Abstract
BACKGROUND AND PURPOSE: Modified coils have failed to improve long-term recanalization of aneurysms. This study examined whether ex vivo transduction of replication-deficient adenovirus containing the bone morphogenetic protein-13 gene (Ad-BMP-13) in fibroblast allografts would improve angiographic results via increased collagen synthesis, compared with fibroblast-coated platinum coils (FBC) and bare platinum coils (PA).
Materials and METHODS: Aneurysms were embolized with Ad-BMP-13-coated coils (n = 20). Rabbits were sacrificed at 14 days and at 1, 3, and 6 months after implantation. Digital subtraction angiography (DSA) evaluated stability after embolization. Histologic specimens were examined with a qualitative grading system. Masson trichrome evaluated collagen deposition. Findings were compared with previously reported controls for PA and FBC in the same model and time points.
RESULTS: The grading system showed a greater total score (P = .0002) in Ad-BMP-13 (6.8 ± 1.6) and FBC (6.3 ± 2.4) compared with PA (4.7 ± 2.4). A group main effects test showed that aneurysm neck tissue coverage in Ad-BMP-13 (2.5 ± 1.1) was higher (P = .0007) than both FBC (1.6 ± 1.4) and PA (0.9 ± 1.1). Ad-BMP-13 had more (P < .0001) collagen deposition than the FBC and PA. One- and 3-month Ad-BMP-13 collagen depositions increased (P < .05) over the FBC and PA. Finally, Ad-BMP-13 showed radiographic stability in 15 (75%) cases, coil compaction in 4 (20%) cases, and progressive occlusion in 1 (5%) case. There were no differences in angiographic results (P = .6522).
CONCLUSION: The Ad-BMP-13-coated coils can improve neck coverage and dome fibrosis in the rabbit model, even in the absence of observed differences in angiographic outcome.
The endovascular treatment of intracranial aneurysms by use of detachable coils is rapidly gaining acceptance as a safe alternative to standard surgical therapy.1 However, treatment of large and giant aneurysms has been problematic owing to low rates of initial complete obliteration of the aneurysm as well as high rates of late aneurysmal recanalization.1,2 The mechanisms responsible for aneurysmal recanalization and coil compaction remain unknown, though previous reports have suggested that it may be because of the inert nature of the platinum.3,4 Thus, investigators have proposed modifications aimed at increasing the coil's “biologic activity,” defined as improved thrombus organization, collagen formation, or endothelialization within and adjacent to the aneurysmal cavity.3–12 Previous groups12 have proposed the delivery of bioactive proteins such as vascular endothelial growth factor (VEGF) to enhance healing of cerebral aneurysms. However, the amount of protein that can be effectively delivered to an aneurysm with use of a platinum microcoil is severely limited, and the effect diminishes with time. These results indicate that prolonged, local synthesis of growth factors might further improve aneurysmal healing. Prolonged production of such growth factors could be achieved with use of ex vivo viral transduction of cellular allografts before implantation.
Our previous experiments implanting fibroblast allografts into aneurysmal cavities have shown that these allografts can enhance cellular proliferation and thrombus organization with an endothelialized membrane bridging the neck early in the time course of study.13 However, we failed to demonstrate enhanced production of collagen; instead, cells were surrounded by loose extracellular matrix. Intra-aneurysmal synthesis of collagenous extracellular matrix is considered to be beneficial for permanent closure of aneurysmal cavities.9
It has been demonstrated that adenovirus-mediated bone morphogenetic protein-13 (Ad-BMP-13) production stimulates robust collagen synthesis in vivo.14 We hypothesize that the ex vivo transduction of fibroblast tissue allografts, before implantation, with replication-deficient Ad-BMP-13, will improve collagen synthesis in experimental aneurysms compared with nontransduced allografts and bare platinum (PA) coils.
Materials and Methods
Male New Zealand white rabbits were used as fibroblast donors, whereas female New Zealand white rabbits were used as recipients. The Institutional Animal Care and Use Committee at this institution approved all procedures before initiation of the study.
Adenovirus Ad-BMP-13 Infected Fibroblasts
The fibroblast preparation and the platinum coils coated with fibroblast have been described previously.13 Recombinant type-5 human adenoviral vectors with complete deletion of the E1A and E1B regions and a partial deletion of the E3 region of the viral genome were used. The therapeutic vector was constructed with the BMP-13 gene under control of a cytomegalovirus promoter (Ad-BMP-13).15
When the tendon-derived cells reached 70% to 80% confluence at about 2 weeks, they were lifted with trypsin, pelleted, and resuspended in 5 mL of culture medium per T25 flask. The cell suspension was then placed in 50 mm x 15 mm culture dishes containing platinum coils (10 mL of suspension per culture dish). These dishes were incubated at 37°C in 5% CO2, with a change of medium on alternate days. To completely immerse the coils, the cultures were maintained with 10 mL of culture medium per plate. When the lumen of the coils seemed to be full of cells, the coils covered with the cells were washed with serum-free medium and were immersed in serum-free medium containing 107 pfu/mL of Ad-BMP-13 (10 mL of 107 pfu/mL gave an MOI of approximately 50 pfu/cfu). When the coil was covered with cells, they were incubated with the virus solution for 24 hours at 37°C in 5% CO2. They were then returned to virus-free medium with 10% FBS and incubated for another 48 to 72 hours. The medium was then replaced with regular culture medium, and the coils covered with cells were ready for implantation.
Creation of Aneurysms and Coil Embolization
Aneurysms were created in 20 female New Zealand white rabbits. Aneurysms were allowed to mature for at least 21 days before embolization. They were then coiled with Ad-BMP-13 transfected fibroblast-coated coils. Methods used for creation of an aneurysm and embolization have been described elsewhere.16,17 Digital subtraction angiography (DSA) was performed after the embolization. Samples were harvested at 14 days (n = 5), 1 month (n = 5), 3 months (n = 5), and 6 months (n = 5) after coil embolization. The results for platinum coil (PA) and fibroblast-coated platinum coils (FBC)-treated controls in the same model at the same time points have been reported previously.13 Aneurysmal sizes (neck, width, and height), volume, and coil-packing attenuation in each group were calculated and compared with use of a 2-way analysis of variance (least squares test; JMP software, SAS Institute, Cary, NC). If an interaction (Group*Duration) was not found, then a main effects test on the group was performed. Any significant interaction or main effect was further investigated with the use of planned comparisons and a Student t post hoc test.
Tissue Harvest
At sacrifice, animals were deeply anesthetized. DSA was performed, followed by euthanasia with use of a lethal injection of pentobarbital. Harvested aneurysms were immediately fixed in 10% neutral buffered formalin. The degree of aneurysmal neck tissue coverage was evaluated as described previously.18 Samples were embedded in paraffin and were sectioned at 1000-μm intervals in a coronal orientation.19 Coil fragments were carefully removed. Sections were re-embedded in paraffin and were sectioned at 5-μm intervals. Sections were stained with hematoxylin-eosin (H&E) and Masson trichrome.
Angiographic Evaluation
All sacrifice angiograms were compared with postembolization angiograms and were assessed for changes in coil configuration or aneurysmal filling. Sacrifice angiograms were then categorized as stable, progressive occlusion, or coil compaction/recanalization compared with the postembolization angiograms. Stable and progressive occlusions were categorized as positive outcomes, whereas compaction was deemed a negative result. A χ2 test was run on the outcomes by the 3 treatment groups.
Histologic Evaluation
Sections were viewed by 2 experienced reviewers, who paid particular attention to the thickness of the cellular layer across the neck of the aneurysms, the collagen matrix deposition, and inflammatory reaction within the aneurysmal dome. An ordinal grading system18 was used to evaluate histologic healing. This scale was devised based on findings at the neck and in the dome. Neck coverage was based both on gross and microscopic inspection. Tissue coverage across the neck as noted on gross inspection, in addition to microscopic findings, which included tissue thickness and tissue type, were graded. The scores of the gross and microscopic inspections were averaged to yield a single neck score. Microcompaction assessment was based on the shape of the coil mass across the neck, from convex to concave. Histologic characteristics in the dome were categorized by the attenuation of cellular infiltration and area of organized tissue. The degree of inflammation was defined and scored as 0) no inflammatory cell infiltration; 1) mild, scant, scattered inflammatory cell infiltration; 2) moderate, patchy inflammatory cell infiltration; and 3) marked, attenuated, diffuse inflammatory cell infiltration.20 These scores, the neck average, microcompaction, and dome characteristics, were added together to obtain a total score representative of the pathologic features of the aneurysm. Ordinal logistic fit tests (JMP, SAS Institute) were performed on the total score data, gross neck scoring, micro neck, dome scoring, and inflammation for all groups. If an interaction (Group*Duration) was not found, then a main effect test on the groups was examined. Any significant interaction or group main effect was further investigated with the use of planned comparisons with a Student t post hoc test (JMP, SAS Institute).
Collagen Deposition
We quantitatively analyzed collagen deposition or fibrosis identified with Masson trichrome staining using a recently described and experimentally verified image analysis technique based on Photoshop software (Adobe Systems, San Jose, Calif).21 The fibrosis ratio, the total area of fibrosis within the aneurysmal cavity and neck divided by the total area of the aneurysmal cavity and neck, was calculated for each aneurysm. We analyzed the data using 2-way analysis of variance (least squares fit; JMP, SAS Institute). If an interaction (Group* Duration) was not found, then a main effect test on groups was examined. Any significant interaction or main effect was further investigated with use of planned comparisons with a Student t post hoc test (JMP, SAS Institute).
Results
Angiography
Aneurysmal volume and coil attenuation.
Aneurysmal sizes and volumes are summarized in Table 1. There were no significant differences in aneurysmal size and volume when interactions were examined, nor was there a group main effect. The mean coil-packing densities in the Ad-BMP-13, PA, and FBC groups were 32.0 ± 9.5%, 30.7 ± 9.8%, and 22.6 ± 8%, respectively. There was a significant group main effect (P = .007) for packing attenuation. The Student t post hoc test showed that the Ad-BMP-13 and PA groups were significantly greater than the FBC group (P < .05). There were no significant differences between the PA and Ad-BMP-13 groups.
Table 1:
Summary of aneurysmal sizes and volume by group*
| Group | Neck (mm) | Width (mm) | Height (mm) | Volume (cm3) |
|---|---|---|---|---|
| PA (n = 18) | 2.7 ± 0.9 | 3.2 ± 0.9 | 7.3 ± .4 | 0.07 ± 0.05 |
| FBC (n = 18) | 2.9 ± 1.0 | 3.8 ± 1.0 | 7.7 ± 2.4 | 0.10 ± 0.07 |
| Ad-BMP-13 (n = 20) | 3.1 ± 0.9 | 3.5 ± 0.7 | 7.8 ± 1.3 | 0.08 ± 0.03 |
Note:—PA indicates the bare platinum coil group; FBC, fibroblast-coated platinum coil group; Ad-BMP-13, bone morphogenetic protein-13 gene transfected fibroblast-coated coil group.
Data are represented as mean±SD. There were no significant differences between groups.
Angiographic findings.
One aneurysm at 1 month showed progressive occlusion. Coil compaction was observed in one of the 5 aneurysms at 3 months and 3 of 5 at 6 months. The remaining 15 aneurysms showed stable occlusion (90% occlusion in 2 and 100% occlusion in 13 aneurysms). The overall scores for the 20 cases included 1 (5%) case of progressive occlusion, 4 (20%) with coil compaction, and 15 (75%) with stable occlusion. Findings were compared with previously reported13 historical controls for PAs and FBCs. Occurrences of coil compaction, stable occlusion, and progressive occlusion in these 3 groups are listed in Table 2. A χ2 test (positive vs negative outcome) was performed on the angiographic data. Both stable and progressive occlusions were considered positive outcomes, whereas compaction was considered negative. There were no significant differences in positive or negative outcomes among the groups (P = .6522).
Table 2:
Angiographic outcomes among different groups
| Group | Coil Compaction | Stable Occlusion | Progressive Occlusion |
|---|---|---|---|
| PA (n = 18) | 4 | 14 | 0 |
| FBC (n = 18) | 2 | 11 | 5 |
| Ad-BMP-13 (n = 20) | 4 | 15 | 1 |
Note:—PA indicates the bare platinum coil group; FBC, fibroblast-coated platinum coil group; Ad-BMP-13, bone morphogenetic protein-13 gene transfected fibroblast-coated coil group. There were no significant differences between groups.
Quantitative Histologic Measurements
Total histologic scores.
The histologic scores are summarized in Table 3. The interaction just failed to be significant (P = .0535). A test of the group main effect was significant (P = .0002). A Student t post hoc test with use of planned comparisons showed that the histologic scores in the Ad-BMP-13 (6.8 ± 1.6) and FBC (6.3 ± 2.4) groups were better than the scores from the control group (4.7 ± 2.4).
Table 3:
Summarized histologic scores*
| Group | 14 Days | 1 Month | 3 Months | 6 Months | Group Total Score (All Time Points) |
|---|---|---|---|---|---|
| PA (n = 18) | 2.5 ± 1.1 | 3.1 ± 2.5 | 5.4 ± 1.4 | 6.8 ± 1.6 | 4.7 ± 2.4† |
| FBC (n = 18) | 7.3 ± 1.9 | 4.7 ± 2.8 | 7.4 ± 2.5 | 6.7 ± 2.0 | 6.3 ± 2.4 |
| Ad-BMP-13 (n = 20) | 6.0 ± 1.6 | 6.6 ± 1.8 | 8.0 ± 1.3 | 6.7 ± 1.0 | 6.8 ± 1.6 |
Note:—PA indicates the bare platinum coil group; FBC, fibroblast-coated platinum coil; Ad-BMP-13, bone morphogenetic protein-13 gene transfected fibroblast-coated coil group.
Data are represented as mean±SD.
PA significantly less than FBC or Ad-BMP-13.
Inspection of neck coverage.
The mean gross neck scores for the Ad-BMP-13, PA, and FBC group were 2.5 ± 1.1, 0.9 ± 1.1, and 1.6 ± 1.4, respectively. The test for an interaction was not significant, but the group main effect was (P = .0007). A Student t post hoc test showed that the degree of neck tissue coverage in the Ad-BMP-13 group was significantly higher than that in the FBC and PA groups (P < .05) (Figs 1 and 2).
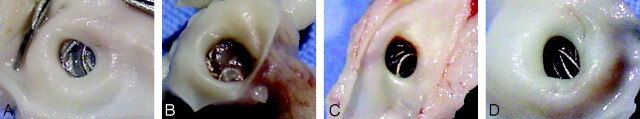
Fig 1.
Gross photographs of neck tissue coverage. A, The 14-day coil loops in Ad-BMP-13–treated aneurysms are embedded within thin translucent membranous tissue, which completely covers the orifice of the neck. B, The Ad-BMP-13 1-month coil loops are embedded within a moderately thick membranous tissue, which completely covers the neck. C–D, The 14-day and 1-month PAs, respectively, show that the coil loops are barely visible at the neck orifice, and no membranous tissue is visible..
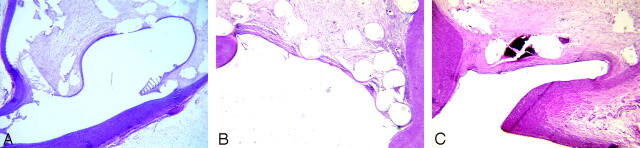
Fig 2.
Photomicrographs of aneurysmal necks at 3 months. A, From the PA group, a thin neointima completely transverses the neck. The tissue appears to be concave to the aneurysm cavity (H&E, original magnification 40×). B, From the FBC group, there is a thin collagenous tissue that completely covers the neck (H&E, original magnification 60×). C, From the Ad-BMP-13 group, there is moderately thick collagenous tissue completely across the neck. (H&E, original magnification 60×). All of the tissue traversing the necks is lined with a monolayer of cells contiguous with the endothelium of the parent arteries.
Collagen formation.
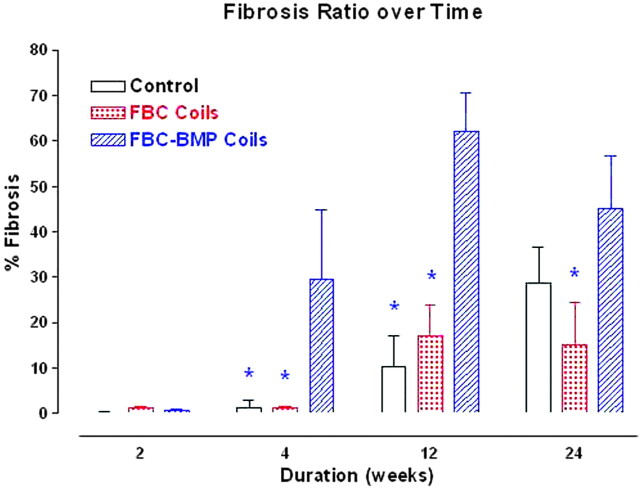
A diffuse, collagenous matrix deposition was noted throughout the aneurysmal dome in 1 of 5 Ad-BMP-13 aneurysms at 14 days. At 1 month, attenuated, diffuse collagen fibers were present in 4 of 5 Ad-BMP-13 aneurysms. Mature, attenuated, diffuse collagen was prominent throughout the aneurysm at 3 and 6 months (Fig 3). For the PA and FBC groups, the collagen was minimal at 14 days. The collagenous matrix remained sparse at 2 months. Thin and loose collagen matrix was evident at 3 and 6 months. The fibrosis ratios are summarized in Table 4. There was a significant interaction (Group*Duration; P = .0402) for collagen deposition. Student t post hoc tests showed that at 1 and 3 months, collagen in the Ad-BMP-13 groups was significantly higher than that in the PA or FBC groups. In addition, at 6 months the Ad-BMP-13 group had significantly greater collagen deposition than the FBC group at 6 months.
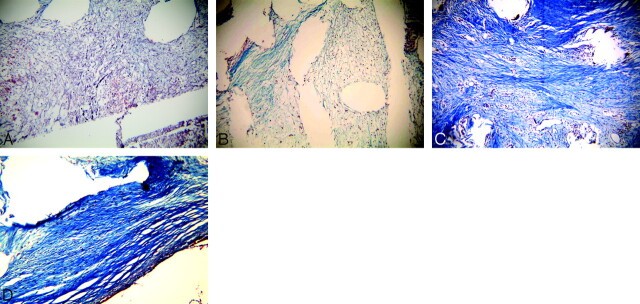
Fig 3.
Photomicrographs of collagen formation. A, From the PA group, there is scant collagen within the aneurysm dome at 1 month (Masson trichrome, original magnification 100×). B, from the FBC group, there is local collagen deposition at 3 months (Masson trichrome, original magnification 100×). C–D, Both from the Ad-BMP-13 group, C shows diffuse, collagenous fibers within the dome at 1 month (Masson trichrome, original magnification 100×), and D shows a denser, more mature collagen within the dome at 6 months (Masson trichrome, original magnification 150×)..
Table 4:
Fibrosis ratio by time
| Group | 14 Days | 1 Month | 3 Months | 6 Months |
|---|---|---|---|---|
| PA (n = 18) | 0.2 | 1.4 | 10.3 | 28.6 |
| FBC (n = 18) | 1.2 | 1.2 | 17 | 15.1† |
| Ad-BMP-13 (n = 20) | 0.7 | 29.5* | 62.2* | 45.1† |
Note:—PA indicates the bare platinum coil group; FBC, fibroblast-coated platinum coil group; Ad-BMP-13, bone morphogenetic protein-13 gene transfected fibroblast-coated coil group.
Variable is significantly different from other groups (P < .05) at 4 weeks and 3 months.
Difference among groups is significant (P < .05) at 6 months.
Inflammatory change.
There was a significant interaction (Group*Duration; P = .0159). Student t post hoc tests showed that at 3 months, inflammation for both Ad-BMP-13 (2.2 ± 0.4) and FBC (2.0 ± 0.0) was significantly greater than that of the PA at 3 months (0.5 ± 0.6). In addition, Ad-BMP-13-treated aneurysms at 1 month (2.0 ± 1.2) had significantly more inflammation than the 1-month FBC (0.7 ± 1.1) aneurysms.
Discussion
Our study demonstrates that collagen deposition was minimal at the early time point (14 days) after implantation with Ad-BMP-13 gene transfected fibroblast-coated coils, bare PAs, and FBCs. Dense, diffuse collagen fibers in the Ad-BMP-13-treated group were noted at 1 month, and the collagen matrix was thicker, more extensive, and more mature with time, whereas the aneurysms treated with PAs or FBCs demonstrated minimal collagen deposition. These results suggest that the improvement in intrasaccular collagenization and fibrosis could result from a genetic, tissue-engineering approach associated with endovascular embolization.
Previous investigators7,22,23 have suggested that platinum coils are biologically inert. On the basis of this theory, various coil modifications have been tested in animal models. In a surgically created aneurysmal model in swine, Dawson et al5 showed that collagen-coated microcoils resulted in an aneurysm completely occluded with a collagen-rich fibrous scar. Szikora et al7 showed that placement of collagen filaments in the lumen of the Guglielmi detachable coil resulted in improved fibrosis in the immediate vicinity of the coil. In the same model, platinum coils covered with a bioabsorbable polymer can accelerate aneurysmal fibrosis.22 However, these methods of coil modification do not address the dearth of fibroblasts in aneurysms embolized with Guglielmi detachable coils. The authors have previously reported that exogenous cells can accelerate thrombus organization in embolized aneurysms6,10 in a small number of subjects in a short duration. The data presented here and published previously13 showed that the total histologic scoring in the Ad-BMP-13 and FBC groups was significantly higher than that in the PA group. Moreover, on the basis of gross and microscopic examinations, neck coverage in the Ad-BMP-13 group was higher than that in the FBC and PA groups (P < .05). The fibrosis ratio in the Ad-BMP-13 group increased significantly with time and was higher than that in the FBC and PA groups at 1 and 3 months, as well as being greater than the FBC group at 6 months. Our previous study13 reported that fibroblast-bearing coils could improve early histologic outcomes but failed to induce collagen formation. Our current study demonstrates that the genetically modified fibroblast with use of BMP-13 gene-bearing coils could improve both the histologic outcome as well as collagen synthesis.
Gene therapy is a technique for correcting defective genes responsible for development of disease. One advantage in gene therapy is the ability to insert the transgene of interest, which can lead to long-term gene expression and protein secretion. BMPs are a group of more than 40 secretor proteins with similarities in their amino acid sequence and are members of the transforming growth factor-beta superfamily. The BMP family regulates the growth and differentiation of a variety of cell types in diverse tissues. BMP-13 has been shown to induce formation of neotendons and neoligaments when applied on a collagen carrier. Our previous in vivo study in rodents with use of adenoviral-mediated gene therapy with BMP-13 demonstrated that BMP-13 can induce robust collagen synthesis.14 In addition, the de novo collagen formed after BMP-13 gene therapy was highly organized. Our current study with use of adenoviral-mediated gene therapy with BMP-13, along with fibroblast allografts and endovascular technique, showed improved aneurysmal fibrosis.
Adenoviral vectors are known to be immunogenic. These data show that Ad-BMP-13 transfected fibroblast-coated coil implantation did not induce a significant inflammatory reaction at the early time point (14 days). At 1 month, the inflammatory response in the Ad-BMP-13 group was significantly higher than that in the FBC, but not the PA, group. At 3 months, the inflammatory response was significantly greater in both the Ad-BMP-13 and FBC groups compared with the control group. In addition, the 3-month Ad-BMP-13 was greater than the 14-day Ad-BMP-13. After 3 months, the inflammatory response in the Ad-BMP-13 group decreased, but not significantly so. The PA group showed a significant increase in inflammatory response from 3 to 6 months, whereas the FBC group remained stable. This suggests that the immune response is short and that it might be addressed with the use of an immunosuppressive agent during the early phase of the experiment.
Aneurysmal size can be responsible for different histologic outcomes after coiling. Wide-necked and giant aneurysms can encounter poor outcome compared with narrow-necked and small-volume aneurysms.1 Aneurysmal neck and volume were compared among groups, and there was no difference. Packing attenuation has been widely accepted as an important determinant of follow-up after coil embolization. Our previous study24 demonstrated that good histologic outcomes were noted in small aneurysms with high packing attenuation. Conversely, larger aneurysms with lower packing attenuation encountered poorer histologic outcomes, which suggest that packing attenuation might have an effect on the histologic response to the coiling after embolization in our current study.
Although rudimentary and not yet validated, the ordinal scale that assessed the neck coverage and dome fibrosis of the aneurysms after coiling revealed that the Ad-BMP-13 and FBC groups had significantly better scores compared with PA. However, it is important to note that at the later time point (6 months), the scores were closer among the 3 groups. It may be that at 6 months tissue response reaches a plateau, when the aneurysms are treated with PA coils alone. Although outcome is the same at 6 months, the Ad-BMP-13 group reaches that plateau sooner, and, from a theoretic standpoint, recanalization becomes less of a threat at an earlier time point after embolization.
The present study had limitations. We did not perform the “empty” gene control (adenovirus vector only, without test gene) and acknowledge that use of this control may partially account for the effect of the immune response in the study. The sample size at some time points in the PA and FBC coil groups was small and unequal. The survival time after embolization was short when viewed clinically. Neither FBC nor Ad-BMP-13 transfected fibroblast-coated coils can be retrieved and repositioned, though this could be easily overcome if commercial groups become interested in the technology.
Conclusion
Although no differences were noted in the angiographic outcomes, ex vivo transduction of fibroblast allografts with replication-deficient adenoviruses containing the BMP-13 gene along with endovascular embolization can, on histologic evaluation, improve neck coverage and dome collagen synthesis in experimental aneurysms.
Fig 4.
A bar chart of percent fibrosis or collagen with time and between groups. The asterisk (*) above a group indicates that it is significantly less (P < .05) than the Ad-BMP-13 group within that same time period.
Footnotes
This work was supported by the National Institutes of Health.
References
- 1.Molyneux A, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17 [DOI] [PubMed] [Google Scholar]
- 2.Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803; discussion 803–04 [DOI] [PubMed] [Google Scholar]
- 3.Murayama Y, Suzuki Y, Vinuela F, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part I: in vitro study. AJNR Am J Neuroradiol 1999;20:1986–91 [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahams JM, Forman MS, Grady MS, et al. Biodegradable polyglycolide endovascular coils promote wall thickening and drug delivery in a rat aneurysm model. Neurosurgery 2001;49:1187–93; discussion 1193–95 [PubMed] [Google Scholar]
- 5.Dawson RC, Krisht AF, Barrow DL, et al. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery 1995;36:133–39; discussion 139–40 [DOI] [PubMed] [Google Scholar]
- 6.Kallmes DF, Williams AD, Cloft HJ, et al. Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology 1998;207:519–23 [DOI] [PubMed] [Google Scholar]
- 7.Szikora I, Wakhloo AK, Guterman LR, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. AJNR Am J Neuroradiol 1997;18:667–72 [PMC free article] [PubMed] [Google Scholar]
- 8.de Gast AN, Altes TA, Marx WF, et al. Transforming growth factor beta-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery 2001;49:690–94; discussion 694–96 [DOI] [PubMed] [Google Scholar]
- 9.Kallmes DF, Fujiwara NH, Yuen D, et al. A collagen-based coil for embolization of saccular aneurysms in a New Zealand White rabbit model. AJNR Am J Neuroradiol 2003;24:591–96 [PMC free article] [PubMed] [Google Scholar]
- 10.Kallmes DF, Borland MK, Cloft HJ, et al. In vitro proliferation and adhesion of basic fibroblast growth factor-producing fibroblasts on platinum coils. Radiology 1998;206:237–43 [DOI] [PubMed] [Google Scholar]
- 11.Murayama Y, Viñuela F, Suzuki Y, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery 1997;40:1233–43; discussion 1243–44 [DOI] [PubMed] [Google Scholar]
- 12.Abrahams JM, Forman MS, Grady MS, et al. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. AJNR Am J Neuroradiol 2001;22:1410–17 [PMC free article] [PubMed] [Google Scholar]
- 13.Dai D, Ding YH, Danielson MA, et al. Endovascular treatment of experimental aneurysms by use of fibroblast-coated platinum coils: an angiographic and histopathologic study. Stroke 2007;38:170–76 [DOI] [PubMed] [Google Scholar]
- 14.Helm GA, Li JZ, Alden TD, et al. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg 2001;95:298–307 [DOI] [PubMed] [Google Scholar]
- 15.Evans CE, Trail IA. Fibroblast-like cells from tendons differ from skin fibroblasts in their ability to form three-dimensional structures in vitro. J Hand Surg [Br] 1998;23:633–41 [DOI] [PubMed] [Google Scholar]
- 16.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol 2000;174:349–54 [DOI] [PubMed] [Google Scholar]
- 17.Cloft HJ, Kallmes DF. Aneurysm packing with HydroCoil Embolic System versus platinum coils: initial clinical experience. AJNR Am J Neuroradiol 2004;25:60–62 [PMC free article] [PubMed] [Google Scholar]
- 18.Dai D, Ding Y, Lewis D, et al. A proposed ordinal scale for grading histology in elastase-induced, saccular aneurysms. AJNR Am J Neuroradiol 2006;27:132–38 [PMC free article] [PubMed] [Google Scholar]
- 19.Dai D, Ding YH, Danielson MA, et al. Modified histologic technique for processing metallic coil-bearing tissue. AJNR Am J Neuroradiol 2005;26:1932–36 [PMC free article] [PubMed] [Google Scholar]
- 20.Ding YH, Dai D, Lewis DA, et al. Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix, and HydroCoil. AJNR Am J Neuroradiol 2005;26:1757–63 [PMC free article] [PubMed] [Google Scholar]
- 21.Dahab GM, Kheriza MM, El-Beltagi HM, et al. Digital quantification of fibrosis in liver biopsy sections: description of a new method by Photoshop software. J Gastroenterol Hepatol 2004;19:78–85 [DOI] [PubMed] [Google Scholar]
- 22.Murayama Y, Tateshima S, Gonzalez NR. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–37 [DOI] [PubMed] [Google Scholar]
- 23.Molyneux AJ, Ellison DW, Morris J, et al. Histological findings in giant aneurysms treated with Guglielmi detachable coils. Report of two cases with autopsy correlation. J Neurosurg 1995;83:129–32 [DOI] [PubMed] [Google Scholar]
- 24.Ding YH, Dai D, Kadirvel R, et al. Relationship between aneurysm volume and histologic healing after coil embolization in elastase-induced aneurysms: a retrospective study. AJNR Am J Neuroradiol 2007. Oct 9; [Epub ahead of print] [DOI] [PMC free article] [PubMed]