Abstract
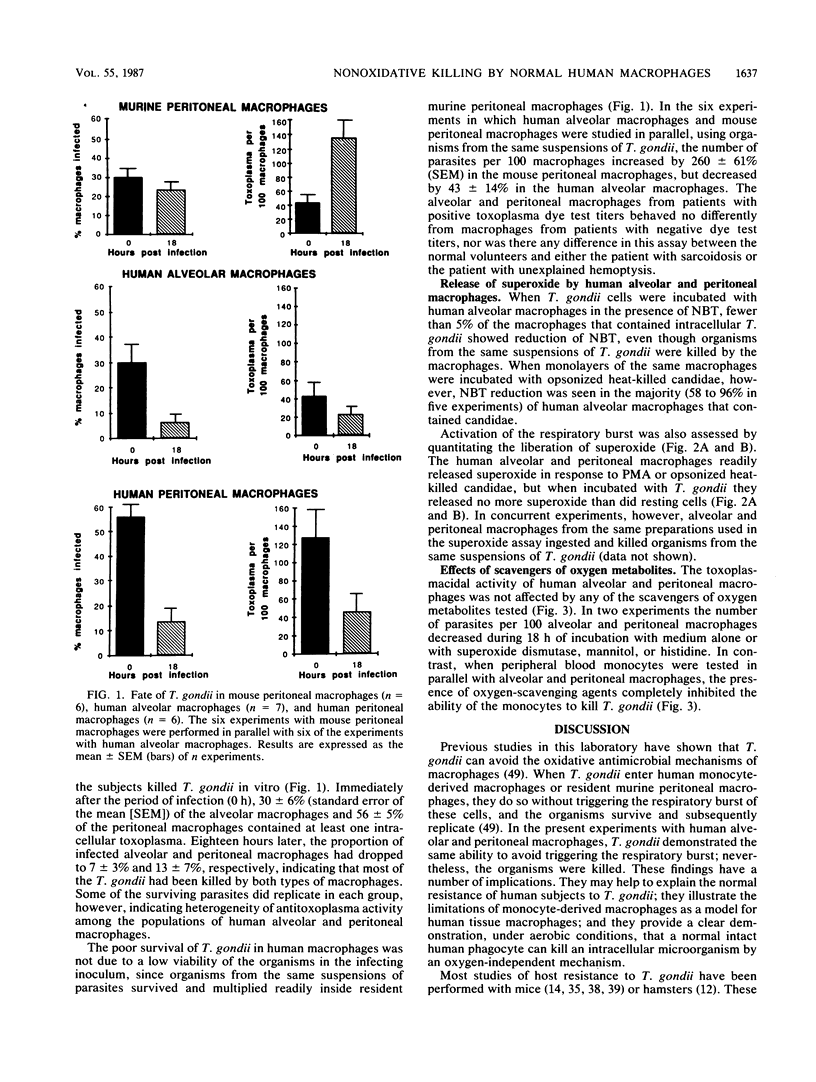
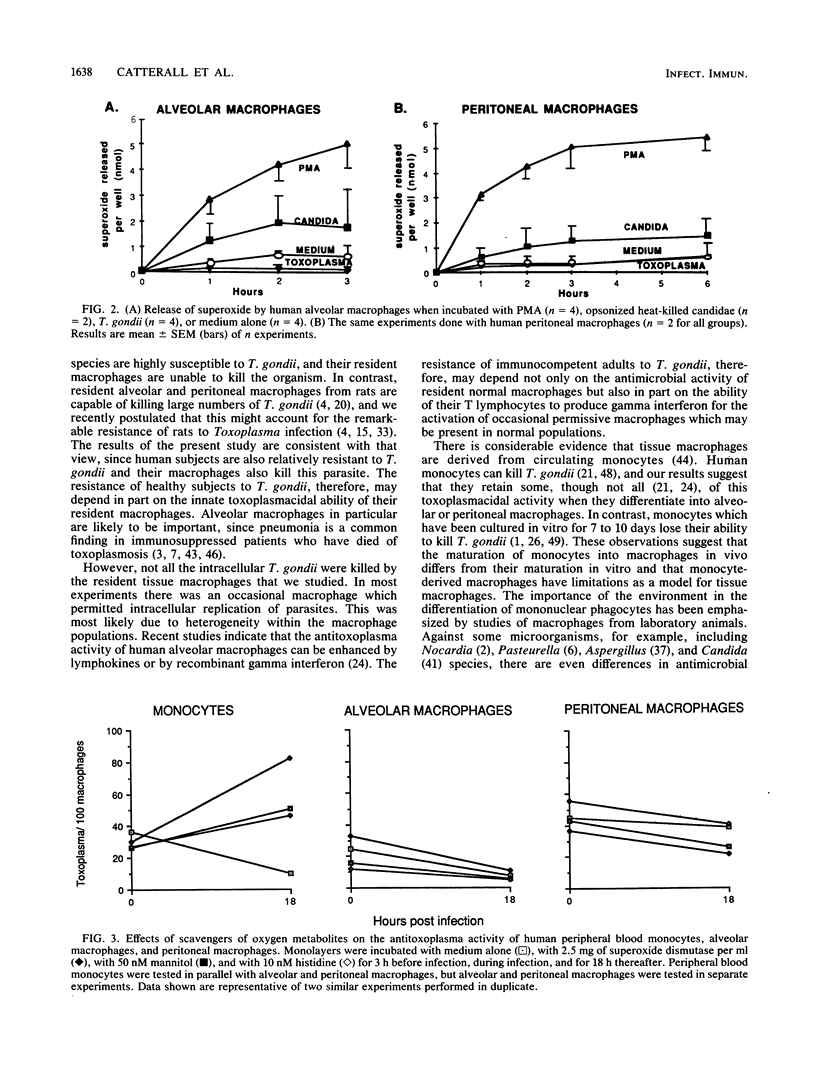
Although Toxoplasma gondii multiplies within normal murine alveolar and peritoneal macrophages, it is killed by normal rat alveolar and peritoneal macrophages. The killing by rat macrophages is by a nonoxidative mechanism. Studies on normal human alveolar macrophages have reported disparate results in regard to their ability to inhibit or kill T. gondii. We considered it of interest to explore further the effect of normal human alveolar and peritoneal macrophages on T. gondii. Unstimulated alveolar macrophages from each of seven individuals demonstrated a marked ability to kill or inhibit multiplication of T. gondii in vitro (e.g., the number of parasites per 100 alveolar macrophages was 31 at time zero and 2 at 18 h, whereas this value increased from 37 at time zero to 183 at 18 h in murine macrophages assayed in parallel). In quantitative assays of superoxide, alveolar macrophages released a substantial amount of superoxide when exposed to phorbol myristate acetate or to candidae. In contrast, alveolar macrophages incubated with T. gondii released no more superoxide than when in medium alone. Scavengers of superoxide anions, hydrogen peroxide, singlet oxygen, and hydroxyl radicals failed to inhibit killing of T. gondii by alveolar macrophages. Peritoneal macrophages from each of six normal women undergoing laparoscopy killed T. gondii in vitro; results of quantitative superoxide assays and scavenger experiments demonstrated that no oxidative burst was triggered in these macrophages by exposure to T. gondii. These data indicate that normal human alveolar and peritoneal macrophages can kill an intracellular parasite by nonoxidative mechanisms and suggest that these mechanisms are important in inhibition or killing of other opportunistic intracellular pathogens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. M., Beaman B. L., Donovan R. M., Goldstein E. Effect of virulent and less virulent strains of Nocardia asteroides on acid-phosphatase activity in alveolar and peritoneal macrophages maintained in vitro. J Infect Dis. 1983 Jul;148(1):117–124. doi: 10.1093/infdis/148.1.117. [DOI] [PubMed] [Google Scholar]
- Catterall J. R., Hofflin J. M., Remington J. S. Pulmonary toxoplasmosis. Am Rev Respir Dis. 1986 Apr;133(4):704–705. doi: 10.1164/arrd.1986.133.4.704. [DOI] [PubMed] [Google Scholar]
- Catterall J. R., Sharma S. D., Remington J. S. Oxygen-independent killing by alveolar macrophages. J Exp Med. 1986 May 1;163(5):1113–1131. doi: 10.1084/jem.163.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J. Drug potentiation of macrophage function. Infect Immun. 1970 Nov;2(5):601–605. doi: 10.1128/iai.2.5.601-605.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Niederbuhl C. J., Campbell S. G. Bactericidal activity of alveolar and peritoneal macrophages exposed in vitro to three strains of Pasteurella multocida. Infect Immun. 1983 Feb;39(2):779–784. doi: 10.1128/iai.39.2.779-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff R. L., Frenkel J. K. Cell-mediated immunity against Besnoitia and toxoplasma in specifically and cross-immunized hamsters and in cultures. J Exp Med. 1974 Mar 1;139(3):560–580. doi: 10.1084/jem.139.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAINSON R. Toxoplasmosis in England. II. Variation factors in the pathogenesis of Toxoplasma infections: the sudden increase in virulence of a strain after passage in multimammate rats and canaries. Ann Trop Med Parasitol. 1955 Dec;49(4):397–416. [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur H., Jones T. C. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J Exp Med. 1978 Jan 1;147(1):157–170. doi: 10.1084/jem.147.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. E., Remington J. S. Mechanisms of killing of Toxoplasma gondii by rat peritoneal macrophages. Infect Immun. 1986 Apr;52(1):151–155. doi: 10.1128/iai.52.1.151-155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R., Estes R., Mack D. G., McLeod E. G. Effects of human alveolar macrophages and peripheral blood monocytes on Toxoplasma gondii. J Infect Dis. 1983 May;147(5):957–957. doi: 10.1093/infdis/147.5.957. [DOI] [PubMed] [Google Scholar]
- Modrzakowski M. C., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes: antagonism of granule cationic proteins by lipopolysaccharide. Infect Immun. 1979 Aug;25(2):597–602. doi: 10.1128/iai.25.2.597-602.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Gellene R. A., Libby D. M., Rothermel C. D., Rubin B. Y. Activation of tissue macrophages from AIDS patients: in vitro response of AIDS alveolar macrophages to lymphokines and interferon-gamma. J Immunol. 1985 Oct;135(4):2374–2377. [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Carriero S. M., Harris A. M., Jaffee E. A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985 Mar;134(3):1982–1988. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Okamura N., Spitznagel J. K. Outer membrane mutants of Salmonella typhimurium LT2 have lipopolysaccharide-dependent resistance to the bactericidal activity of anaerobic human neutrophils. Infect Immun. 1982 Jun;36(3):1086–1095. doi: 10.1128/iai.36.3.1086-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- REMINGTON J. S., JACOBS L., KAUFMAN H. E. Studies on chronic toxoplasmosis; the relation of infective dose to residual infection and to the possibility of congenital transmission. Am J Ophthalmol. 1958 Nov;46(5 Pt 2):261–268. [PubMed] [Google Scholar]
- Remington J. S., Krahenbuhl J. L., Mendenhall J. W. A role for activated macrophages in resistance to infection with Toxoplasma. Infect Immun. 1972 Nov;6(5):829–834. doi: 10.1128/iai.6.5.829-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S. Toxoplasmosis in the adult. Bull N Y Acad Med. 1974 Feb;50(2):211–227. [PMC free article] [PubMed] [Google Scholar]
- Ryning F. W., Remington J. S. Effect of alveolar macrophages on Toxoplasma gondii. Infect Immun. 1977 Dec;18(3):746–753. doi: 10.1128/iai.18.3.746-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. I., Davis C. E. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983 Dec;42(3):1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Pelster B., Suzuki N., Piekarski G., Brandis H. Immunity to Toxoplasma gondii induced in vitro in non-immune mouse macrophages with specifically immune lymphocytes. J Immunol. 1975 Oct;115(4):1151–1158. [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L., Weidner E. Lymphokine activation of J774G8 cells and mouse peritoneal macrophages challenged with Toxoplasma gondii. Infect Immun. 1985 Sep;49(3):760–764. doi: 10.1128/iai.49.3.760-764.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Okamura N. Oxygen independent microbicidal mechanisms of human polymorphonuclear leukocytes. Adv Exp Med Biol. 1983;162:5–17. doi: 10.1007/978-1-4684-4481-0_2. [DOI] [PubMed] [Google Scholar]
- Steigbigel R. T., Lambert L. H., Jr, Remington J. S. Phagocytic and bacterial properties of normal human monocytes. J Clin Invest. 1974 Jan;53(1):131–142. doi: 10.1172/JCI107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthanthiran M., Solomon S. D., Williams P. S., Rubin A. L., Novogrodsky A., Stenzel K. H. Hydroxyl radical scavengers inhibit human natural killer cell activity. Nature. 1984 Jan 19;307(5948):276–278. doi: 10.1038/307276a0. [DOI] [PubMed] [Google Scholar]
- Tourani J. M., Israël-Biet D., Venet A., Andrieu J. M. Unusual pulmonary infection in a puzzling presentation of AIDS. Lancet. 1985 Apr 27;1(8435):989–989. doi: 10.1016/s0140-6736(85)91769-6. [DOI] [PubMed] [Google Scholar]
- Vel W. A., Namavar F., Verweij A. M., Pubben A. N., MacLaren D. M. Killing capacity of human polymorphonuclear leukocytes in aerobic and anaerobic conditions. J Med Microbiol. 1984 Oct;18(2):173–180. doi: 10.1099/00222615-18-2-173. [DOI] [PubMed] [Google Scholar]
- Vietzke W. M., Gelderman A. H., Grimley P. M., Valsamis M. P. Toxoplasmosis complicating malignancy. Experience at the National Cancer Institute. Cancer. 1968 May;21(5):816–827. doi: 10.1002/1097-0142(196805)21:5<816::aid-cncr2820210506>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Haas J. E. Cellular defenses against Toxoplasma gondii in newborns. J Clin Invest. 1984 Jun;73(6):1606–1616. doi: 10.1172/JCI111367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Activity of human blood leukocytes against Toxoplasma gondii. J Infect Dis. 1979 Dec;140(6):890–895. doi: 10.1093/infdis/140.6.890. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston D. J., Territo M. C., Ho W. G., Miller M. J., Gale R. P., Golde D. W. Alveolar macrophage dysfunction in human bone marrow transplant recipients. Am J Med. 1982 Dec;73(6):859–866. doi: 10.1016/0002-9343(82)90777-x. [DOI] [PubMed] [Google Scholar]
- van Furth R., Raeburn J. A., van Zwet T. L. Characteristics of human mononuclear phagocytes. Blood. 1979 Aug;54(2):485–500. [PubMed] [Google Scholar]



