Abstract
Objectives
Accumulating evidence suggests that a hypercoagulable state influences early graft failure after off-pump coronary artery bypass (OPCAB). We hypothesized that regional myocardial ischemia caused by obligatory periods of coronary occlusion during OPCAB is an important trigger for this prothrombotic state.
Methods
Using a series of biomarkers, 60 consecutive patients undergoing OPCAB were monitored for myocardial injury (myoglobin), inflammation (TNF-α, IL-8) and thrombosis (thrombin generation-F1.2, contact activation pathway-FXII-a, platelet derived microparticles-via flow cytometry). The transcardiac gradients of these markers were determined by assaying both arterial and coronary sinus blood just after protamine administration. Intramyocardial pH was monitored continuously during coronary occlusion in a subset (N=30 grafts, 11 patients). The influence of management strategies affecting hemostasis (e.g. antiplatelet therapy, anti-fibrinolytics, peak ACT during heparinization) was analyzed.
Results
Ischemic injury, depicted by the transcardiac myoglobin gradient, significantly correlated with intramyocardial acidosis during coronary occlusion (R=0.96, p<0.0001) and predicted the transcardiac gradients of TNF-α (R=0.83, p<0.001) and F1.2 (R=0.72, p<0.0001). Transcardiac F1.2 strongly correlated with TNF-α (R=0.73, p=0.01) and IL-8 (R=0.51, p=0.02). Patients receiving aprotinin (N=20) showed significantly lower transcardiac gradients for myoglobin (4.1±7.5 v. 72.9±108.8% change, p=0.002), F1.2 (31±37 v. 89±149%, p=0.03), FXII-a (2.6±4.1 v. 19.2±34%, p=0.04) and microparticles (7±3.9 v. 12.9±8%, p=0.01).
Conclusions
Strong correlations between myocardial ischemia and the transcardiac gradients of markers for inflammation and thrombosis suggest that even brief episodes of coronary occlusion in the beating heart may have pathophysiologic consequences. Aprotinin, but not other factors that influence the coagulation system, appears to mitigate this process during OPCAB.
Keywords: coronary artery bypass, hypercoagulabilty, ischemia, OPCAB, aprotinin
Introduction
Off-pump coronary artery bypass graft surgery (OPCAB) circumvents the need for global myocardial ischemia inherent with CABG performed on the arrested heart and requires only brief periods of regional ischemia during the coronary anastomoses. On occasion, this regional ischemia is tolerated poorly and results in arrhythmias and myocardial stunning, but this risk is minimal when coronary occlusion is kept to <10–15 minutes per anastomosis (1). As a result, randomized studies comparing off- vs. on-pump CABG have documented improvements in myocardial injury and early hemodynamics after OPCAB (2–4). However, the impact of regional warm ischemia on inflammation and coagulation after OPCAB has not been studied.
Thrombin mediates a variety of inflammatory and thrombotic processes associated with ischemia-reperfusion injury. In prior OPCAB studies, we noted a burst of thrombin production from the heart minutes after protamine administration (5), reminiscent of the well-described “rebound hypercoagulable state” that occurs in unstable angina patients after heparin withdrawal (6). The prothrombin cleavage product, F1.2, has been established as a surrogate marker of a prothrombotic state through significant associations with the risk of acute vein graft failure (7), myocardial injury (8) after CABG, and major adverse cardiovascular events (9). Thrombin production can be mitigated by enhanced antiplatelet therapy (10) as well as the use of higher heparin doses during cardiopulmonary bypass (11). Aprotinin, used primarily for its hemostatic properties, has been suggested to have anti-thrombotic effects via inhibition of the thrombin receptor, protease activated receptor-1 (PAR-1) (12–15). Although other PAR-1 antagonists have been documented to reduce major adverse cardiovascular events (16), there are few clinical reports to suggest an antithrombotic effect from aprotinin despite administration to thousands of on-pump CABG patients. In contrast to conventional CABG, we hypothesized that OPCAB creates a series of pathologic findings in response to regional warm ischemia (e.g. local activation of thrombin, platelets and the contact activation pathway) that provide a more favorable model for demonstrating the clinical effects of PAR-1 inhibition by aprotinin.
Methods
Patient selection and enrollment
Following IRB approval (UMB protocol #25350), sixty consecutive patients underwent off-pump CABG at our institution. All subjects provided informed consent before enrollment into this prospective, observational study of patients undergoing OPCAB with no aprotinin (n=40) or off-pump CABG with a modified full dose regimen of aprotinin (n=20), as previously described (15). Assignment to the aprotinin group was nonrandomized. Hemodynamic instability, acute coronary syndromes or situations in which complete revascularization was not possible served as exclusion criteria. Patients with chronic renal insufficiency (creatinine > 2.0 mg/dL), and allergy to radiographic contrast were also excluded from enrollment. Demographics, preoperative risk factors and medications, intra- and postoperative data were prospectively recorded into a relational database.
Treatments
A modified full-dose regimen was used as previously described. Heparin dose was calculated by protamine titration using a HMS heparin assay (0.0 to 2.5 mg/kg cartridge, Medtronic, Minneapolis, Minnesota) to maintain levels greater than 2μg/mL and a kaolin-based ACT greater than 300 seconds. The heparin effect was partially reversed by administering half the dose of protamine calculated by the HMS device. The algorithm for intraoperative and postoperative blood product transfusions was based on thrombelastography analysis, as described (17). All patients received aspirin (325 mg orally each morning and within 6 hours after intensive care unit arrival) as the sole postoperative platelet inhibitor. Glucose levels were maintained at less than 150 mg/dL using routine insulin infusions.
Surgery and Perioperative Management
After median sternotomy, the left internal thoracic artery was used in all patients; the saphenous vein was harvested using an endoscopic (VasoView6®, Guidant Systems, Inc., Minneapolis, MN) or open approach, based on anatomical considerations. Conduits were stored in heparinized saline after harvest. Proximal anastomoses were performed using a partial occluding aortic clamp. All distal anastomoses were facilitated by suction-based exposure and stabilizing devices (Octopus 4.3®, Medtronic, Inc., Minneapolis, MN) without intracoronary shunts. Shed mediastinal blood was collected intraoperatively using a cellsaving device (Cobe BRAT 2; Arvada, CO), processed, and retransfused.
Intraoperative blood flow analysis
Blood flow and flow waveform were measured in each graft using transit time ultrasound (Transonic, Inc, Ithica, NY). Waveforms were analyzed for pulsatility index and percentage diastolic flow using digital data acquisition software (WinDaq™, DATAQ Instruments, Inc, Dayton, OH).
Blood sample collection
Blood samples, collected in tubes containing 3.2% citrate, were obtained preoperatively from the arterial line. Postoperatively, 30 minutes after heparin reversal with half dose protamine, blood was obtained from the systemic arterial line and coronary sinus (CS). Additional systemic blood was collected on postoperative days 1 and 3. Platelet-poor plasma was obtained by rapid centrifugation (2000g) and stored at −80 C°.
Myocardial Injury and Acidosis
Cardiac troponin I (Tn-I) and myoglobin were both assessed using ELISA kits (Life Diagnostics, Inc., West Chester, PA). Regional (i.e. anterior and posterior ventricular wall) changes in pH were monitored in real time using the Khuri™ pH monitoring system (Terumo Corp, Tokyo Japan).
Assays for Coagulation and Inflammation
Thrombin formation was evaluated by assaying postoperative systemic and CS samples for the presence of prothrombin fragment 1.2 (F1.2) (Dade Behring, Marburg, Germany). The contact activation pathway was evaluated by assaying FXII-a (American Diagnostica, INC., Stamford, CT). Inflammation was assessed via interleukin-8 (IL-8) and tumor necrosis factor-α (TNF-α) using ELISA (Bender MedSystems, Vienna, Austria). Comparison of the concentration of these markers in the CS to a simultaneously obtained aortic (Ao) sample allowed for calculation of % transcardiac change as follows: (CS-Ao)/Ao × 100.
Assays for Platelet Function
Thrombelastography (TEG™, Haemoscope, Niles, IL)
Maximum amplitude (MA) of the TEG trace was measured after 30 seconds of activation by kaolin or tissue factor (0.0001% Innovin®, Dade-Behring).
Whole blood aggregometry (Chronolog, Hawerton, PA)
Impedance changes (Ω) were assessed at 6 minutes following the addition of 1μg/ml and 5μg/ml collagen as described (7).
Whole blood flow cytometry: (Becton-Dickinson FACScan, Franklin Lakes, NJ)
Citrated blood samples were diluted with modified Tyrode Buffer containing 5mM GPRP (Centerchem Inc., Norwalk, CT) and then incubated with antibodies against CD41-FITC (Serotec, Raleigh, NC) and AnnexinV-APC (BD Biosciences, San Jose, CA). After 20 minutes incubation, the samples were fixed with 1% paraformaldehyde and stored at 4°C until analysis within 72 hours by FACS. Both, forward scatter and sideward light scatter were set at logarithmic gain, and platelet derived microparticles were identified. The %annexin-positive microparticle events compared to total CD41 positive events was calculated as described (18).
Endothelial Integrity
Surplus segments from each bypass conduit were stored in balanced salt solution at room temperature for 30–60 minutes, embedded in cutting compound (Tissue-Tek® O.C.T., Redding, CA) and then frozen in liquid nitrogen. Four separate 5 micron thick sections were assessed for the expression of an EC marker, CD31 (R&D System, Inc., Minneapolis, MN) using immunohistochemistry. The %vessel circumference positive for CD31 was calculated in each section using image analysis software (Bioquant Nova Prime, Nashville, TN) and EC integrity defined by the average %CD31 staining for all sections analyzed (7).
Graft Patency
Bypass graft patency was determined by blinded review of a 64 detector row, CT angiography (CTA) scan (420 ms rotation, 100–150 mL contrast agent IV at 5 cc/s) using retrospective ECG gating. Patency was defined as any flow through the entire graft regardless of the presence of stenosis. The graft was classified as nonpatent if a stump was seen or if there was no contrast in an area known by operative report to contain a graft.
Statistics
The primary endpoint of this trial was a comparison of markers of regional hypercoagulability and inflammation after OPCAB with and without the use of aprotinin. A previous investigation of aprotinin by our group (15) showed a 3-fold decrease in transcardiac F1.2 compared to control. Therefore, 20 patients per group would provide a >80% power to detect a similar difference in F1.2 and other markers in this cohort at p=0.05, assuming a standard deviation of 25%†. Groups were compared by means of the unpaired Student’s t-test for continuous variables and the Fisher’s exact test for categorical variables. The area under the concentration-time curves was compared for markers that were measured serially (e.g. cTn-I). Correlations were examined using linear regression for parametric data. Statistical analysis was performed using the InStat™ statistical package with the assistance of a biostatistician.
Results
Patient population
OPCAB patients that received aprotinin were similar to the control group in all analyzed clinical variables (Table 1) and received equal number of grafts per group (3.1±0.4 vs. 3.2±0.5). None of the patients in this cohort required conversion to on-pump techniques and 100% underwent follow-up of graft patency prior to hospital discharge. There were no deaths and no evidence of hypersensitivity reaction to aprotinin in any of the patients during the study.
Table 1.
Comparison of Baseline Characteristics
| Control (n=40) | Aprotinin (n=20) | p Value | |
|---|---|---|---|
| Patient Characteristics | |||
| Age (yr) | 67.5±10.5 | 67.6±11.3 | NS |
| BMI | 29.1±6.4 | 28.4±4.4 | NS |
| COPD | 18% | 5% | NS |
| Current Smoker | 33% | 25% | NS |
| Diabetes | 40% | 40% | NS |
| Gender (M) | 63% | 60% | NS |
| History of Carotid Artery Disease | 23% | 25% | NS |
| Hypercholesterolemia | 80% | 75% | NS |
| Hypertension (>90 and/or >140) | 88% | 70% | NS |
| No. of Diseased Territories | 2.8±0.6 | 2.9±0.5 | NS |
| Peripheral Vascular Disease | 18% | 20% | NS |
| Previous Heart Surgery | 5% | 15% | NS |
| Previous Myocardial Infarction | 43% | 65% | NS |
| Previous Stroke | 3% | 10% | NS |
| Previous TIA | 8% | 20% | NS |
| Medications At Surgery | |||
| ACE inhibitor | 65% | 60% | NS |
| Aspirin | 93% | 100% | NS |
| Beta blocker | 85% | 70% | NS |
| Preoperative clopidogrel | 30% | 40% | NS |
| Intravenous heparin | 50% | 60% | NS |
| Intravenous nitrates | 15% | 25% | NS |
Myocardial Acidosis/Ischemia and Regional Hypercoagulability
Despite equivalent periods in which the coronary arteries were occluded during OPCAB, the subset of patients that underwent tissue pH monitoring showed a wide variation in the degree of acidosis that developed in the regional myocardium (Fig. 1). The mean pH drop during native vessel occlusion showed a significant correlation with the transcardiac gradient of myoglobin assessed 30 minutes after the completion of grafting (R=0.96, p<0.001). Myocardial injury, as defined by the trancardiac myoglobin release, showed a strong correlation with the transcardiac gradient of F1.2 (R=0.72, p<0.0001). This F1.2 gradient, in turn, showed significant correlations with the transcardiac gradients of TNF-α (R=0.73, p=0.01), IL-8 (R=0.51, p=0.02) and FXIIa (R=0.29, p=0.03) but not with the release of platelet derived microparticles.
Figure 1. Correlation between regional acidosis and myoglobin release.
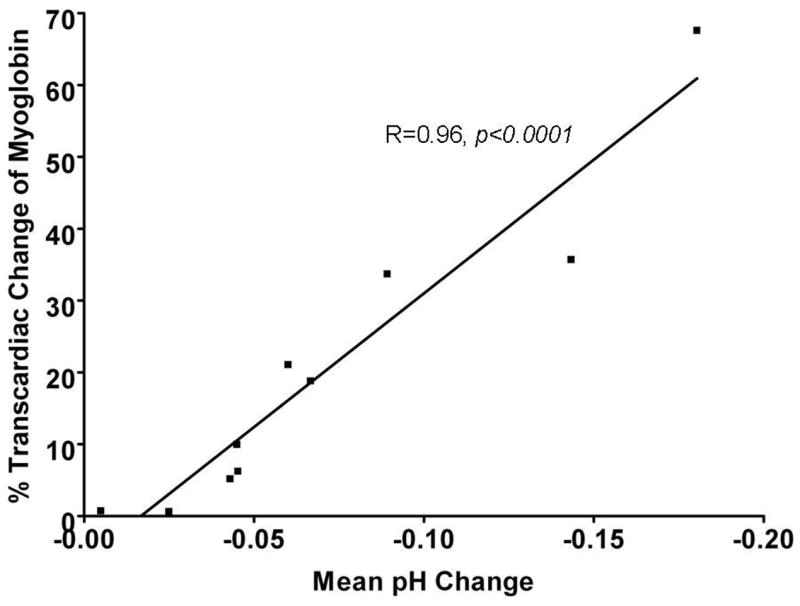
Tissue pH was monitored during the distal anastomoses performed during OPCAB. We noted varying degrees of intramyocardial acidosis despite coronary occlusion times that were nearly identical between anastomoses and always <15 minutes. Myoglobin release was measured at the completion of OPCAB, 30 minutes after protamine administration. A transcardiac gradient was calculated by analyzing myoglobin levels from blood samples obtained simultaneously from the aorta and coronary sinus. A strong linear correlation between the mean regional pH change intraoperatively and transcardiac myoglobin release postoperatively.
Effects of Aprotinin
When comparing perioperative myocardial injury for patients in the aprotinin vs. control groups, we noted a significant reduction in transcardiac myoglobin gradient measured postoperatively in those treated with aprotinin. However, Tn-I release was not significantly different between groups (Fig. 2). Compared to control, the aprotinin group showed statistically significant decreases in the transcardiac gradients of F1.2, FXII-a, and platelet derived microparticles released into the CS (Fig. 3). We found no statistically significant difference in systemic platelet function between the groups at any measured time point (Table 2).
Figure 2. Myocardial injury after OPCAB in the aprotinin vs. control groups.
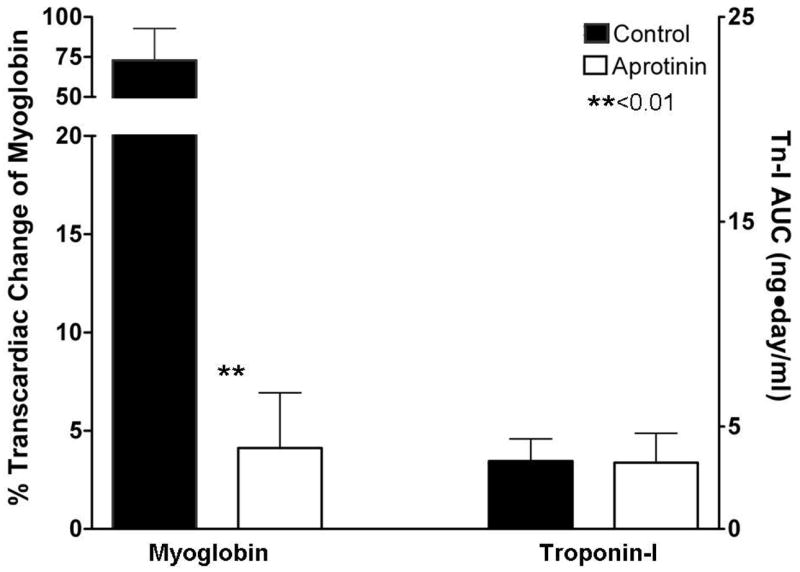
Myocardial injury was measured by analyzing the trancardiac gradient of myoglobin at 30 minutes following the completion of OPCAB and by comparing the area under the concentration-time curve of cardiac troponin I (cTn-I) levels measured at 6, 24 and 72 hours. While transcardiac myoglobin release was significantly reduced in the aprotinin vs. control group, cTn-I was not significantly different.
Figure 3. Differences in thrombosis markers in the aprotinin and control groups.
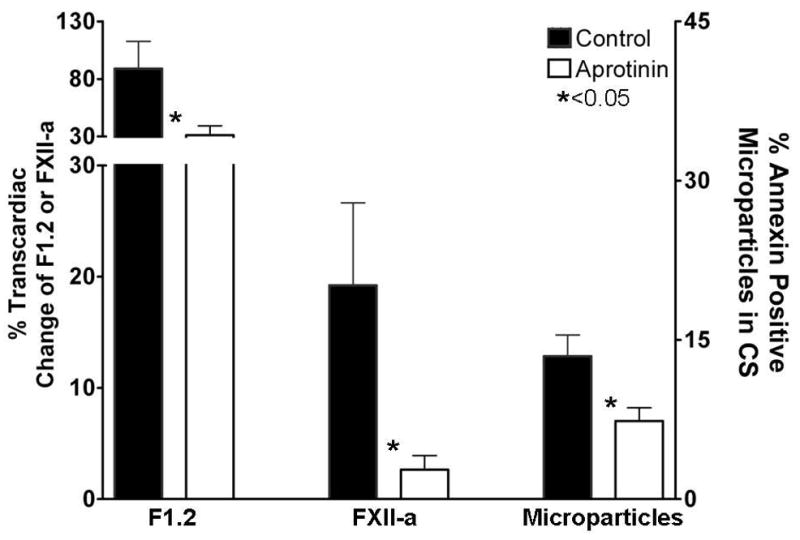
Thrombin production and the contact activation pathway were monitored after OPCAB by measuring the trancardiac gradients of F1.2 and FXII-a. Platelet activation within the coronary circulation was assessed by determining the proportion of CD41+, Annexin+ microparticles released from the heart, normalized against the number of platelets in the sample. The aprotinin group showed significantly reduced thrombin formation (F1.2), inhibition of contact activation (FXII-a) and reduced microparticle formation within the heart after OPCAB.
Table 2.
Platelet Function
| Control | Aprotinin | P value | |
|---|---|---|---|
| POSTOP | |||
| TEG with Kaolin (MA) | 57±9 | 60±11 | NS |
| TEG with Tissue Factor (MA) | 65±10 | 64±6 | NS |
| WBA with 1μg/ml Collagen (Ohms) | 5.6±3.9 | 5.1±2.5 | NS |
| WBA with 5μg/ml Collagen (Ohms) | 9.9±5.5 | 11±6.1 | NS |
| POD1 | |||
| TEG with Kaolin (MA) | 60±7 | 61±6 | NS |
| TEG with Tissue Factor (MA) | 64±10 | 69±4 | NS |
| WBA with 1μg/ml Collagen (Ohms) | 7.1±4.1 | 6.6±3.2 | NS |
| WBA with 5μg/ml Collagen (Ohms) | 13.4±5.1 | 13.8±3 | NS |
| POD3 | |||
| TEG with Kaolin (MA) | 68±7 | 66±8 | NS |
| TEG with Tissue Factor (MA) | 73±7 | 75±4 | NS |
| WBA with 1μg/ml Collagen (Ohms) | 8.3±4.1 | 7.2±3.5 | NS |
| WBA with 5μg/ml Collagen (Ohms) | 13.4±4.3 | 14±5.1 | NS |
Other management strategies affecting hemostasis such as receiving clopidogrel until surgery or developing a peak ACT of >400 seconds, did not appear to significantly decrease any of the transcardiac coagulation markers (Table 3).
Table 3.
Other Hemostatic Management Strategies
| Peak ACT (sec) | Receiving until Surgery… | |||||
|---|---|---|---|---|---|---|
| >400 (N=23) | <400 (N=37) | P Value | Clopidigrel (N=19) | No Clopidigre (N=41) | P Value | |
| Transcardiac F1.2 (%) | 94±160 | 75±125 | NS | 90±146 | 80±138 | NS |
| Transcardiac FXII-a (%) | 11±15 | 16±37 | NS | 7±15 | 16±35 | NS |
| Coronary Sinus Microparticles (%) | 10±12 | 6±7 | NS | 6±3 | 10±16 | NS |
Graft Quality and Patency
The quality of the conduits and their anastomoses appeared to be similar between the control and aprotinin groups as defined by similar endothelial integrity (38±37 vs. 38±39% luminal surface positive for CD31, p=NS); and measurements of blood flow (37±17 vs. 42±21 ml/min, p=NS), %diastolic flow (51±19 vs. 60±4%, p=NS), and pulsatility index (2.1±0.9 vs. 3±2, p=NS). Flow in the venous grafts in both groups showed inverse correlations with the transcardiac gradient of myoglobin (R=−0.65, p<0.05) and the risk of developing the thrombin “burst” after protamine (i.e. >two-fold transcardiac gradient in F1.2, Fig. 4). Although we noted an interesting trend in the incidence of vein graft failure defined by postoperative CT angiography (5% vs. 0% failure for control vs. aprotinin), the results did not reach statistical significance.
Figure 4. Increased risk of the thrombin burst in patients with low flow bypass grafts.
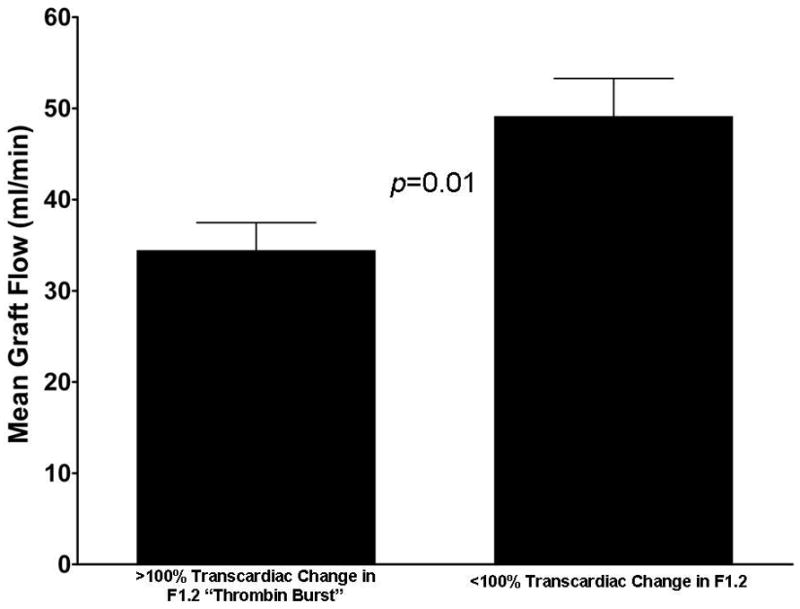
Most patients showed at least some increase in F1.2 in the coronary sinus vs. the aortic blood after OPCAB. However, the “thrombin burst”, defined as a two-fold or greater transcardiac increase in F1.2, was noted in only 18 (30%) patients. Mean blood flow within the venous grafts was significant reduced in those patients who developed the thrombin burst at the end of the case compared to those with F1.2 levels less than this threshold.
Discussion
Perhaps due to differences in collateral formation or ischemic preconditioning (1), we noted a wide variability in the cardiac release of myoglobin during this study despite the requirement of similar periods of coronary occlusion between OPCAB patients in this cohort. As a low-molecular-weight protein, myoglobin leaks from injured myocytes earlier than traditional markers such as cTn-I (19), thus providing a more sensitive measure of injury. Patients with more severe myocardial injury, in turn, developed a local inflammatory and hypercoagulable state as depicted by heightened arterial-coronary sinus (i.e. transcardiac) gradients for TNF, IL-8, F1.2, FXII-a, and platelet derived microparticles. We previously established that regional thrombin production defined by these assays is exacerbated when CABG is performed off- compared to on-pump (5). The main finding of the current study was that myocardial injury induced by warm ischemia, a feature unique to OPCAB, appears to be an important trigger to explain this difference. In addition, the strong correlation noted between the gradient of F1.2 with most of the other markers that were analyzed supports a role for thrombin as critical link between coagulation and inflammation occurring within the heart during OPCAB.
We also analyzed the impact of different therapeutic approaches employed in our cohort that might have an influence on hemostasis and thrombotic risk. A portion of our cohort was receiving clopidogrel until surgery and/or developed a higher intraoperative ACT level (e.g. >400 seconds). In these patients, we found no difference in the regional production of thrombin or any other marker compared to those treated with aspirin monotherapy and intraoperative ACT levels <400 sec. These findings contradict other reports suggesting that these strategies are effective ways to reduce thrombin production (10,11). However, these investigations analyzed thrombin production in the systemic circulation. Analysis of CS blood provides a more sensitive and specific assay of events within the upstream coronary circulation (20,21). We speculate that the application of this method in our study improved our pathophysiological insight into conditions that influence the risk of graft thrombosis after OPCAB.
Aprotinin administered prior to skin incision at full dose was associated with a significant reduction in the transcardiac gradient of the coagulation markers that were analyzed when compared to the control group. Activation of PAR-1 is likely an important avenue through which the platelet is able to respond to thrombin and participate in the feedback thrombin burst necessary for graft thrombosis. Aprotinin has been shown to block PAR-1 in vitro (12) and in vivo (14). Nevertheless, conventional wisdom suggests that aprotinin is a prothromobic agent because of its potent hemostatic effects. Establishing a clear definition of the hypercoagulable state is a vital step towards better understanding the relationship between the use of aprotinin and risk of thrombosis.
On the other hand, aprotinin also inhibits fibrinolysis, an important mechanism through which the coronary circulation keeps thrombus formation in check. This has raised considerable concern that aprotinin might increase the risk of thrombosis (22). The safety of aprotinin remains an important and controversial issue in cardiothoracic surgery, with conflicting evidence arising from randomized clinical trials (23) and observational studies (22) during on-pump CABG regarding issues such as renal dysfunction and even death. However, these nonrandomized observational trials have recently been reviewed by an FDA advisory panel and deemed statistically flawed in their analysis. As a result of the reanalysis of the raw data from these studies, the panel reached significantly more favorable conclusions regarding the safety of aprotinin‡. There has also been a more recent meta analysis by the Cochrane group (24) looking at over 200 randomized controlled trials and over 20,000 patients that found no increased risk of myocardial infarction, stroke, graft thrombosis or death with aprotinin use. On the other hand, renal dysfunction does appear to be a consistent risk that emerges in studies analyzing the safety of aprotinin use. The continued marketing of aprotinin for cardiac surgery is likely going to require further more in depth study of this important issue.
Another plausible approach to investigate would have been to prevent warm ischemic injury to the myocardium by the routine use of intracoronary shunts during the distal anastomoses. Shunts were not used in this cohort out of concern for injury to the coronary endothelium during placement (25). Therefore, we are not able to address the merits of this idea.
In previous studies, our group has demonstrated that endothelial disruption in the bypass conduit directly relates to regional thrombin production after OPCAB (7). In this study, we add lower intraluminal blood flow within the grafts as an additional risk factor (Fig. 4). Platelets and coagulation factors are known to react to abnormalities in flow and endothelium by participating in thrombin generation. There is not likely to be a systematic difference in the endothelial quality or blood flow within the coronary circulation during on- and off-pump CABG. Therefore, technical differences between these procedures may help explain the discrepancy in thrombin production. Warm myocardial ischemia is an issue that is unique to OPCAB and has been shown to be a more potent stimulant of thrombin production than cold ischemia in a variety of experimental and clinical studies (26). We feel that our data point to this issue is a primary stimulus of the hypercoagulable state after OPCAB.
Limitations
The main limitations of this study were the small number of subjects that were analyzed and nonrandomized study design. Given insufficient numbers, we were unable to perform a multivariate analysis to confirm that aprotinin was an independent predictor of regional hypercoagulability and were underpowered to establish a link to hard endpoints such as postoperative SVG failure and MI. Our study screened for factors that are most likely to influence regional hypercoagulability such as anastomotic and endothelial quality of each SVG and platelet function using three separate assays. However, the nonrandomized use of aprotinin is likely to have introduced confounding factors that remain unaccounted for in our analysis. In addition, the clinical intrusiveness of the protocol limited our coronary sinus measurements to a single 30 minute time point after protamine administration, which may have underestimated the incidence of the thrombin burst reaction after OPCAB. Finally, because this study was performed only at a single center, generalizability to other centers may be limited by institution specific practices that effect thrombin production such as the aggressive use of the cell saver during OPCAB and SVG harvesting and preparation techniques. For these reasons, our findings are best interpreted as “hypothesis generating” pending the completion of an ongoing prospective clinical trial to investigate the relationship between regional hypercoagulability markers and early graft failure.
Conclusion
In the present study, we demonstrated that intramyocardial acidosis following obligatory periods of warm ischemia during OPCAB was associated with the activation of a regional hypercoagulable and inflammatory state. Intraoperative aprotinin use during in OPCAB was found to attenuate these findings, perhaps via inhibitory effects on PAR-1 and/or other serine proteases. These data support further investigation of a novel role for aprotinin (or other PAR-1 antagonists) during surgical revascularization: as an adjunct to help abrogate the postoperative hypercoagulable state after OPCAB, and potentially improve early graft patency.
Acknowledgments
R.P. is funded by grants from the NIH (R01HL084080), American Heart Association (Scientist Development Grant, 043518N), University of Maryland (intramural grant), Tobacco Restitution Fund at the University of Maryland and Bayer Pharmaceutical Corp (phase IV grant).
Footnotes
http://www.fda.gov/OHRMS/DOCKETS/AC/07/slides/2007-4316s1-12-FDA-Levenson.ppt, accessed on 10/15/07.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nathoe HM, Buskens E, Jansen EWL, Suyker WJL, Stella PR, Lahpor JR, van Boven W-J, van Dijk D, Diephuis JC, Borst C, Moons KGM, Grobbee DE, de Jaegere PPT. Role of Coronary Collaterals in Off-Pump and On-Pump Coronary Bypass Surgery. Circ. 2004;110:1738–1742. doi: 10.1161/01.CIR.0000143105.42988.FD. [DOI] [PubMed] [Google Scholar]
- 2.Puskas JD, Williams WH, Duke PG, Staples JR, Glas KE, Marshall JJ, Leimbach M, Huber P, Garas S, Sammons BH, McCall SA, Petersen RJ, Bailey DE, Chu H, Mahoney EM, Weintraub WS, Guyton RA. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial Injury, transfusion requirements and length of stay: A prospective randomized comparison comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:797–808. doi: 10.1067/mtc.2003.324. [DOI] [PubMed] [Google Scholar]
- 3.Ascione R, Williams S, Lloyd CT, Sundaramoorthi T, Pitsis AA, Angelini GD. Reduced postoperative blood loss and transfusion requirement after beating-heart coronary operations: A prospective randomized study. J Thorac Cardiovasc Surg. 2001;121:689–696. doi: 10.1067/mtc.2001.112823. [DOI] [PubMed] [Google Scholar]
- 4.Nuttall GA, Erchul DT, Haight TJ, Ringhofer SN, Miller TL, Oliver WC, Zehr KJ, Schroeder DR. A comparison of bleeding and transfusion in patients who undergo coronary artery bypass grafting via sternotomy with and without cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2003;17:447–451. doi: 10.1016/s1053-0770(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 5.Kon ZN, Kwon MH, Collins MJ, Kallam S, Sangrampurkar R, Ozeki T, Brown EN, Romar LG, Pierson RN, Gammie JS, Brown JM, Griffith BP, Poston RS. Off-Pump Coronary Artery Bypass Leads to a Regional Hypercoagulable State Not Detectable Using Systemic Markers. Innovations. 2006;1:232–8. doi: 10.1097/01.imi.0000242160.21278.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker RC, Spencer FA, Li Y, Ball SP, Ma Y, Hurley T, Hebert J. Thrombin generation after the abrupt cessation of intravenous unfractionated heparin among patients with acute coronary syndromes: Potential mechanisms for heightened prothrombotic potential. J Am Coll Cardiol. 1999;34(4):1020–1027. doi: 10.1016/s0735-1097(99)00322-8. [DOI] [PubMed] [Google Scholar]
- 7.Poston RS, Gu J, Brown JM, Gammie JS, White C, Nie L, Pierson RN, Griffith BP. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006;131(1):122–30. doi: 10.1016/j.jtcvs.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Raivio P, Kuitunen A, Suojaranta-Ylinen R, Lassila R, Petaja J. Thrombin generation during reperfusion after coronary artery bypass surgery associates with postoperative myocardial damage. J Thromb Haemost. 2006;4(7):1523–9. doi: 10.1111/j.1538-7836.2006.02028.x. [DOI] [PubMed] [Google Scholar]
- 9.Eikelboom JW, Weitz JI, Budajd A, Zhaoe F, Coplande I, Maciejewskid P, Johnstonb M, Yusufet S. Clopidogrel does not suppress blood markers of coagulation activation in aspirin-treated patients with non-ST-elevation acute coronary syndromes. Eur Heart J. 2002;23(22):1771–9. doi: 10.1053/euhj.2000.3234. [DOI] [PubMed] [Google Scholar]
- 10.Merlini PA, Repetto A, Andreoli AM, Ricci S, Lombardi A, Finardi A, Dipasquale G, Lamponi M, Mannucci PM, Ardissino D. Effect of abciximab on prothrombin activation and thrombin generation in patients with acute myocardial infarction also receiving reteplase. Am J Cardiol. 2004;93(2):195–8. doi: 10.1016/j.amjcard.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Despotis GJ, Gravlee G, Filos K, Levy J. Anticoagulation monitoring during cardiac surgery: a review of current and emerging techniques. Anesthesiology. 1999;91(4):1122–31. doi: 10.1097/00000542-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Landis RC, Asimakopoilos G, Poullis M, Haskard DO, Taylor KM. The antithrombotic and anti-inflammatory mechanisms of action of aprotinin. Ann Thorac Surg. 2001;72:2169–75. doi: 10.1016/s0003-4975(01)02821-1. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich W. Reducing thrombin formation during cardiopulmonary bypass: is there a benefit of the additional anticoagulant action of aprotinin? J Cardiovasc Pharmacol. 1996;27(Suppl 1):S50–7. doi: 10.1097/00005344-199600001-00011. [DOI] [PubMed] [Google Scholar]
- 14.Khan TA, Bianchi C, Voisine P, Sandmeyer J, Feng J, Sellke FW. Aprotinin inhibits protease-dependent platelet aggregation and thrombosis. Ann Thorac Surg. 2005;79(5):1545–50. doi: 10.1016/j.athoracsur.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Poston RS, White C, Gu J, Brown J, Gammie J, Pierson RN, Lee A, Connerney I, Avari T, Christenson R, Tandry U, Griffith BP. Aprotinin shows both hemostatic and antithrombotic effects during off-pump coronary artery bypass grafting. Ann Thorac Surg. 2006;81:104–110. doi: 10.1016/j.athoracsur.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 16.Moliterno DJ. Results of a Multinational Randomized, Double-Blind, Placebo-Controlled Study of a Novel Thrombin Receptor Antagonist SCH 530348 in Percutaneous Coronary Intervention. American College of Cardiology meeting; New Orleans. March 24th 2007. [Google Scholar]
- 17.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–9. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PG, ten Have K, Eijsman L, Hack CE, Sturk A. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circ. 1997;96:3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 19.Roberts R, Fromm RE. Management of acute coronary syndromes based on risk stratification by biochemical markers: an idea whose time has come. Circ. 1998;98(18):1831–3. doi: 10.1161/01.cir.98.18.1831. [DOI] [PubMed] [Google Scholar]
- 20.Scharn DM. Activation of platelets in blood perfusing angioplasty damaged coronary arteries. Cardiovasc Surg. 2002;10:566. doi: 10.1161/01.atv.12.12.1475. [DOI] [PubMed] [Google Scholar]
- 21.Laskey WK, Zawacki K, Lim R, Herzog W. Effects of local heparin administration on coronary thrombin activity during percutaneous transluminal coronary angioplasty. Catheter Cardiovasc Interv. 1999;48(1):84–8. doi: 10.1002/(sici)1522-726x(199909)48:1<84::aid-ccd17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 23.Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128:442–448. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 24.Henry DA, Carless P, Moxey A, O’connell D, Stokes B, McClelland B, Laupacis A, Fergusson D. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2007;(4):CD001886. doi: 10.1002/14651858.CD001886.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Dygert JH, Thatte HS, Kumbhani DJ, Najjar SF, Treanor PR, Khuri SF. Intracoronary shunt-induced endothelial cell damage in porcine heart. J Surg Res. 2006;131(2):168–74. doi: 10.1016/j.jss.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Wolberg AS, Meng ZH, Monroe DM, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56(6):1221–8. doi: 10.1097/01.ta.0000064328.97941.fc. [DOI] [PubMed] [Google Scholar]


