin into the cells that forms podosome-like rings and then applied varying stresses via the magnetic bead on the cell top (Fig. 4A, B). Elevating the magnitudes of the applied stresses from 8.7 to 17.5 and to 26.2 Pa increased the peak displacements of the podosome α-actinin rings from 48±18.7 nm to 53.2±14.6 nm and to 87.4±17.7 nm, respectively (Fig. 4C, D). It is interesting that the relationship between the applied stress and the maximum induced α-actinin displacements was nonlinear, a typical feature found when probing focal adhesions [6, 7]. However, our recent published data show that biochemical activities of enzymes such as Src can increase rapidly within 300 ms at remote cytoplasmic sites after stress application [16], suggesting that some of the induced displacements of podosomes at different magnitudes of stresses in the same cell could be due to indirect biochemical changes and associated structural changes (remodeling) at or near podosomes. If this were true, it would be another piece of evidence that podosomes act as mechanosensors. Detailed biochemical studies are needed to test this idea in the future.
Figure 4. Mechanical stresses are transmitted outside-in to the podosomes.
(A) An RGD-coated 4.5-µm bead (arrow) was attached to the apical surface of a BHK cell transfected with EGFP-α-actinin. (B) The cell exhibited a large podosome α-actinin ring at the basal surface at the periphery of the cell and a small faint ring inside. (C) Direct displacements of podosome rings were quantified using a sensitive synchronous detection method [38. 39] in response to varying oscillatory stresses (0.8 Hz). Maximum displacements of the podosomes increased from ~30 nm at 8.7 Pa and 17.5 Pa to ~50 nm at 26.2 Pa. Displacements at sites other than the rings are due to stress-induced diaplacements of α-actinin associated with other cytoskeletal structures. The pink dot represents the bead center position. (D) Quantitative analyses of podosome maximum displacements. Average peak displacements were 48±18.7 nm, 53.2±14.6 nm, and 87.4±17.7 nm, respectively with increasing stresses. (*, p<0.01; n = 5 cells, mean ± se) Note that the relationship between the applied stress and the maximum α-actinin displacements was nonlinear.
Of four different integrin-dependent adhesions (focal adhesions, focal complexes, fibrilar adhesions, and podosomes), podosomes are the least understood in terms of dynamics and physical features. Previous reports show that inhibition of myosin activity promotes formation of podosomes [4, 17]. In contrast, we find that myosin inhibition leads to podosomes disassembly. We do not know why our data are different from those published data, but 3 different inhibitors of myosin-dependent tension (blebbistatin, ML7, and Y-27832) all disassembled podosomes and prevented formation of new podosomes, which are consistent with our previous report [5]. We wonder whether these are cell-type and/or species related differences, or they depend on how podosomes are initiated. Alternatively, it is possible that dependence of podosome dynamics on myosin activity is tightly regulated. No matter what, it is clear that podosome dynamics depends not only on actin dynamics but also on myosin activity.
In the current study, we present evidence for podosomes as bona fide mechanosensors capable of transmitting mechanical stresses inside-out and outside-in. These results are surprising since one would not expect to observe such a high magnitude of tractions by these small, very dynamic, dot-like structures (~0.5–1 µm), and to find that they are so sensitive to substrate rigidity and to applied stresses. At this time we do not know what exact molecule(s) inside the podosomes is responsible for sensing forces, but integrins, zyxin, paxillin, α-actinin, and vinculin are all candidates for early sensing. It is likely that myosin-II motor proteins in the podosomes or close to the podosomes are the primary component of the sensing apparatus. Our findings on the vital role of myosin-dependent tension in self-organized podosome formation suggest that local interactions between myosin II motor proteins [18] and actins might be crucial to provide global order to these self-organized structures in living cells. Our current work extends the previously published work [5]. In light of a previous paper that only quantifies tractions generated by focal adhesions [9], our work suggests that one may not be able to ignore those tractions generated by dynamic adhesion structures such as podosomes. A report shows that tractions in fibroblasts are regulated by Rho-dependent kinase but not by MLC kinase [19]. Our current data that MLC kinase inhibition by ML7 can also lead to disappearance of podosome rings and inhibition of podosome tractions suggest that tractions exerted by these dynamic structures may be controlled by different myosin IIs [4, 18] when compared with those by more stable and large adhesion structures such as focal adhesions. The exact physiological significance of the mechanosensing capability of podosomes is not clear at the present time, but it might be related to the need for the cell to probe the local physical properties of the substrate rapidly in order to adjust its intracellular mechanical/biochemical activities to adapt to its physical microenvironment. More work is needed in elucidating how individual signaling molecules [20] interact with myosin motor proteins in regulating podosome dynamics and mechanics, which may play important roles in cell invasion.
Supplementary Material
Acknowledgments
We thank Dr. R. Tsien of University of California at San Diego for the gift of mCherry-actin. This work was supported by NIH grant GM072744 (N.W.) and University of Illinois (N.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Badowski C, Pawlak G, Grichine A, Chabadel A, Oddou C, Jurdic P, Pfaff M, Albigès-Rizo C, Block MR. Paxillin Phosphorylation Controls Invadopodia/Podosomes Spatiotemporal Organization. Mol. Biol. Cell. 2008;19:633–645. doi: 10.1091/mbc.E06-01-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samanna V, Ma T, Mak TW, Rogers M, Chellaiah MA. Actin polymerization modulates CD44 surface expression, MMP-9 activation, and osteoclast function. J. Cell Physiol. 2007;213:710–720. doi: 10.1002/jcp.21137. [DOI] [PubMed] [Google Scholar]
- 4.van Helden SF, Oud MM, Joosten B, Peterse N, Figdor CG, van Leeuwen FN. PGE2-mediated podosome loss in dendritic cells is dependent on actomyosin contraction downstream of the RhoA-Rho-kinase axis. J. Cell Sci. 2008;121:1096–1106. doi: 10.1242/jcs.020289. [DOI] [PubMed] [Google Scholar]
- 5.Collin O, Tracqui P, Stephanou A, Usson Y, Clément-Lacroix J, Planus E. Spatiotemporal dynamics of actin-rich adhesion microdomains: influence of substrate flexibility. J. Cell Sci. 2006;119:1914–1925. doi: 10.1242/jcs.02838. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 7.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 8.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahaluc D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 10.Smilenov LB, Mikhailov A, Pelham RJ, Marcantonio EE, Gundersen GG. Focal adhesion motility revealed in stationary fibroblasts. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- 11.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 12.Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr. Opin. Cell Biol. 2006;18:472–481. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 14.Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 2000;279:1345–1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 15.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 16.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. U S A. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beningo KA, Hamao K, Dembo M, Wang YL, Hosoya H. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch Biochem Biophys. 2006;456:224–231. doi: 10.1016/j.abb.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luxenburg C, Parsons JT, Addadi L, Geiger B. Involvement of the Src-cortactin pathway in podosome formation and turnover during polarization of cultured osteoclasts. J Cell Sci. 2006;119:4878–4888. doi: 10.1242/jcs.03271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.