Abstract
Background
Nuclear factor-κB (NF-κB) includes a family of signal-activated transcription factors which normally regulate responses to injury and infection, but which are aberrantly activated in many carcinomas.
Objective
To review the activation and role of NF-κB in pathogenesis and as a target for treatment and prevention in carcinoma.
Methods
Evidence from experimental, epidemiologic, pre-clinical studies and clinical trials cited in the literature are reviewed.
Results/conclusion
Cumulative evidence implicates NF-κB in cell survival, inflammation, angiogenesis, spread and therapeutic resistance during tumor development, progression and metastasis of carcinomas. Non-specific natural and synthetic agents that inhibit NF-κB have demonstrated activity and safety in prevention or therapy. NF-κB activating kinases and the proteasome are under investigation for targeted prevention and therapy of carcinoma.
Keywords: aspirin, BMS-345541, bortezomib, carcinoma, CHS-828, dexamethasone, Inhibitor-kappaB, MLN-120B, NF-kappaB, NSAIDs, proteasome, sulindac
1. INTRODUCTION
Originally named for its ability to act as an enhancer element of the immunoglobulin kappa light chain gene in B-cells, NF-κB's role has significantly expanded with demonstration of its contribution in other cells to development, injury, inflammation and the pathogenesis of malignancy. In cancer, NF-κB activation has been linked to cell proliferation, survival, invasion, and angiogenesis, making it a potentially desirable target for therapy. Already, agents that inhibit NF-κB have been shown to reduce tumor growth as well as induce apoptosis in malignant cells. Furthermore, NF-κB has become a potential target for chemoprevention as there is increasing evidence of the critical role it plays in tumorigenesis. As our understanding of the molecules and mechanisms that regulate this pathway increases, so will our ability to selectivity target and inhibit NF-κB with existing and new agents. Recent development of specific small molecule inhibitors has yielded a plethora of agents for both preclinical and clinical studies. Additionally, greater understanding of the activity and uses of existing immunosuppressive and natural compounds that alter NF-κB activity may lead to improved chemoprevention as well as adjuvant therapies to traditional cytotoxic agents and radiation regimens. Thus, interventions that target and attenuate NF-κB activation may broaden our armamentarium in the fight against cancer for use as single agent or combination therapy.
2. NUCLEAR FACTOR-κB (NF-κB) COMPLEXES
2.1 NF-κB family members
There are five members of the NF-κB family, including NF-κB1 (p105/p50), NF-κB2 (p100/p52), REL A (p65), cREL, and RELB, which can associate with one another to form various heterodimeric and homodimeric combinations1, 2. These proteins share a highly conserved REL homology domain that accounts for their ability to dimerize and bind specific DNA sequences in the promoters of many genes. This family of transcription factors is regulated through compartmentalization, with cytoplasmic sequestration in an inactive form maintained by C-terminal or distinct peptides (Inhibitor-kappaBs, IκBs), that contain ankyrin repeats which inhibit nuclear localization and DNA binding. Signal activation by phosphorylation and degradation of these IκBs enables nuclear translocation and promoter binding. Further post-translational modifications, including phosphorylation and acetylation of NF-κB subunits, their co-factors and chromatin structure, can determine whether the complex serves to transactivate or repress gene function1, 2.
2.2 Classical and alternative pathways
The signal activation of these NF-κB and IκB family members has been characterized into two main pathways; a classical or canonical pathway, and an alternative or non-canonical pathway 1, 2(Fig. 1). The classical pathway involves NF-κB1 (p105) which is processed to p50 and bound to RELA (p65) and IκB (inhibitor Kappa B) in the cytoplasm. IκBα is phosphorylated at Serine 32 and 36 by the complex of IKKs (IκB kinases) made up of IKKα, IKKβ, and IKKγ. IKKα and IKKβ are catalytic subunits that share a 52% overlap in there sequence, while IKKγ serves as a regulatory subunit, modulating the activity of the other IKK subunits. This phosphorylation step targets IκBα for ubiquitination by an E3 ligase and degradation by the 26S proteasome, releasing the bound NF-κB1 to be processed and to translocate to the nucleus1, 2. Processing of the precursor of NF-κB1 (p105) requires ubiquitin-dependent proteolysis of their C-terminus transcription modulating domain and ankyrin repeats to yield p50. A serine-threonine protein kinase, CK2, has also been shown to mark the IκBα subunit for degradation by phosphorylation of its C-terminal end3. CK2 also contributes to the aberrant activation of IKKβ and phosphorylation of IκBα4. The classical pathway is known to be stimulated by many proinflammatory cytokines including Tumor Necrosis Factor-alpha (TNF-α), Interleukin-1 (IL-1), bacterial LPS, and growth factors that act through Epidermal Growth Factor Receptor (EGFR), other growth factor receptors, and non-receptor tyrosine kinases 1, 2, 5-7 (Bagain manuscript in preparation).
Fig. 1.
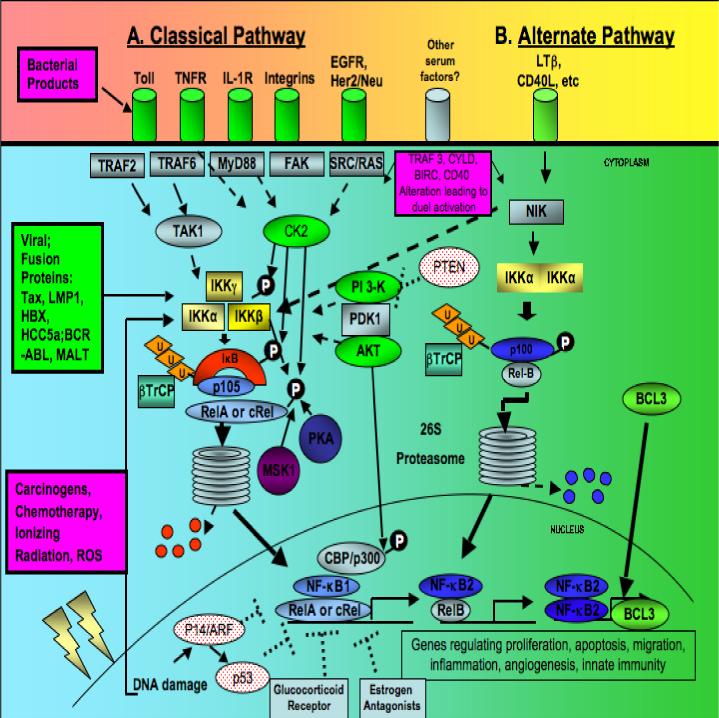
NF-κB activation in pathogenesis of carcinoma. A. The classical pathway, involving p105 (NF-κB1) and RelA activation. B. The alternative pathway stimulated through NIK and IKKα homodimers, involving NF-κB2 and RelB. Abbreviations. NIK, NF-κB inducing kinase; IL1R, interleukin 1 receptor; EGFR, epidermal growth factor receptor; TNFR, Tumor necrosis factor receptor; TRAF, TNF receptor-associated factor; TAK, transforming growth factor-β-activated kinase; FAK, focal adhesion kinase; CK2, casein kinase 2; PI3K, phophatidylinositol 3-kinase; PDK, 3 phosphoinositide-dependent protein kinase; PKA, protein kinase A; MSK1, mitogen and stress activated protein kinase 1.
The alternative pathway, originally defined in cells of the hematopoietic lineage, is triggered by stimuli such as CD40L and lymphotoxin β receptor, which activate NF-κB inducing kinase (NIK). NIK then induces IKKα/ IKKα homodimers, which activates the processing of p100/RelB into NF- κB2 p52/RelB heterodimers via the proteasome1, 2. As in classical pathway signaling, the process appears to involve a ubiquitin-dependent process, however involvement of IKKβ and IKKγ is not needed in the alternative pathway1, 2.
The transactivating function of NF-κB family members can be altered as a result of other post-translational modifications or interactions with various co-factors. RELA (p65) and other family members may be altered by phosphorylation at various sites on their domain. RELA has been shown to be phosphorylated at serine 536 by IKKβ8, at serine 529 by CK29, and serine 276 by PKA10. Additionally, stress activated protein kinase (MSK1)11 has been shown to promote activation of RELA. Lastly a series of interactions with AKT, CBP/p300 and p14ARF/p53 may also differentially regulate NF-κB activation or repression of downstream genes12-14.
3. NF-κB AND CARCINOMA
3.1 NF-κB, infection, inflammation, and carcinogens in the development of cancer
Experimental and epidemiologic evidence indicates that pathogen, carcinogen, and inflammation-induced activation of NF-κB plays direct or indirect roles in cellular promotion, transformation and progression of both experimental and human cancers15, 16. Evidence implicating pathogen-induced NF-κB activation in the development of cancer originated with identification of the REV-T viral oncogene that causes avian reticuloendothelial lymphomatosis, v-Rel16, 17, which shares a Rel transactivation domain with the mammalian homologues RELA (p65), cREL and RELB.
Figure 1 summarizes a number of alterations and signal pathways that have been implicated in activation of NF-κB in human cancers (reviewed in 18). These include Epstein Barr virus gene LMP-1, and human T lymphocyte virus gene Tax, which encode proteins that activate NF-κB and gene programs that contribute to the pathogenesis of lymphomas, nasopharyngeal carcinomas and adult T-cell leukemias, respectively. Human papilloma virus (HPV) genes E6 and E7 genes have also been implicated in NF-κB activation, and are associated with premalignant and malignant lesions of the cervix, oropharynx and larynx, where HPV associated neoplasms are prevalent. In hepatocellular carcinoma, hepatitis B X protein and hepatitis C 5A and core proteins have been shown to activate NF-κB. Gastric H. pylori and colonic bacteria in patients with ulcerative colitis have also been implicated in NF-κB activation in epithelia and inflammatory leukocytes, and promotion of gastric and colon carcinomas.
Several known chemical and physical carcinogens implicated in the initiation and promotion of human cancer can also activate NF-κB. Specifically, nicotine and carcinogens in tobacco and betel nut (areca), which are linked to the pathogenesis of both head and neck as well as lung malignancies, induce AKT and NF-κB activation, promoting cell proliferation, survival and inflammation15, 16, 19. Nicotine has been reported to directly activate these pathways via nicotinic receptors and AKT 19. Chemotherapy and radiation-induced DNA damage has been reported to induce NF-κB activation via a nuclear to cytoplasmic signaling mechanism involving sumoylation of the IKK complex20. TNF, gamma radiation, and certain chemotherapeutic drugs, induce several NF-κB target anti-apoptotic genes (TRAFs, IAPs and Bcl-2 and Bcl-XL) that protect cells from therapeutic injury by these agents16.
3.2 Aberrant activation of NF-κB in carcinoma
Aberrant activation of NF-κB is prevalent in cell lines and tumor tissue specimens, and contributes to malignant progression and therapeutic resistance in most of the major forms of human cancer. As recently reviewed18, NF-κB/RELs are constitutively activated in human carcinomas of the breast, head and neck, esophagus, cervix, prostate, lung, colon and pancreas.
Aberrant activation of upstream tyrosine receptor and non-receptor kinases via IKKs or other kinases represents the most common etiology for NF-κB activation in epithelial and lymphoid malignancies (Fig. 1) 18. Autocrine or paracrine activation of NF-κB resulting from overexpression of TGF-α, Epidermal Growth Factor (EGF), Her2/Neu, IL-1, Hepatocyte Growth Factor (HGF), and integrin family ligands and receptors, has been reported. EGFR and her2/neu signaling involving PI3K, IKK and CK2 has been demonstrated in breast cancer. IL-1/IL-1R, Transforming Growth Factor-α (TGF-α)/EGFR, PI3K, AKT, CK2 and IKK have been shown to mediate activation in head and neck squamous cell carcinomas. Activation via CK2 and IKK is observed in colon carcinomas. PI3K/AKT and IKK are important in signal activation of NF-κB and cell survival in many cancers. Constitutive activation of NF-κB p52:p52 due to overexpression and association with the transactivating family member Bcl-3 has been detected in breast carcinomas (Fig. 1D).
Although established human carcinomas frequently demonstrate activation and a promotional role of NF-κB, several experimental studies in murine models suggest that early in carcinogenesis, NF-κB and IKKs may play an inhibitory rather than promotional role in carcinoma development. Inhibition of NF-κB with activation of oncogenic ras in human keratinocytes was reported to promote development of malignant human epidermal lesions resembling squamous cell carcinoma in mice21. Targeted deficiency of IKKβ in liver was reported to increase susceptibility of mice to diethylnitrosamine (DEN)-induced hepatocarcinogenesis22. Targeted deficiency of IKKγ/NEMO in liver parenchymal cells resulted in chronic liver disease resembling human nonalcoholic steatohepatitis and spontaneous hepatic carcinoma23. However, others have reported that inhibition of NF-κB inhibits murine and human squamous cell carcinoma tumorigenesis in mice18, or cholestatic hepatitis associated hepatocellular carcinomas in Mdr2 knockout mice24. These differing results suggest the possibility that the cancer promoting role of NF-κB in certain contexts may be tissue or carcinogenesis dependent, or may involve additional steps that are not well understood.
Among such steps, loss of the inhibitory effects of certain tumor suppressor genes may be important in function of NF-κB as a prosurvival factor in carcinomas (Fig. 1). Evidence indicates that activation of functional ARF, ATR, and Chk2 normally mediates alternative phosphorylation of RELA, and p53 competes for CBP/p300, maintaining repression of NF-κB prosurvival genes when the ARF-ATR-p53 pathway and proapoptotic target genes are activated 14. Inactivation of this proapoptotic pathway by hypermethylation of p14ARF/p16INK4A or mutation or HPV inactivation of p53 are among the most common alterations in squamous cell carcinomas and other human cancers 14. Loss of the inhibitory effects of TGFβ signaling on NF-κB and inflammatory gene expression represents another common event that is potentially important in development of squamous cell carcinomas25. Loss of PTEN expression is associated with increased PI 3-kinase/AKT signal activation of NF-κB and c-MYC in prostate carcinoma cells26.
Direct mutation or altered expression of NF-κB/IκB molecules has only rarely been reported in human cancers, such as in Hodgkins lymphomas, where mutations of IκB that favor activation have been identified27, 28. Further, recent studies in human cancer indicate that the classical and alternate pathways may not be activated individually as identified in their tissues of origin in knockout mice (i.e. classical in epithelia and hematopoietic development, alternate pathway in lymphoid development)1. In some circumstances, both pathways may be co-activated via the same mechanisms. Annunziata et al., demonstrated that various genetic or epigenetic alterations affecting NIK, TRAF3, CYLD, BIRC2/BIRC3, CD40, NFKB1, or NFKB2 are common and can promote activation of the classical and alternative pathways in human multiple myeloma (MM) tumors and cell lines 29. They provided evidence that NIK may activate not only the alternate pathway via IKKα, but also the classical pathway via a previously uncharacterized IKKβ-dependent mechanism. As a result, many MM tumors were found to be sensitive to IKKβ inhibitors. Additionally, Allen et al., recently demonstrated co-activation of classical and alternate pathway NF-κB subunits in head and neck squamous cell carcinomas30. They showed that proteasome inhibitor bortezomib more effectively inhibited nuclear localization of RELA and p50 than cREL or alternate pathway subunits NF-κB2 or RELB in tumor specimens and lines from patients with HNSCC. In addition, IKKα has recently been implicated in promoting activation of the mTOR pathway 31. Together, these results highlight the need to develop pharmacologic agents for IKKα and other targets in the alternate pathway, in addition to IKKβ and proteasome inhibitors, which have been the subject of greatest interest to date.
NF-κB has been shown to regulate many of the genes differentially expressed and implicated in cell proliferation, survival, migration, and tumorigenesis and metastasis in cancer. NF-κB related gene signatures have been identified and associated with malignant phenotype in squamous cell carcinomas32-37, Hodgkins and certain non-Hodgkins lymphomas38, 39, and inflammatory breast cancer40. Targets of NF-κB important in cell proliferation and survival include prominent oncogenes such as cyclin D, Bcl-XL, and Inhibitors of Apoptosis (IAPs) 35-37, 41-43. Expression of key angiogenesis factors and adhesion molecules such as GRO1, IL-8 and VEGF are directly or indirectly enhanced by NF-κB activation5-7, 44. Together, these genes contribute to the increase in proliferation, survival, inflammation and angiogenesis that leads to rapid tumorigenesis and metastasis.
4. MOLECULAR TARGETED THERAPY
As knowledge of the pathways and complexes involved in the activation of NF-κB has grown, so has the list of possible targets for its inhibition. Several key targets exist including the IKK's, the 26S proteasome, CK2, and PPAR-γ. While all of these have been shown to play a role in NF-κB activation, the contribution of each may vary from cancer to cancer and thus the efficacy of targeting them. These targets also vary in their specificity toward NF-κB. Inhibition of the IKK's has been demonstrated to have a relatively specific inhibition of NF-κB. In contrast, targets such as the proteasome, PI3K, PKA and CK2, while potentially efficacious for inhibiting NF-κB, but also affect other pathways, making their therapeutic effects more complex to understand.
4.1 Proteasome Inhibition
For the canonical NF-κB transcription factors (i.e., NF-κB1/RELA) to be translocated from the cytoplasm to nucleus and bind DNA targets, IκB must be ubiquitinated by the SCF-β-TrCP ubiquitin ligase complex and then degraded by the proteasome. The proteasome is a 26S multiprotein complex that consists of a 19S regulatory subunit and a 20S catalytic subunit that contains six unique ATP-dependent serine protease sites which hydrolyze proteins into small polypeptides', leading to their inactivation45. Through its regulation of protein recycling, the proteasome is involved in many processes that are important in cancer such as cell-cycle progression, apoptosis and angiogenesis46,47. Very specific and potent proteasome inhibitors have been engineered by coupling boronic acid to dipeptides48. PS-341, or bortezomib, a dipeptide boronate, is the most well studied proteasome inhibitor that has currently entered clinical development 39. Bortezomib has been shown to inhibit proliferation as well as induce apoptosis in a variety of carcinomas, such as head and neck30, 46, 49, prostate47, pancreatic50, gastric51, and ovarian52 carcinomas. The anti-tumor properties of bortezomib correlate in part with its ability to inhibit the degradation of the IκBα46, 53. However, targeted NF-κB inhibitors such as the IKK inhibitor PS-1145, do not entirely reproduce all of bortezomib's therapeutic activities, therefore implicating the significance of additional proteins which are also regulated by proteasome inhibitors54. Global inhibition of protein degradation resulting in endoplasmic reticulum induced stress responses has recently been implicated in sensitivity and resistance to proteasome inhibition50. New allosteric inhibitors of the proteasome are also in development which can preferentially inhibit the degradation of specific proteasome targets, such as IκBα55. These results from preclinical studies as well as clinical trials demonstrate that proteasome inhibitors can potently inhibit proliferation and induce apoptosis in a variety of otherwise resistant cancer cells, and sensitize tumors to adjuvant chemotherapy and radiation therapy. However, the low response rates in monotherapy trials of proteasome inhibitors in patients with carcinomas have been disappointing, when compared to those in MM and other B cell malignancies 18. Emerging evidence suggests that pre-existing and/or induced activation of other prosurvival signal pathways and transcription factors in carcinomas contributes to resistance to proteasome inhibitors30. In addition to the activation and relatively lesser effect of proteasome inhibition on the alternate pathway subunits, there is activation of other prosurvival pathways, including MAPK (ERK, JNK, p38) and STAT3, which are not inhibited by proteasome inhibitor therapy in tumors or cell lines from patients with head and neck squamous cell carcinoma30, 56. Further studies reveal that proteasome inhibitors can induce JNK, ERK and AP-activation and in resistant human HNSCC and other malignancies56. Proteasome inhibitors are also being studied in combination with other chemotherapy agents and radiation in which proteasome and NF-κB inhibition enhances cytotoxicity18, 30, 57.
4.2 IKK Inhibition
The IKK complex is a target shown to be a dedicated activator of NF-κB activity1, 2. Rational design and construction has yielded several potent and selective inhibitors. Most have a predominant effect on IKKβ, but there are some that show activity against IKKα as well. CHS-828 is probably the most clinically advanced of the IKKβ inhibitors. A phase I trial with single daily dose for 5 days every 28 days for refractory solid tumors showed dose limiting toxicities of nausea, vomiting, diarrhea, fatigue and urogenital tract mucositis58. There were no observed tumor responses. A second trial CHS-828 was given 1 day every 3 weeks at increasing doses. At the 500mg dose, 2/3 of patients experienced a dose limiting toxic effect59.
BMS-345541 targets the allosteric sites of IKKα and IKKβ60. Yang has been able to show the of IKK inhibition as effective therapy in experimental melanoma models, achieving apoptosis through a mitochondrial mediated pathway61. In a subsequent study, via that knockdown of endogenous IKKβ, Yang showed it significantly reduced the growth of the melanoma lesions and knockdown of either IKKα or IKKβ prolongs the life span of immunocompetent mice62.
The inhibitor MLN120B showed activity against multiple myeloma cells in a SCID mouse model63. It was able to cause 25% to 90% inhibition of cell growth in vitro and augment TNF induced toxicity. Additionally, MLN120B augmented growth inhibition initiated by doxorubicin and melphalan in multiple myeloma cell lines RPMI 8226 and INA6.
PS-1145, a beta-carboline, has shown activity in HeLa cells (cervical carcinoma) by reducing NF-κB activity64. In prostate cancer, PS-1145 has been able to reduce proliferation and invasiveness and attributable to NF-κB activity reducution65. Another study demonstrated that PS-1145 in combination with docetaxel resulted in cell death and lower IL-6 levels in prostate cancer.66 However, PS-1145 and MLN 120B did not show significant anti-tumor activity in most cultured HNSCC lines (J Ricker, L Nottingham and C Van Waes, unpublished observations), and this was associated with incomplete NF-κB inhibition, consistent with a role of non-canonical IKKα and NF-κB activation observed in these cancers4, 30.
BAY 11−7085, an irreversible inhibitor of IκBα phosphorylation was shown to inhibit NF-κB activation and have anti-tumor effects in vitro and in vivo against Caov-3 ovarian cancer models67, 68. In combination with paclitaxel or cisplatin, Bay 11−7085 showed increased cell death, by reducing the normally induced NF-κB activation. Additionally, further synergy against intra-abdominal dissemination and production of ascites in athymic nude mice inoculated intra-peritoneally with the ovarian cancer cell line, as the further evidence for the use of NF-κB targeted inhibition in combination with cytotoxic agents.
Because IKKβ plays an important role in macrophage IL-1β response to pathogens, there remains concern that highly efficient or prolonged IKKβ blockade could lead to unexpected complications. Also to date, no studies of specific IKKα inhibitor activity or toxicity in cancer have been published. Considering that IKKα is part of the canonical IKK complex and implicated in several nuclear mechanisms that enhance expression of genes activated by the classical pathway, and is a key activator of the alternative pathway, its inhibition may also merit investigation as a modulator of NF-κB targeted in cancer therapy.
4.3 CK2 Inhibition
CK2 (formerly casein kinase II) is a protein kinase with multiple substrates that include NF-κB, WNT, PI3K and many other signal pathways69.Initially, CK2 was shown to activate NF-κB in the context of UV-radiation. We recently demonstrated it also plays a significant role in activation of IKKβ and RELA in head and neck cancer cells4. CK2 and its targets regulate broad cellular processes , including maintenance of cell viability, protection of cells from apoptosis, and tumorgenesis, and elevated CK2 activity has been established in a number of cancers.
Most CK2 inhibitors, as derivatives of tetrabromobenzimidazole/triazole and indoloquinazolines have their selectivity by binding a small hydrophobic pocket adjacent to the ATP/GTP binding site. One study showed induction of cell death in anti-estrogen resistant human breast cancer cells by the protein kinase CK2 inhibitor DMAT70. Specifically this study pointed to reduced amounts of BCL2 in estrogen independent breast cancer cells as a reason for CK2 susceptibility and not necessarily a reduction in NF-κB. DMAT showed synergy in inhibiting tumorigenesis in vivo when combined with the tyrosine kinase inhibitor Imatinib while showing minimal kidney, liver and bone marrow toxicity71.
Apigenin is a natural plant flavone in parsley onions, oranges, tea and chamomile, making it a potential anti-oxidant and anti-inflammatory chemoprevenative agent72. It exhibits CK2 inhibitory affects, stabilizing IκBα and down regulating NF-κB activity. It has been shown to have anti-CK2 activity in both prostate and breast cancer73, 74 and decreased the volume and weight of prostate tumor xenografts upon treatment75.
A more novel approach to therapy using antisense oligonucleotides may yet provide another specific way of targeting this molecule. Ahmed and colleagues have shown therapeutic effects on prostate and head and neck cancer both in vivo and in vitro, with a relative increase in sensitivity of cancer cells compared to normal tissue76, 77. Nanocapusule delivery mechanisms as vectors to promote tumor-specific uptake of the oligonucleotides may increase the selectivity and potency of antisense or siRNAs targeting CK2.
4.4 Selective estrogen receptor modulators (SERMS)
Selective estrogen receptor (ER) modulators, commonly referred to as SERMS, are a class of compounds that interact with subsets of estrogen receptors with discriminating tissue specificity. The relationship between estrogen receptor ligands and inflammation had long been known in clinical practice, in that excess estrogen, especially in pregnancy, was known to subdue symptoms of inflammatory diseases like Crohn's disease and ulcerative colitis. ER ligands have been developed which selectively inhibit NF-κB and thereby inflammation without induction of the classic effects of estrogen78. Estrogen has been hypothesized to promote breast cancer, and activated NF-κB has been detected in a majority of breast cancers which lacked ER receptor expression. DNA bound NF-κB was practically absent in ER positive specimens,79 supporting the hypothesis of the negative effects of ER on NF-κB activation. Tamoxifen, a SERM with wide clinical application, has been shown to inhibit TNF induced NF-κB activation in human embryonic kidney cells and KBM-5 leukemic cell line, through inhibiting IKK activity and suppressing IκBα degradation.80
4.4 Peroxisome proliferator-activated receptor (PPAR)
Peroxisome proliferator-activated receptor (PPAR)-γ is a nuclear receptor and transcription factor of the steroid superfamily. It has recently been implicated as a regulator of cellular proliferation and inflammatory responses. The ligands of PPAR-γ include several prostanoids, such as 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2), polyunsaturated fatty acids, a variety of nonsteroidal anti-inflammatory drugs (NSAIDs), and a new class of oral antidiabetic agents, the thiazolidinediones (TZDs)81, 82. Su et al demonstrated in colon carcinoma Caco-2 cells that 15d-PGJ2 inhibits the nuclear translocation and subsequent DNA binding of NF-κB via an IκB-α–dependent pathway by inhibiting the immune response–induced degradation of IκB-α.83 PPAR-γ ligands can also inhibit tumor-associated angiogenesis by blocking the production of ELR+CXC chemokines, which is mediated through antagonizing NF-κB activation.84
5. ANTI-INFLAMMATORY AND IMMUNOMODULATING AGENTS
The connection between chronic inflammation and cancer has been at the center of many recent genetic and epidemiologic studies. Many NSAIDs have been studied for the anti-inflammatory role in chemoprevention, such as aspirin, sulindac, and more selective COX-2 inhibitors.85 Additionally, other agents such as sulfasalazine and glucocorticoids have been shown to inhibit inflammation and NF-κB activation.
5.1 Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
While NSAIDs prevent the synthesis of prostaglandins by inhibiting cyclooxygenase (COX) activity, some of their anti-inflammatory and cancer-preventative effects may be independent of COX, through inhibition of NF-κB activation. This inhibition can take place at multiple levels of the pathway, including IKK activity, proteasome-mediated degradation of IκB, as well as the nuclear activity and DNA binding of the transcription factors. It has been demonstrated that high concentrations of aspirin and sodium salicylate can inhibit the LPS-induced NF-κB-dependent transcription by preventing IκBα degradation86. This effect was later confirmed on TNF-α-induced activation of NF-κB and attributed to the inhibition of both IKKα and IKKβ kinase activity87. IKKβ inhibition is due to a competitive effect of aspirin and salicylates with ATP binding to IKKβ, reducing the phosphorylation of IκB and subsequent NF-κB activation86.
Anti-tumor and preventative effects of aspirin and other NSAIDs have been demonstrated in a number of studies. Aspirin inhibited the growth of colorectal carcinoma cell lines both in vitro and in vivo88, and regular aspirin use has been shown to prevent colorectal adenomas, reducing the risk of colorectal carcinoma in clinical trials89, 89. Variable inhibition of NF-κB activity has been found with several other NSAIDs via inhibition of IKK activity. Independent of COX-inhibition, Sulindac inhibits growth of polyps and precancerous lesions in the colon, especially in association with familial adenomatous polyposis. Sulindac can prevent IκBα degradation and subsequent NF-κB nuclear translocation by inhibiting the catalytic activity of IKKβ85. In NSCLC cells, sulindac enhanced TNF-α-mediated apoptosis by inhibiting NF-κB activation91. Ibuprofen has been shown to inhibit the constitutive activation of IKKα and NF-κB in androgen-independent prostate tumor cells92.
A novel mechanism for aspirin's inhibitory effects on NF-κB-driven transcription has been recently suggested. In colon cancer cells, aspirin's long-term suppressive effects on NF-κB were associated with the inhibition of IκBα degradation to prevent relocalization of RELA into the nucleolus93. By sequestering RELA away from target promoters, aspirin reduced the transcription of NF-κB-dependent anti-apoptotic genes, promoting apoptosis92. Further, selective COX-2 inhibitor celecoxib suppressed NF-κB activation, RELA phosphorylation, and nuclear translocation in human non-small cell lung carcinoma by inhibiting IKK activity94. The same study demonstrated that the COX-2 promoter, which is regulated by NF-κB, was also inhibited by celecoxib. Thus, inhibiting NF-κB has the additional anti-tumor effect of decreasing the expression of NF-κB downstream target gene, Cox-2. Recently, Meloxicam, a preferential COX-2 inhibitor that has a higher degree of COX-1 inhibition than selective COX-2 inhibitors such as celecoxib, induced apoptosis in esophageal squamous cell carcinoma (SCC) in patients through inhibiting NF-κB regulation of COX-295, which is similar to the mechanisms by which aspirin affected esophageal SCC TE-13 cells in vitro96.
These results suggest that NSAIDs effects on NF-κB activity may be both drug and cell type specific; that NSAIDs exhibit potential chemopreventative activity against various carcinomas such as lung cancer, colon cancer, prostate cancer, and esophageal cancer; and that such effects could be mediated at least partially by regulating NF-κB activity96, 97.
5.2 Sulfasalazine
Sulfasalazine, which has been routinely used for the treatment of rheumatoid arthritis and inflammatory bowel disease, is an anti-inflammatory derivative of 5-amino salicylic acid (5-ASA). Mechanistically, sulfasalazine has been shown to inhibit NF-κB activation98 via a direct inhibition of ATP binding to IKKα and β99. Further, while its metabolite 5-ASA lacks intrinsic IKK-inhibitor properties, it was shown to reduce p65 phosphorylation and NF-κB-dependent transcription in Caco-2 cells, a colon adenocarcinoma cell line100. Recently, in human renal cell carcinoma, sulfasalazine demonstrated inhibition of NF-κB and blocked tumor cell growth by inducing cell apoptosis in vitro101. Clinical trials using sulfasalazine initiated to assess its anti-cancer potential for treatment in recurrent malignant gliomas have not demonstrated significant activity as a single agent.
5.3 Glucocorticoids
Glucocorticoids represent another class of agents with strongly established anti-inflammatory and anti-tumor activities, at least partially mediated through inhibition of NF-κB102, such as inhibition of IKK activity, DNA binding, and transcription of the IκBα gene103. Glucocorticoids are commonly administered and have activity for the treatment of hematopoietic malignancies particularly dependent on NF-κB activation, including certain leukemias, lymphomas and multiple myeloma. To date however, little evidence suggests similar efficacy of glucocorticoids in carcinoma therapy.
5.4 Immunosuppressive Agents
Like glucocorticoids, immunosuppressive agents have been studied less extensively in carcinomas than in lymphoid tumors. However, thalidomide and its analogues, classified as immunomodulatory drugs, have shown promising activity in hematological malignancies such as chronic lymphocytic leukemia and myelodysplastic syndromes as well as in some solid tumors such as prostate cancer, melanoma and progressive brain tumors104. These agents have been shown to possess potent anti-NF-κB properties. Thalidomide and its analogues have demonstrated perhaps the most promising results in multiple myeloma therapy105.
Rapamycin and its derivative compounds, in complex with their cellular receptor FK506-binding protein (FKBP12), act on cell growth and proliferation mainly by inhibiting the mammalian target of rapamycin (mTOR)106. However rapamycin might serve as a potent new agent to overcome drug resistance through its inhibition of NF-κB. In melanoma cells, rapamycin inhibited IKK activity and decreased NF-κB nuclear translocation induced by doxorubicin treatment.107
6. NATURAL AGENTS AS INHIBITORS OF NF-κB
Natural agents or their derivatives have been attractive sources of medicines for centuries. Most of them have been in use in crude formulations as folk medicines in diverse cultures all over the world. An added attraction of natural agents is that they are relatively inexpensive and culturally more accepted. There are a number of natural compounds which show inhibitory effects on NF-κB that may warrant investigation of their potential for prevention of cancer or other diseases associated with inflammation.
Curcumin
Curcumin (diferuloylmethane) 1,7-bis-(4-hydroxy-3-methoxy phenyl)-1,6-heptadiene-3,5-dione, is a polyphenol derived from the plant Curcuma longa, commonly called turmeric. Turmeric is a member of the ginger family. Its rhizomes produce a brilliant yellow dye. The primary bioactive constituents in turmeric have been found to be the phenolic curcuminoids, the most important of which is curcumin (diferuloylmethane). The role of curcumin in inhibiting NF-κB has been well studied in cancer cell lines, such as ovarian 108, breast109, head and neck cancer 110, lung111, prostate112 and others. Many head and neck squamous cell carcinomas constitutively express active NF-κB, and treatment with curcumin inhibited NF-κB as monitored by DNA binding, IKK activation, and p65 nuclear translocation, thus leading to suppression of expression of the NF-κB regulated proteins Bcl-2, IL-6, cyclin D1, COX-2, MMP-9110. This was associated with inhibition of proliferation and induction of apoptosis in these cell lines110, 113.
Resveratrol
Resveratrol, is a polyphenol (trans-3, 4′, 5-trihydroxystilbene) abundant in red grapes, berries, and peanuts. As early as 1997, resveratrol was found to be a potent chemopreventive agent, blocking the initiation, promotion, and progression of tumors induced by the aryl hydrocarbon dimethylbenz(a)anthracene (DMBA).114 Since that time, resveratrol has been shown to inhibit the growth of a wide variety of tumor cells, including lymphoid and myeloid; cancers of breast, prostate, and thyroid; melanoma; head and neck squamous cell carcinomas; and ovarian and cervical carcinomas.
Resveratrol has been shown to mediate down-regulation of various proliferative and antiapoptotic gene products, including cyclin D1, cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, and TRAF2 through suppression of constitutively active NF-κB through inhibition of IKK and the phosphorylation of IκBα and of p65 in human myeloma cell lines. 115
Green tea and EGCG
Green tea is a common drink in many Eastern as well as Western countries, with remarkable anti-inflammatory and cancer chemopreventive effects found in many animal tumor studies, cell culture systems, and epidemiologic investigations.116 Epigallocatechin 3-gallate (EGCG), the major polyphenol present in green tea, has been shown to inhibit TNF-alpha induced degradation of IκB and activation of NF-κB in human epidermoid carcinoma (A431) cells and in normal human epidermal keratinocytes at high concentrations.117
Parthenolide
Parthenolide is a sesquiterpene lactone present in several medicinal plants that have been used in folk medicine for their anti-inflammatory and analgesic properties. In vitro, it inhibits NF-κB by preventing the TNF-alpha-induced IKKβ activation. Recently, parthenolide inhibited pancreatic cancer cell growth in BxPC-3, PANC-1, and MIA PaCa-pancreatic carcinoma cell lines118. Parthenolide treatment also increased the amount of the inhibitory protein, IκBα, and decreased NF-κB DNA binding activity. The effect of this inhibition was found to be synergistic with sulindac, an NSAID.118
Kambekaurin
Isodon japonicus is a medicinal plant that has been used in folk medicine in China, Japan, and Korea as a remedy for gastrointestinal disorder, tumor, and inflammatory diseases. In an effort to identify the compound(s) that account for the anti-inflammatory property of Isodon japonicus, Hwang et al have reported several kaurane diterpenes from this plant with significant inhibitory effects on the NF-κB activation, prominent among them being kambekaurin, in the treatment of cancer.119 Treatment of cells with kamebakaurin prevented the tumor necrosis factor-alpha (TNF-α)-induced expression of antiapoptotic NF-κB target genes encoding c-IAP1 (hiap-2) and c-IAP2 (hiap-1), members of the inhibitor of apoptosis family, and Bfl-1/A1, a prosurvival Bcl-2 homologue, and augmented the TNF-α-induced caspase 8 activity, thereby resulting in sensitizing MCF-7 cells to TNF-α-induced apoptosis.120
Other Natural Inhibitors of NF-κB
Silymarin and silibinin (a component of silymarin) form another class of compounds that can effectively target the NF-κB pathway. Silybinin was shown to inhibit constitutive activation of NF- κB in DU145 human prostate adenocarcinoma cells.121 It was also found to have chemopreventive effects on N-butyl-N-(4-hydroxybutyl) nitrosamine induced bladder cancers in mice. 122
CONCLUSIONS AND EXPERT OPINION
The alterations in multiple signal pathways shown to positively and negatively regulate NF-κB activation are consistent with its frequent activation and important functional role in carcinoma. The redundancy in alterations that may lead to activation can also explain in part why drugs targeting receptors and kinases further upstream are effective primarily in cancers in which limited alterations and a dominant role of these signaling pathways are established. Agents with broad activities such as NSAIDS, corticosteroids and proteasome inhibitors have already demonstrated evidence of efficacy and safety in prevention or therapy of certain cancers, often before the role of NF-κB among their mechanisms of action was identified. Similarly, many natural products initially identified as having beneficial dietary or anti-inflammatory medicinal effects have been shown to have inhibitory effects on activation of NF-κB and NF-κB associated inflammation, and are the subject of renewed interest, possibly as chemopreventative agents. The important role of CK2, IKKs, ubiquitinating enzymes and proteasome in integrating these signals and the final steps of activation have recently made these important molecules for study as potential targets for prevention and therapy.
Although there is now considerable evidence for the role of IKKs, NF-κB and the proteasome in cancer, and beneficial therapeutic and preventative effects of inhibitors of these targets, their role in cellular homeostasis and immunity has been implicated in some of the toxicities and narrow therapeutic windows observed with these agents. Concerns about NF-κB's and proteasome's broad expression and important functions as with DNA and hormone receptor targeted agents have not prevented careful development of some of the most important anti-cancer drugs, including corticosteroids, NSAIDs, proteasome inhibitors and SERMs, which have cytoplasmic or nuclear IKK or NF-κB inhibitory effects. Libraries of siRNAs, natural, and synthetic products are currently being exploited for the discovery of key targets involved in NF-κB activation in specific tissue and cancer subtypes, and to discover and identify drugs with different tissue selectivities and lower toxicity profiles for use in prevention or therapy. Combinations with existing cytotoxic drugs, radiation are also being explored to take advantage of sensitization that results from NF-κB inhibition during DNA damage, in an effort to further enhance the efficacy of these agents. Increased appreciation and understanding of the activation of several different prosurvival signal activated transcription factors in many carcinomas has resulted in improved understanding of the mechanism of drug resistance, and recognition of the need to investigate combinations of these targeted agents for preventive or therapeutic activity. Many natural products while active in GI tract or skin have been found to have poor systemic bioavailability. Efforts to utilize classical and newer engineering approaches in medicinal chemistry to modify these products holds promise for development of additional agents for systemic uses.
Fig. 2.
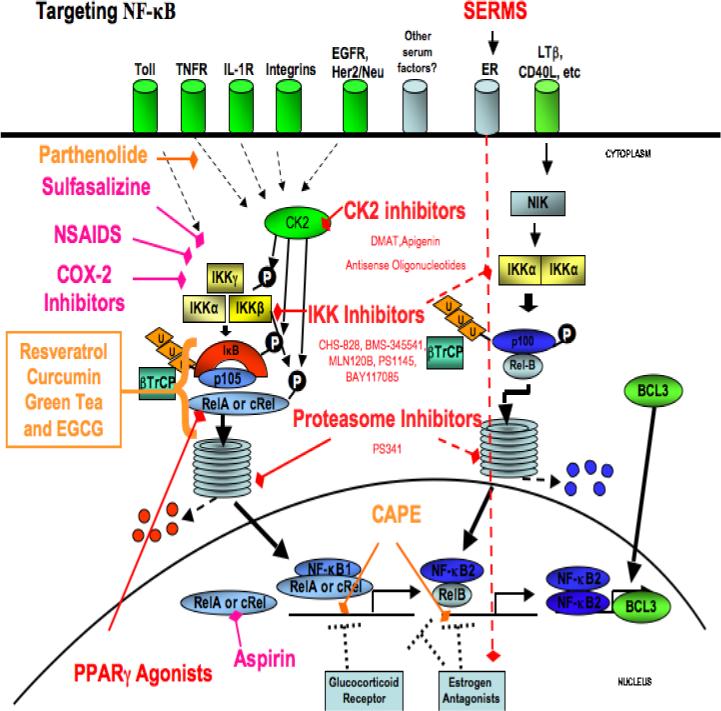
Targeting NF-κB in carcinoma. Red text highlights specific molecular targeted therapeutic agents. Pink text highlights anti-inflammatory agents that show inhibition of NF-κB. Orange text highlights natural compounds that exhibit anti-NF-κB properties. Abbreviations. NIK- NF-κB inducing kinase; IL1R, interleukin 1 receptor; EGFR, epidermal growth factor receptor; TNFR, Tumor necrosis factor receptor; TRAF, TNF receptor -associated factor; TAK transforming growth factor-β-activated kinase; FAK, focal adhesion kinase; CK2, casein kinase 2.
Acknowledgements
The authors would like to thank Drs. Shivaani Kummar and James F. Battey for reading the manuscript.
Supported by NIDCD intramural project Z01-DC-00016, NCI-Millennium Pharmaceuticals Cooperative Research and Development Agreement 00676 (CVW), NIH-Pfizer Clinical Research Training Program (MB), and Howard Hughes Medical Institute Research Scholars Program (JC).
Footnotes
Disclosures
Dr. Van Waes has Cooperative Research and Development Agreements between NIH and Millennium Pharmaceuticals for investigation of proteasome and IKK inhibitors.
REFERENCES
- 1.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008 Feb 8;132(3):344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunological reviews. 2006 Apr;210:171–86. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 3.Barroga CF, Stevenson JK, Schwarz EM, Verma IM. Constitutive phosphorylation of I kappa B alpha by casein kinase II. Proceedings of the National Academy of Sciences of the United States of America. 1995 Aug 15;92(17):7637–41. doi: 10.1073/pnas.92.17.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu M, Yeh J, Van Waes C. Protein kinase casein kinase 2 mediates inhibitor-kappaB kinase and aberrant nuclear factor-kappaB activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res. 2006 Jul 1;66(13):6722–31. doi: 10.1158/0008-5472.CAN-05-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancroft CC, Chen Z, Yeh J, Sunwoo JB, Yeh NT, Jackson S, et al. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappaB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer. 2002 Jun 1;99(4):538–48. doi: 10.1002/ijc.10398. [DOI] [PubMed] [Google Scholar]
- 6.Loukinova E, Chen Z, Van Waes C, Dong G. Expression of proangiogenic chemokine Gro 1 in low and high metastatic variants of Pam murine squamous cell carcinoma is differentially regulated by IL-1alpha, EGF and TGF-beta1 through NF-kappaB dependent and independent mechanisms. Int J Cancer. 2001 Dec 1;94(5):637–44. doi: 10.1002/ijc.1514. [DOI] [PubMed] [Google Scholar]
- 7.Wolf JS, Chen Z, Dong G, Sunwoo JB, Bancroft CC, Capo DE, et al. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res. 2001 Jun;7(6):1812–20. [PubMed] [Google Scholar]
- 8.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999 Oct 22;274(43):30353–6. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000 Oct 20;275(42):32592–7. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 10.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Molecular cell. 1998 Apr;1(5):661–71. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). The EMBO journal. 2003 Mar 17;22(6):1313–24. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001 Jun 1;276(22):18934–40. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 13.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr., Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000 Mar;20(5):1626–38. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tergaonkar V, Perkins ND. p53 and NF-kappaB crosstalk: IKKalpha tips the balance. Molecular cell. 2007 Apr 27;26(2):158–9. doi: 10.1016/j.molcel.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006 Nov 30;72(11):1605–21. doi: 10.1016/j.bcp.2006.06.029. *A useful review of the role of inflammation in cancer
- 16.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001 Feb;107(3):241–6. doi: 10.1172/JCI11991. *Role of NF-κB in chemotherapy resistance is established
- 17.Hiscott J, Kwon H, Genin P. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J Clin Invest. 2001 Jan;107(2):143–51. doi: 10.1172/JCI11918. *Important oncogenic viruses that activate NF-κB
- 18.Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007 Feb 15;13(4):1076–82. doi: 10.1158/1078-0432.CCR-06-2221. **Recent brief review of role of NF-κB in cancer, and as a target for prevention and therapy
- 19.Tsurutani J, Castillo SS, Brognard J, Granville CA, Zhang C, Gills JJ, et al. Tobacco components stimulate Akt-dependent proliferation and NFkappaB-dependent survival in lung cancer cells. Carcinogenesis. 2005 Jul;26(7):1182–95. doi: 10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 20.Huang TT, Wuerzberger-Davis SM, Wu ZH, Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell. 2003 Nov 26;115(5):565–76. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 21.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003 Feb 6;421(6923):639–43. doi: 10.1038/nature01283. *Paper providing evidence NF- κB may be protective in early carcinogenesis
- 22.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10544–51. doi: 10.1073/pnas.0603499103. *Paper providing evidence NF- κB may be protective in early carcinogenesis
- 23.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. The role of NF-kappaB in hepatocarcinogenesis: promoter or suppressor? J Hepatol. 2007 Aug;47(2):307–9. doi: 10.1016/j.jhep.2007.05.006. *Paper providing evidence NF- κB may be protective in early carcinogenesis
- 24.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004 Sep 23;431(7007):461–6. doi: 10.1038/nature02924. *Paper providing evidence NF- κB may promote early carcinogenesis
- 25.Loukinova E, Chen Z, Van Waes C, Dong G. Expression of proangiogenic chemokine Gro 1 in low and high metastatic variants of Pam murine squamous cell carcinoma is differentially regulated by IL-1alpha, EGF and TGF-beta1 through NF-kappaB dependent and independent mechanisms. Int J Cancer. 2001 Dec 1;94(5):637–44. doi: 10.1002/ijc.1514. *Paper providing evidence loss of TGFβ regulation may contribute to NF- κB role in promotion of inflammatory gene expression observed in progression.
- 26.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004 Nov 11;23(53):8571–80. doi: 10.1038/sj.onc.1207902. *PTEN, another tumor suppressor whose loss promotes PI3K and NF-κB activation
- 27.Emmerich F, Meiser M, Hummel M, Demel G, Foss HD, Jundt F, et al. Overexpression of I kappa B alpha without inhibition of NF-kappaB activity and mutations in the I kappa B alpha gene in Reed-Sternberg cells. Blood. 1999 Nov 1;94(9):3129–34. [PubMed] [Google Scholar]
- 28.Wood KM, Roff M, Hay RT. Defective IkappaBalpha in Hodgkin cell lines with constitutively active NF-kappaB. Oncogene. 1998 Apr 23;16(16):2131–9. doi: 10.1038/sj.onc.1201735. [DOI] [PubMed] [Google Scholar]
- 29.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer cell. 2007 Aug;12(2):115–30. doi: 10.1016/j.ccr.2007.07.004. **Important paper demonstrating mechanisms and common occurrence of co-activation of both NF-κB pathways in MM
- 30.Allen C, Saigal K, Nottingham L, Arun P, Chen Z, Van Waes C. Bortezomib-induced apoptosis with limited clinical response is accompanied by inhibition of canonical but not alternative NF-κB pathway subunits in head and neck cancer. Clin Cancer Res. 2008;14 doi: 10.1158/1078-0432.CCR-07-4470. in press. *Paper demonstating co-activation and differential sensitivity of canonical and non-canonical NF-κB to proteasome inhibitor in patient tumors
- 31.Dan HC, Adli M, Baldwin AS. Regulation of mammalian target of rapamycin activity in PTEN-inactive prostate cancer cells by I kappa B kinase alpha. Cancer Res. 2007 Jul 1;67(13):6263–9. doi: 10.1158/0008-5472.CAN-07-1232. **Paper indicating a novel alternative role of IKKα affecting mTOR pathway involved in protein synthesis
- 32.Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006 Aug 15;66(16):8210–8. doi: 10.1158/0008-5472.CAN-06-1213. *NF-κB gene signature associated with high risk of cancer recurrence
- 33.Dong G, Loukinova E, Chen Z, Gangi L, Chanturita TI, Liu ET, et al. Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res. 2001 Jun 15;61(12):4797–808. * NF-κB gene signature associated with tumor progression
- 34.Loercher A, Lee TL, Ricker JL, Howard A, Geoghegen J, Chen Z, et al. Nuclear factor-kappaB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res. 2004 Sep 15;64(18):6511–23. doi: 10.1158/0008-5472.CAN-04-0852. * NF-κB promotes global gene expression and malignant phenotype in cancer
- 35.Lee TL, Yang XP, Yan B, Friedman J, Duggal P, Bagain L, et al. A novel nuclear factor-kappaB gene signature is differentially expressed in head and neck squamous cell carcinomas in association with TP53 status. Clin Cancer Res. 2007 Oct 1;13(19):5680–91. doi: 10.1158/1078-0432.CCR-07-0670. [DOI] [PubMed] [Google Scholar]
- 36.Yan B, Chen G, Saigal K, Yang X, Jensen ST, Van Waes C, et al. Systems biology-defined NF-kappaB regulons, interacting signal pathways and networks are implicated in the malignant phenotype of head and neck cancer cell lines differing in p53 status. Genome biology. 2008 Mar 11;9(3):R53. doi: 10.1186/gb-2008-9-3-r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan B, Yang X, Lee TL, Friedman J, Tang J, Van Waes C, et al. Genome-wide identification of novel expression signatures reveal distinct patterns and prevalence of binding motifs for p53, nuclear factor-kappaB and other signal transcription factors in head and neck squamous cell carcinoma. Genome biology. 2007;8(5):R78. doi: 10.1186/gb-2007-8-5-r78. **A series of papers demonstrating interaction of NF-kB related gene alterations with key cancer pathways
- 38.Hinz M, Lemke P, Anagnostopoulos I, Hacker C, Krappmann D, Mathas S, et al. Nuclear factor kappaB-dependent gene expression profiling of Hodgkin's disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. The Journal of experimental medicine. 2002 Sep 2;196(5):605–17. doi: 10.1084/jem.20020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staudt LM. Gene expression profiling of lymphoid malignancies. Annual review of medicine. 2002;53:303–18. doi: 10.1146/annurev.med.53.082901.103941. [DOI] [PubMed] [Google Scholar]
- 40.Van Laere SJ, Van der Auwera I, Van den Eynden GG, Elst HJ, Weyler J, Harris AL, et al. Nuclear factor-kappaB signature of inflammatory breast cancer by cDNA microarray validated by quantitative real-time reverse transcription-PCR, immunohistochemistry, and nuclear factor-kappaB DNA-binding. Clin Cancer Res. 2006 Jun 1;12(11 Pt 1):3249–56. doi: 10.1158/1078-0432.CCR-05-2800. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol Cell Biol. 2000 Apr;20(8):2687–95. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999 Aug;19(8):5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998 Sep 11;281(5383):1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 44.Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nature reviews. 2002 Sep;2(9):664–74. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams J. Proteasome inhibition: a novel approach to cancer therapy. Trends Mol Med. 2002;8(4 Suppl):S49–54. doi: 10.1016/s1471-4914(02)02315-8. [DOI] [PubMed] [Google Scholar]
- 46.Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001 May;7(5):1419–28. *paper implicating NF-κB as important target of proteasome inhibitor in cancer
- 47.Williams S, Pettaway C, Song R, Papandreou C, Logothetis C, McConkey DJ. Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts. Mol Cancer Ther. 2003 Sep;2(9):835–43. [PubMed] [Google Scholar]
- 48.Iqbal M, Chatterjee S, Kauer JC, Das M, Messina P, Freed B, et al. Potent inhibitors of proteasome. J Med Chem. 1995 Jun 23;38(13):2276–7. doi: 10.1021/jm00013a002. [DOI] [PubMed] [Google Scholar]
- 49.Lun M, Zhang PL, Pellitteri PK, Law A, Kennedy TL, Brown RE. Nuclear factor-kappaB pathway as a therapeutic target in head and neck squamous cell carcinoma: pharmaceutical and molecular validation in human cell lines using Velcade and siRNA/NF-kappaB. Ann Clin Lab Sci. 2005;35(3):251–8. Summer. [PubMed] [Google Scholar]
- 50.Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Andtbacka RH, Dunner K, Jr., et al. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 2006 Apr 1;66(7):3773–81. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 51.Adachi M, Zhang Y, Zhao X, Minami T, Kawamura R, Hinoda Y, et al. Synergistic effect of histone deacetylase inhibitors FK228 and m-carboxycinnamic acid bis-hydroxamide with proteasome inhibitors PSI and PS-341 against gastrointestinal adenocarcinoma cells. Clin Cancer Res. 2004 Jun 1;10(11):3853–62. doi: 10.1158/1078-0432.CCR-03-0806. [DOI] [PubMed] [Google Scholar]
- 52.Zhu H, Zhang L, Dong F, Guo W, Wu S, Teraishi F, et al. Bik/NBK accumulation correlates with apoptosis-induction by bortezomib (PS-341, Velcade) and other proteasome inhibitors. Oncogene. 2005 Jul 21;24(31):4993–9. doi: 10.1038/sj.onc.1208683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lun M, Zhang PL, Siegelmann-Danieli N, Blasick TM, Brown RE. Intracellular inhibitory effects of Velcade correlate with morphoproteomic expression of phosphorylated-nuclear factor-kappaB and p53 in breast cancer cell lines. Ann Clin Lab Sci. 2005;35(1):15–24. Winter. [PubMed] [Google Scholar]
- 54.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002 May 10;277(19):16639–47. doi: 10.1074/jbc.M200360200. *paper implicating NF-κB as important target of proteasome inhibitor in cancer
- 55.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001 Aug;8(8):739–58. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Ricker JL, Malhotra PS, Nottingham L, Bagain L, Lee TL, Yeh NT, r Van Waes C. Bortezomib exhibits differential anti-tumor activity in head and neck tumor xenografts and cells which correspond to proteasome, NF-κB and AP-1 activities in vitro. Mol Cancer Ther. 2008;7 doi: 10.1158/1535-7163.MCT-07-2046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Waes C, Chang AA, Lebowitz PF, Druzgal CH, Chen Z, Elsayed YA, et al. Inhibition of nuclear factor-kappaB and target genes during combined therapy with proteasome inhibitor bortezomib and reirradiation in patients with recurrent head-and-neck squamous cell carcinoma. International journal of radiation oncology, biology, physics. 2005 Dec 1;63(5):1400–12. doi: 10.1016/j.ijrobp.2005.05.007. *First in human study of proteasome inhibitor with radiation and pilot studies of phospho-RELA and NF-κB regulated genes in tumor and cytokines in serum as markers of response to proteasome inhibition
- 58.Hovstadius P, Larsson R, Jonsson E, Skov T, Kissmeyer AM, Krasilnikoff K, et al. A Phase I study of CHS 828 in patients with solid tumor malignancy. Clin Cancer Res. 2002 Sep;8(9):2843–50. [PubMed] [Google Scholar]
- 59.Ravaud A, Cerny T, Terret C, Wanders J, Bui BN, Hess D, et al. Phase I study and pharmacokinetic of CHS-828, a guanidino-containing compound, administered orally as a single dose every 3 weeks in solid tumours: an ECSG/EORTC study. Eur J Cancer. 2005 Mar;41(5):702–7. doi: 10.1016/j.ejca.2004.12.023. *Phase I trial of IKKβ inhibitor
- 60.Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003 Jan 17;278(3):1450–6. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin Cancer Res. 2006 Feb 1;12(3 Pt 1):950–60. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang J, Pan WH, Clawson GA, Richmond A. Systemic targeting inhibitor of kappaB kinase inhibits melanoma tumor growth. Cancer Res. 2007 Apr 1;67(7):3127–34. doi: 10.1158/0008-5472.CAN-06-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hideshima T, Neri P, Tassone P, Yasui H, Ishitsuka K, Raje N, et al. MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clin Cancer Res. 2006 Oct 1;12(19):5887–94. doi: 10.1158/1078-0432.CCR-05-2501. [DOI] [PubMed] [Google Scholar]
- 64.Castro AC, Dang LC, Soucy F, Grenier L, Mazdiyasni H, Hottelet M, et al. Novel IKK inhibitors: beta-carbolines. Bioorganic & medicinal chemistry letters. 2003 Jul 21;13(14):2419–22. doi: 10.1016/s0960-894x(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 65.Yemelyanov A, Gasparian A, Lindholm P, Dang L, Pierce JW, Kisseljov F, et al. Effects of IKK inhibitor PS1145 on NF-kappaB function, proliferation, apoptosis and invasion activity in prostate carcinoma cells. Oncogene. 2006 Jan 19;25(3):387–98. doi: 10.1038/sj.onc.1209066. [DOI] [PubMed] [Google Scholar]
- 66.Domingo-Domenech J, Oliva C, Rovira A, Codony-Servat J, Bosch M, Filella X, et al. Interleukin 6, a nuclear factor-kappaB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-kappaB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin Cancer Res. 2006 Sep 15;12(18):5578–86. doi: 10.1158/1078-0432.CCR-05-2767. [DOI] [PubMed] [Google Scholar]
- 67.Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of inhibitor of nuclear factor-kappaB phosphorylation increases the efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Clin Cancer Res. 2004 Nov 15;10(22):7645–54. doi: 10.1158/1078-0432.CCR-04-0958. [DOI] [PubMed] [Google Scholar]
- 68.Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004 May 28;279(22):23477–85. doi: 10.1074/jbc.M313709200. [DOI] [PubMed] [Google Scholar]
- 69.Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008 Jan;1784(1):33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 70.Yde CW, Frogne T, Lykkesfeldt AE, Fichtner I, Issinger OG, Stenvang J. Induction of cell death in antiestrogen resistant human breast cancer cells by the protein kinase CK2 inhibitor DMAT. Cancer letters. 2007 Oct 28;256(2):229–37. doi: 10.1016/j.canlet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Mishra S, Pertz V, Zhang B, Kaur P, Shimada H, Groffen J, et al. Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. Leukemia. 2007 Jan;21(1):178–80. doi: 10.1038/sj.leu.2404460. [DOI] [PubMed] [Google Scholar]
- 72.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review). Int J Oncol. 2007 Jan;30(1):233–45. [PubMed] [Google Scholar]
- 73.Hessenauer A, Montenarh M, Gotz C. Inhibition of CK2 activity provokes different responses in hormone-sensitive and hormone-refractory prostate cancer cells. Int J Oncol. 2003 Jun;22(6):1263–70. [PubMed] [Google Scholar]
- 74.Landesman-Bollag E, Song DH, Romieu-Mourez R, Sussman DJ, Cardiff RD, Sonenshein GE, et al. Protein kinase CK2: signaling and tumorigenesis in the mammary gland. Mol Cell Biochem. 2001 Nov;227(1−2):153–65. [PubMed] [Google Scholar]
- 75.Shukla S, Gupta S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol Cancer Ther. 2006 Apr;5(4):843–52. doi: 10.1158/1535-7163.MCT-05-0370. [DOI] [PubMed] [Google Scholar]
- 76.Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anti-cancer drugs. 2005 Nov;16(10):1037–43. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Slaton JW, Unger GM, Sloper DT, Davis AT, Ahmed K. Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model. Mol Cancer Res. 2004 Dec;2(12):712–21. [PubMed] [Google Scholar]
- 78.Chadwick CC, Chippari S, Matelan E, Borges-Marcucci L, Eckert AM, Keith JC, Jr., et al. Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proceedings of the National Academy of Sciences of the United States of America. 2005 Feb 15;102(7):2543–8. doi: 10.1073/pnas.0405841102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):10137–42. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takada Y, Bhardwaj A, Potdar P, Aggarwal BB. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene. 2004 Dec 9;23(57):9247–58. doi: 10.1038/sj.onc.1208169. [DOI] [PubMed] [Google Scholar]
- 81.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995 Dec 1;83(5):803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 82.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995 Oct 13;270(41):23975–83. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 83.Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, et al. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999 Aug;104(4):383–9. doi: 10.1172/JCI7145. *Early evidence of potential for PPAR ligands as preventive agents
- 84.Keshamouni VG, Arenberg DA, Reddy RC, Newstead MJ, Anthwal S, Standiford TJ. PPAR-gamma activation inhibits angiogenesis by blocking ELR+CXC chemokine production in non-small cell lung cancer. Neoplasia. 2005 Mar;7(3):294–301. doi: 10.1593/neo.04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappaB pathway. J Biol Chem. 1999 Sep 17;274(38):27307–14. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 86.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998 Nov 5;396(6706):77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 87.Yan F, Polk DB. Aminosalicylic acid inhibits IkappaB kinase alpha phosphorylation of IkappaBalpha in mouse intestinal epithelial cells. J Biol Chem. 1999 Dec 17;274(51):36631–6. doi: 10.1074/jbc.274.51.36631. [DOI] [PubMed] [Google Scholar]
- 88.Stark LA, Din FV, Zwacka RM, Dunlop MG. Aspirin-induced activation of the NF-kappaB signaling pathway: a novel mechanism for aspirin-mediated apoptosis in colon cancer cells. FASEB J. 2001 May;15(7):1273–5. [PubMed] [Google Scholar]
- 89.Chan AT, Giovannucci EL, Schernhammer ES, Colditz GA, Hunter DJ, Willett WC, et al. A prospective study of aspirin use and the risk for colorectal adenoma. Ann Intern Med. 2004 Feb 3;140(3):157–66. doi: 10.7326/0003-4819-140-3-200402030-00006. [DOI] [PubMed] [Google Scholar]
- 90.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003 Mar 6;348(10):883–90. doi: 10.1056/NEJMoa021633. **Highlights clinical potential of NSAIDs for prevention of premalignant colorectal adenomas
- 91.Berman KS, Verma UN, Harburg G, Minna JD, Cobb MH, Gaynor RB. Sulindac enhances tumor necrosis factor-alpha-mediated apoptosis of lung cancer cell lines by inhibition of nuclear factor-kappaB. Clin Cancer Res. 2002 Feb;8(2):354–60. [PubMed] [Google Scholar]
- 92.Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999 Dec 2;18(51):7389–94. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 93.Stark LA, Dunlop MG. Nucleolar sequestration of RelA (p65) regulates NF-kappaB-driven transcription and apoptosis. Mol Cell Biol. 2005 Jul;25(14):5985–6004. doi: 10.1128/MCB.25.14.5985-6004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shishodia S, Koul D, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-kappa B activation through inhibition of activation of I kappa B alpha kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J Immunol. 2004 Aug 1;173(3):2011–22. doi: 10.4049/jimmunol.173.3.2011. [DOI] [PubMed] [Google Scholar]
- 95.Liu JF, Zhang SW, Jamieson GG, Zhu GJ, Wu TC, Zhu TN, et al. The effects of a COX-2 inhibitor meloxicam on squamous cell carcinoma of the esophagus in vivo. Int J Cancer. 2008 Apr 1;122(7):1639–44. doi: 10.1002/ijc.23288. [DOI] [PubMed] [Google Scholar]
- 96.Liu JF, Jamieson GG, Drew PA, Zhu GJ, Zhang SW, Zhu TN, et al. Aspirin induces apoptosis in oesophageal cancer cells by inhibiting the pathway of NF-kappaB downstream regulation of cyclooxygenase-2. ANZ J Surg. 2005 Nov;75(11):1011–6. doi: 10.1111/j.1445-2197.2005.03596.x. [DOI] [PubMed] [Google Scholar]
- 97.Dasgupta K, Di Cesar D, Ghosn J, Rajan R, Mahmud S, Rahme E. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J. 2006 Mar-Apr;12(2):130–5. [PubMed] [Google Scholar]
- 98.Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998 Mar 1;101(5):1163–74. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000 Nov;119(5):1209–18. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- 100.Egan LJ, Mays DC, Huntoon CJ, Bell MP, Pike MG, Sandborn WJ, et al. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999 Sep 10;274(37):26448–53. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- 101.Sourbier C, Danilin S, Lindner V, Steger J, Rothhut S, Meyer N, et al. Targeting the nuclear factor-kappaB rescue pathway has promising future in human renal cell carcinoma therapy. Cancer Res. 2007 Dec 15;67(24):11668–76. doi: 10.1158/0008-5472.CAN-07-0632. [DOI] [PubMed] [Google Scholar]
- 102.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003 Aug;24(4):488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 103.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995 Oct 13;270(5234):286–90. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 104.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004 Apr;4(4):314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 105.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999 Nov 18;341(21):1565–71. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 106.Chen J, Fang Y. A novel pathway regulating the mammalian target of rapamycin (mTOR) signaling. Biochem Pharmacol. 2002 Oct 1;64(7):1071–7. doi: 10.1016/s0006-2952(02)01263-7. [DOI] [PubMed] [Google Scholar]
- 107.Romano MF, Avellino R, Petrella A, Bisogni R, Romano S, Venuta S. Rapamycin inhibits doxorubicin-induced NF-kappaB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur J Cancer. 2004 Dec;40(18):2829–36. doi: 10.1016/j.ejca.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 108.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, et al. Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by Targeting the Nuclear Factor-{kappa}B Pathway. Clin Cancer Res. 2007 Jun 1;13(11):3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 109.Lee KW, Kim JH, Lee HJ, Surh YJ. Curcumin inhibits phorbol ester-induced up-regulation of cyclooxygenase-2 and matrix metalloproteinase-9 by blocking ERK1/2 phosphorylation and NF-kappaB transcriptional activity in MCF10A human breast epithelial cells. Antioxidants & redox signaling. 2005 Nov-Dec;7(11−12):1612–20. doi: 10.1089/ars.2005.7.1612. [DOI] [PubMed] [Google Scholar]
- 110.Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. 2004 Sep 20;111(5):679–92. doi: 10.1002/ijc.20333. [DOI] [PubMed] [Google Scholar]
- 111.Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003 Jul;24(7):1269–79. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- 112.Kumar AP, Garcia GE, Ghosh R, Rajnarayanan RV, Alworth WL, Slaga TJ. 4-Hydroxy-3-methoxybenzoic acid methyl ester: a curcumin derivative targets Akt/NF kappa B cell survival signaling pathway: potential for prostate cancer management. Neoplasia. 2003 May-Jun;5(3):255–66. doi: 10.1016/S1476-5586(03)80057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alok Bharti C, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochemical Pharmacology. 2002;64:883–8. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 114.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997 Jan 10;275(5297):218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 115.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007 Mar 15;109(6):2293–302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 116.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 117.Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys. 2000 Apr 15;376(2):338–46. doi: 10.1006/abbi.2000.1742. [DOI] [PubMed] [Google Scholar]
- 118.Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol Cancer Ther. 2005 Apr;4(4):587–94. doi: 10.1158/1535-7163.MCT-04-0215. [DOI] [PubMed] [Google Scholar]
- 119.Hewamana S, Alghazal S, Lin TT, Clement M, Jenkins C, Guzman ML, et al. The NF-{kappa}B subunit, Rel A, is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukaemia and represents a promising therapeutic target. Blood. 2008 Jan 28; doi: 10.1182/blood-2007-11-125278. [DOI] [PubMed] [Google Scholar]
- 120.Lee JH, Koo TH, Hwang BY, Lee JJ. Kaurane diterpene, kamebakaurin, inhibits NF-kappa B by directly targeting the DNA-binding activity of p50 and blocks the expression of antiapoptotic NF-kappa B target genes. J Biol Chem. 2002 May 24;277(21):18411–20. doi: 10.1074/jbc.M201368200. [DOI] [PubMed] [Google Scholar]
- 121.Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits constitutive and TNFalpha-induced activation of NF-kappaB and sensitizes human prostate carcinoma DU145 cells to TNFalpha-induced apoptosis. Oncogene. 2002 Mar 7;21(11):1759–67. doi: 10.1038/sj.onc.1205240. [DOI] [PubMed] [Google Scholar]
- 122.Tyagi A, Raina K, Singh RP, Gu M, Agarwal C, Harrison G, et al. Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Mol Cancer Ther. 2007 Dec;6(12 Pt 1):3248–55. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]


