Abstract
Current cognitive models suggest that the processing of dynamic facial attributes, including social signals such as gaze direction and facial expression, involves the superior temporal sulcus, whereas the processing of invariant facial structure such as the individuals’ identity involves the fusiform face area. Where facial attractiveness, a social signal that may emerge from invariant facial structure, is processed within this dual–route model of face perception is uncertain. Here, we present two studies. First, we investigated the explicit judgments of facial attractiveness and attractiveness-motivated behaviour in patients with acquired prosopagnosia, a deficit in familiar face recognition usually associated with damage to medial occipitotemporal cortex. We found that both abilities were impaired in these patients, with some weak residual ability for attractiveness judgments found only in those patients with unilateral right occipitotemporal or bilateral anterior temporal lesions. Importantly, deficits in attractiveness perception correlated with the severity of the face recognition deficit. Second, we performed a functional magnetic resonance imaging study in healthy subjects that included an implicit and explicit processing of facial attractiveness. We found increased neural activity when explicitly judging facial attractiveness within a number of cortical regions including the fusiform face area, but not the superior temporal sulcus, indicating a potential contribution of the fusiform face area to this judgment. Thus, converging neuropsychological and neuroimaging evidence points to a critical role of the inferior occipitotemporal cortex in the processing of facial attractiveness.
Keywords: Aesthetic, Attraction, Face perception, Fusiform, Prosopagnosia, Temporal
INTRODUCTION
Faces are a rich class of visual stimuli that provide a variety of information about an individual, such as their identity, gender, age and race. Faces also convey information relevant for social interactions such as an individual’s emotional state or direction of eye-gaze. An understanding of the neural mechanisms involved in processing these different aspects of faces is the focus of extensive research (Haxby et al., 2000).
Neuropsychological (e.g.(Bowers et al., 1985, de Gelder et al., 2000) and, more recently, neuroimaging (e.g., (Kanwisher et al., 1997, Ishai et al., 1999, Hoffman and Haxby, 2000) findings suggest that selective brain regions within the occipito-temporal cortex make distinct contributions to the complex process of face perception. The superior temporal sulcus (STS), a region located in the lateral occipitotemporal cortex, is suggested to process dynamic facial properties (Puce et al., 1998, Hoffman and Haxby, 2000): that is, structural elements that can change from moment to moment, such as the direction of gaze and emotional expressions, both of which play important roles in social interactions (Allison et al., 2000). On the other hand, the fusiform face area (FFA), a region located in the medial occipitotemporal cortex, is preferentially involved in processing temporally-invariant facial structure: that is, elements that remain constant over such dynamic variations, which provide the chief cues to properties such as identity and gender (Sergent et al., 1992, Kanwisher et al., 1997, George et al., 1999, Hoffman and Haxby, 2000).
Less explored than identity, expression and gaze direction is another facial property: attractiveness. Perceptions of facial attractiveness can influence many judgments we make about others, including their desirability and personality (Dion et al., 1972), likelihood of mating success (Thornhill et al., 1995, Pashos and Niemitz, 2003), earning potential (Frieze et al., 1991), and competency in school and work (Dipboye et al., 1975). Given the contribution of facial attractiveness to these social judgments, some have postulated that facial attractiveness may be another property processed by the superior temporal sulcus (Winston et al., 2007). Indeed, according to Winston and colleagues (Winston et al., 2007), the role of the superior temporal sulcus in facial attractiveness judgements may be consistent with the “intention-detection” of the observer, that is the process of assessing the attractiveness of a conspecific as the social evaluation of another’s intentions toward ourselves. However, one might also argue that, unlike the social signals of gaze direction and expression, attractiveness is based more on temporally invariant rather than dynamic aspects of facial structure: in general, perceptions of physical beauty persist despite moment-to-moment fluctuations in facial expression or gaze. Indeed, while a pleasant expression can contribute to attractiveness, current research suggests that the most important factors in attractiveness are averageness, symmetry, and sexual dimorphism, facial properties that are stable over time (Rhodes, 2006). In this respect, attractiveness may be more similar in processing demands to the properties of identity and gender, and may therefore require the contribution of the FFA as well. The present study aims at testing this specific hypothesis.
In order to provide converging evidence for our hypothesis, we have performed two studies, which used a neuropsychological and a neuroimaging approach respectively. In the first study, we assessed whether patients with prosopagnosia, i.e. the inability to recognize the identity of familiar faces (Barton, 2003, Harris and Aguirre, 2007), retain the ability to process facial attractiveness. Acquired prosopagnosia usually results from bilateral or unilateral right medial occipitotemporal lesions, often involving the right fusiform gyrus (Meadows, 1974, Damasio et al., 1982, Bouvier and Engel, 2006). The extent of lateral occipitotemporal damage in these subjects is more variable, which may account for the observation that the perception of facial expression can be spared in these subjects (Bowers et al., 1985, de Gelder et al., 2000). If the perception of facial attractiveness is consistently impaired in this population, and even correlates with the severity of the impairment in facial identity processing, this would support the hypothesis that a medial occipitotemporal processing stream which includes the fusiform gyrus contributes to the perception of facial attractiveness, as well as the impairment in identity recognition.
Although lesion studies can provide important information on the role of certain cortical regions for a given cognitive function, the large size and variable nature of lesions across individuals makes it difficult to confidently ascribe a particular function to a specific cortical region of interest. For this reason, we performed a second study in healthy individuals, in which we used functional magnetic resonance imaging (fMRI) to further examine the contribution of the STS and FFA during the processing of facial attractiveness. According to the critical role of the STS and FFA in processing emotional expressions (social interaction cues) (Puce et al., 1998, Hoffman and Haxby, 2000) and invariant facial attributes (i.e., identity and gender) (Sergent et al., 1992, Kanwisher et al., 1997, George et al., 1999, Hoffman and Haxby, 2000) respectively, we performed a region-of-interest analysis to specifically examine the neural activity within these two regions while participants processed faces of varying degrees of attractiveness in both an implicit and explicit manner.
EXPERIMENTAL PROCEDURES
Neuropsychological study
Participants
We tested 8 subjects with prosopagnosia and 19 healthy control subjects. All subjects had normal or corrected-to-normal vision and gave informed consent in a manner consistent with the principles of the Declaration of Helsinki. The study protocols were approved by the institutional review boards of the University of British Columbia and Vancouver General Hospital.
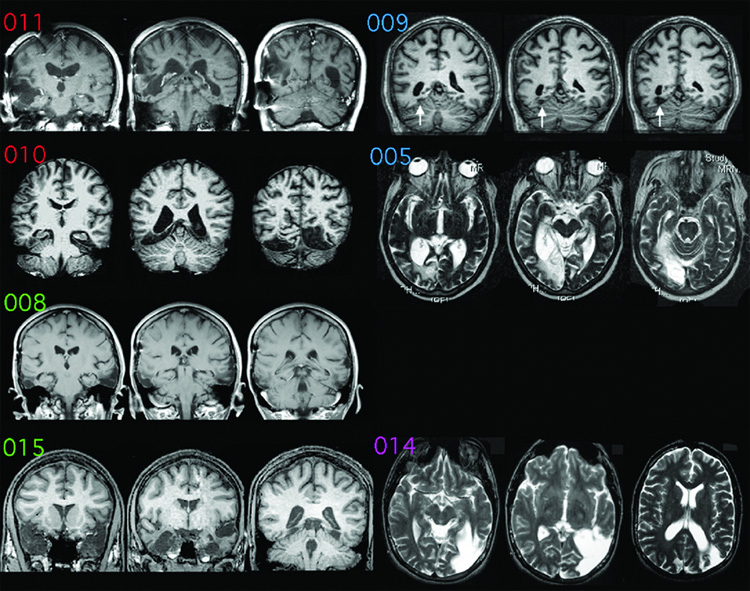
In Table 1 we report the prosopagnosic subjects’ scores on the Warrington Recognition Memory Test (Warrington, 1984), the Benton Face Recognition Test (Benton and Van Allen, 1972), and the assessment of facial recognition on a famous face familiarity test (Barton et al., 2001). Representative MRI scans are shown in Figure 1, and anatomic templates in Figure 2. More detailed case information on most of these subjects can be found in previous reports using the same subject identification numbers (Barton et al., 2004a, Barton et al., 2004b, Barton and Cherkasova, 2005).
Table 1.
Subjects’ demographic and lesion data. BFRT = Benton Face Recognition Test (x/54). WRMT (F) = Warrington recognition memory test faces component (x/50). WRMT (W) = Warrington recognition memory test word component (x/50). d' = discriminative power on famous face familiarity test (Barton et al., 2001).
| Subject | Site of Lesion | Gender | Age (years) | BFRT | WRMT(F) | WRMT(W) | d' |
|---|---|---|---|---|---|---|---|
| 001 | Unknown Unilateral(right) medial |
Male | 25 | 39 | 30 | 45 | −0.61 |
| 005 | occipotemporal bilateral anterio |
Male | 63 | 35 | 33 | 42 | 0.67 |
| 008 | temporal unilateral (right) medial |
Female | 37 | 25 | 13 | 45 | 0.68 |
| 009 | occipotemporal bilateral |
Male | 50 | 43 | 33 | 43 | 0.88 |
| 010 | occipitotemporal bilateral |
Male | 42 | 37 | 24 | 48 | −0.22 |
| 011 | occipitotemporal unilateral (left) lateral |
Male | 52 | 32 | 33 | 45 | −0.18 |
| 014 | occipotemporal bilateral anterior |
Male | 29 | 34 | 33 | 47 | 0.9 |
| 015 | temporal | Male | 24 | 45 | 27 | 45 | 0.88 |
Figure 1.
Magnetic Resonance (MR) images of the prosopagnosic subjects. By convention, right hemispheres are on the left of each image. Scans of subjects 005 and 014 are displayed in axial sections; the others are displayed in coronal sections. The MR images refer to (from top to bottom): left row = bilateral lesions, subjects 011, 010, 008, and 015; right row = unilateral lesions, subjects 009, 005, 014. White arrows for subject 009 show his small infarct. There is no image for subject 001, who had no visible lesion. Letter coloring refers to lesion category: red = bilateral occipitotemporal, green = bilateral anterior temporal, blue = unilateral right occipitotemporal, purple = unilateral left occipitotemporal.
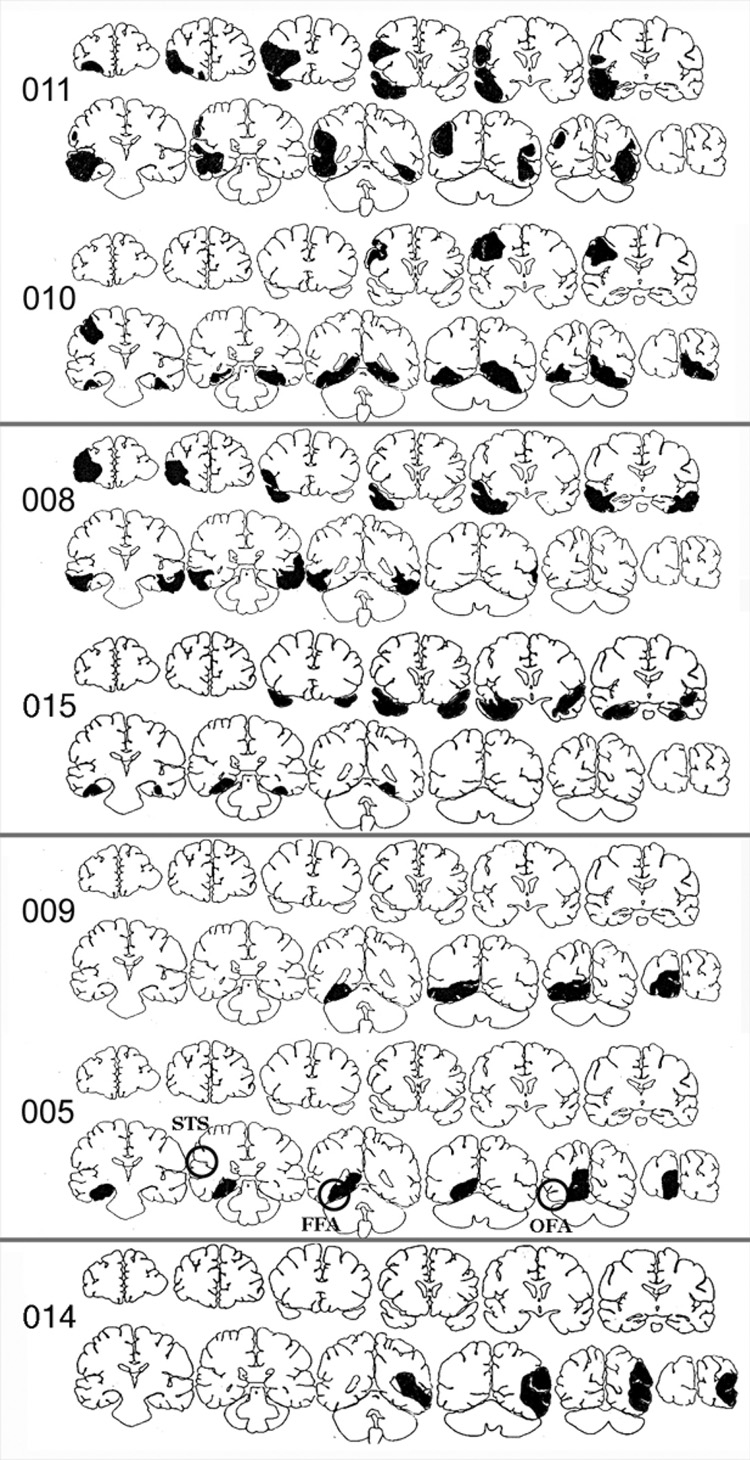
Figure 2.
Coronal template drawings of lesions of the prosopagnosic subjects. Rings in the drawing for subject 005 show the approximate location of the superior temporal sulcus (STS), fusiform face area (FFA), and occipital face area (OFA).
Subject 001, a 25-year-old right-handed male, suffered from cardiopulmonary arrest and coma at 18 months (Kosslyn et al., 1995). A structural MRI did not reveal any lesion (Hadjikhani and de Gelder, 2002); however, he demonstrated severe deficits in perceiving facial configuration similar to prosopagnosic subjects with lesions of the fusiform face area (Kosslyn et al., 1995). Regarding perception of expression, he scored 24/36 on the Revised Eyes Test (Baron-Cohen et al., 2001) (www.autismresearchcentre.com/tests), which is within the normal range (control mean = 26.2, sd = 3.6).
Subjects 010 and 011 have bilateral, primarily occipitotemporal, lesions. Subject 010 is a 42-year-old right-handed male who has bilateral posterior occipitotemporal lesions following an automobile accident 21 years earlier, resulting in a subdural hematoma with associated prosopagnosia and right hemianopia. On the faces portion of the DANVA-2 (diagnostic assessment of non-verbal accuracy, (www.dyssemia.com/danva), a test that requires subjects to indicate which of four emotions corresponds to an expression on a test face, he made 12 errors, which is better than 95% limits for chance (15 errors) but slightly above the normal range for his age (mean =4.8 errors, s.d. = 2.6). Nevertheless, his lesion involves neither the right nor the left superior temporal sulcus (Figure 1). Subject 011, a subject of numerous prosopagnosic studies (Etcoff et al., 1991, Farah et al., 1995a, Farah et al., 1995b), is a 53-year-old right-handed male who sustained a severe head injury in an automobile accident at the age of 18 resulting in damage predominantly to the right anterior temporal and medial occipitotemporal cortex and a small left medial occipitotemporal lesion. Subject 011 had difficulty with the Revised Eyes Test, scoring 7/36, but on the faces portion of the DANVA-2 he made 10 errors, which is better than 95% limits for chance (15 errors) and within the normal range for his age (mean = 6.3 errors, sd = 2.7). His MRI scan (Figure 1) does suggest potential compromise of the right superior temporal sulcus as well as the fusiform gyrus.
Subjects 005 and 009 have unilateral right medial occipitotemporal lesions. Subject 005 is a 63-year-old right-handed male who had a right medial occipitotemporal stroke 5 years prior to testing. He had difficulty on the Revised Eyes Test, scoring 16/36. However, on the face portion of the DANVA-2, he made only 9 errors, which is within the normal range for his age (mean = 6.3 errors, sd = 2.7) and better than the 95% limits for chance (15 errors). Subject 009 is a 50-year-old right-handed male who suffered a right posterior cerebral arterial infarct that affected the fusiform face area, as shown in an fMRI study (de Gelder et al., 2003), at the age of 49, with resultant prosopagnosia and left hemianopia. A previous report showed that subject 009 performed normally on an experimental test involving matching expressions from the faces of different individuals (de Gelder et al., 2003).
Subjects 008 and 015 have bilateral damage primarily affecting anterior temporal structures. Subject 008 is a 37-year-old right-handed female, who experienced a severe closed head injury with damage to the left temporal lobe and a subsequent right temporal lobe resection 14 years prior to testing. On the Revised Eyes Test (Baron-Cohen et al., 2001) she scored 22/36, within the normal range (control mean = 26.2, sd = 3.6). Subject 015 is a 24-year-old right-handed male who contracted herpes simplex encephalitis 3 years prior, resulting in bilateral anteromedial temporal lobe damage, with additional symptoms of topographagnosia (i.e. difficulty in orienting in the environment). We have previously shown that subject 015 does have a remaining right fusi form face area and right superior temporal sulcus with fMRI (Fox et al., 2007). He also performed normally on the Florida Affect Battery, and on an experimental test of expression discrimination (Fox et al., 2007). Both subjects 008 and 015 have normal visual fields.
Subject 014 is a 29-year-old left-handed male who suffered from perinatal hypoxia resulting in lifelong prosopagnosia, visual field deficits and communication problems. His MRI scan shows an unusual left-sided lesion involving the inferior occipitotemporal, lateral occipitotemporal and occipitoparietal cortex.
Coronal template drawings of the lesions visible in seven subjects are shown in Figure 2. While conclusions cannot be definitive without functional imaging, the structural data suggest involvement of the right superior temporal sulcus in subject 011, and of the left superior temporal sulcus in subject 014. In subjects 009 and 010, lesion sites may also involve the right occipital face area. Among patients with documented brain damage, the right fusiform face area is likely involved in all subjects except 008 and 015, with more anterior temporal lesions, and in subject 014, with an anomalous left hemispheric lesion.
Control subjects included 19 healthy heterosexual participants (8 female). Male subjects had a mean age of 38.3 years (sd = 13.9), which was not significantly different from the male prosopagnosic subjects, whose mean age was 40.7 years (sd = 15.1). Female subjects had a mean age of 35.1 years (sd = 13.5), similar to the single prosopagnosic female who was 37 year-sold.
Stimuli and Tasks
Stimuli consisted of two sets of 40 anonymous human faces categorized as “beautiful” and “average” according to the aesthetic judgment given by a different group of subjects in a prior report (Aharon et al., 2001). All stimuli were digitized at 600 dpi in 8-bit grayscale, spatially downsampled, and cropped to fit in an oval window sized 310–350 pixels wide by 470 pixels high. Each stimulus set consisted of 20 male and 20 female faces. The stimuli were presented in a random order, one at a time, on a computer screen positioned approximately 57 cm away from the subjects’ eyes. Subjects performed two different tasks: the “Explicit rating of attractiveness” task and the “Attractiveness-motivated behavior” task. During the “Explicit rating of attractiveness” task, participants were asked to rate each face on a computerized visual analogue scale with the extremes marked as “not attractive” and “very attractive”. The cursor began at a marked midpoint and could be moved anywhere between either extreme by pressing one of two keys. Ratings were scaled to integers between 0 (not attractive) and 12 (very attractive). These integers were not visible to the participants. While performing the “Attractiveness-motivated behavior” task, participants were aware that they would see a series of aces that would each be displayed for 8 seconds unless they intervened. If the participants wished the picture to disappear sooner they were instructed to toggle between the “z” and “x” keys of the keyboard. On the other hand, if they wanted the picture to remain on the screen they were instructed to toggle between the “n” and “m” keys. Each pair of key presses increased or decreased the total viewing time according to the following formula:
where Extreme Viewing Time was 0 seconds for key-presses reducing the viewing time and 16 seconds for key-presses increasing the viewing time, and K was a scaling constant set at 40. When the elapsed time exceeded the new viewing time, the picture disappeared and the next trial began. A slider was displayed to the left of each picture indicating the total viewing time at any moment. This task-performance procedure is identical to the one adopted in a previous study (Aharon et al., 2001).
Data Analysis
With respect to the “Explicit rating of attractiveness” task we calculated, for each participant, the average score given to the four categories of stimuli, i.e. average male, beautiful male, average female, beautiful female. Then, we performed two ANOVAs (JMP IN 5.1 Software; SAS Institute Inc) using a general linear model with these scores as repeated measures, and Observer-gender (male, female), Stimulus-gender (same as observer, opposite of observer) and Aesthetic-class (average, beautiful) as independent factors. The first ANOVA included only the healthy ontrol subjects in order to control for the validity of the aesthetic categories and the gender effects reported in a previous study (Aharon et al., 2001). Then, we performed the second ANOVA aiming at investigating whether or not prosopagnosic subjects differed from the control subjects in the ability to perceive facial attractiveness. In this second ANOVA we included the Group (prosopagnosic, control) as another independent factor. Similar analyses were performed with respect to the “Attractiveness-motivated behaviour” task.
In addition, we performed different analyses that did not depend upon the use of the aesthetic categories determined by controls used in the prior normative studies, which were not age-matched to our prosopagnosic subjects. Using our own control subjects, we calculated the mean attractiveness rating for each of the 80 faces. Then, we calculated for each prosopagnosic subject the correlation coefficient (r) of their ratings with the mean ratings given by the controls, which would be significant if residual attractiveness perception is present. Therefore, we asked whether the mean correlation coefficient for the prosopagnosic group was significantly different than zero. To adjust for the asymmetry of the r distribution, we first transformed the r values using Fisher's Z(r) = ln{(1+r)/(1−r)}. Moreover, to assess individually whether or not any prosopagnosic subject had any residual appreciation of facial attractiveness at all, we performed two different analyses: first, we examined whether the difference between their mean ratings of average faces differed significantly from their ratings of beautiful faces; second, we evaluated whether, in our second analysis of correlation coefficients, a subject’s ratings for all faces showed a significant correlation with the mean ratings of the control subjects.
Finally, we performed correlation analyses to assess whether or not the processing of facial aesthetics relates to the processing of facial identity. We had previously quantified the prosopagnosic defect in these subjects by using a test of famous face familiarity; and by using signal detection theory obtained a d’ for each subject, an index of their power to discriminate between familiar and unfamiliar faces. Likewise, the correlation coefficient of a prosopagnosic’s attractiveness ratings with the mean ratings of controls can serve as an index of aesthetic facial processing in a prosopagnosic subject. If aesthetic judgements about faces are related to identity judgements, then we should expect a significant correlation between the indices of aesthetic processing (Fisher's Z(r)) and identity processing.
Similarly ANOVAs and correlation analyses were performed with respect to the “Attractiveness-motivated behavior” task, with the exposure time of the stimuli as the repeated dependent variable.
Neuroimaging study
Participants
Eleven right-handed healthy subjects (6 females; mean age ± sd: 24.09 years ± 2.17) participated in the fMRI study. All participants provided informed consent in accordance with the Declaration of Helsinki, as approved by the institutional review board of the University of British Columbia. All subjects had normal or corrected-to-normal vision. Data collected from one subject was excluded from all analyses due to excessive head motion.
Stimuli and Tasks
Participants underwent three functional scans. The first scan was a functional localizer designed to define face-selective brain regions to be used for a restricted Region of Interest (ROI) analysis of the subsequent two experimental scans. During the functional localizer scan, subjects viewed photographs of objects and faces (neutral and expressive), which were presented in separate blocks. Within each block subjects were asked to perform a one-back task, which consisted of indicating a repeated image by pressing a button. The localizer scan began and ended with 12 seconds of fixation during which a cross was present on the center of the screen. Following the initial block of fixation, image blocks (12 seconds) were interleaved with fixation blocks (12 seconds) for the entire duration of the scan (448 seconds). Six blocks of each image category (object, neutral face, and expressive face) were presented in a counterbalanced order. Each image block consisted of 15 images (12 novel and 3 repeated) presented centrally for 500ms, with an inter-stimulus-interval (ISI) of 300ms. Neutral and expressive faces were taken from the authors’ personal collection, whereas object images, which included only non-living objects (e.g., television, basketball), were gathered from the internet. All images were converted to grayscale and resized to a fixed width of 400 pixels.
Following the localizer scan, subjects participated in two experimental scans, which were separated only to ensure an appropriate duration for each individual scan. As in the localizer scan, each experimental scan began and ended with 12 seconds of fixation. Following the initial block of fixation, experimental blocks (28 seconds) were interleaved with fixation blocks (12 seconds) for the duration of the scan (336 seconds). Each experimental block consisted of 14 face images presented centrally for 1300ms, with an ISI of 700ms. These face images were randomly selected from a sample of 96 images comprising three aesthetic categories (32 Attractive, 32 Average, 32 Unattractive). Attractive and Average faces were obtained from the images used in Experiment 1, while Unattractive faces were gathered from the internet. During alternating experimental blocks subjects were asked to perform one of two different behavioural tasks, namely the Explicit or Implicit task. During the Explicit task, participants were required to make a three-alternative forced-choice attractiveness rating (Attractive, Average, Unattractive), an explicit processing of facial attractiveness. During the Implicit task, they performed a one-back same-different task, in which they were required to press a button when the image presented was identical to the previous one, an implicit processing of facial attractiveness. During the central 4 seconds of the fixation block, immediately preceding each experimental block, the fixation cross was replaced with the words “Attractiveness” or “Repeated Image” instructing the subjects to perform, on the upcoming experimental block, either the explicit or implicit task, respectively. Face images were digitized at 600 dpi in 8-bit grayscale, spatially downsampled, and cropped to fit in an oval window sized 400 pixels wide. As the implicit one-back task necessitated the repetition of certain images, these same images were repeated during the explicit task, but in a different randomized presentation order. This resulted in a balanced design where the same 108 images (including 12 repeated images) were seen during both tasks.
Data Acquisition
All scans were acquired in a 3.0 Tesla Phillips scanner. Images were presented using Presentation 9.81 software and were rear-projected onto a mirror mounted on the head coil. Whole brain anatomical scans were acquired using a T1-weighted echoplanar imaging (EPI) sequence, consisting of 170 axial slices of 1mm thickness (1mm gap) with an in-plane resolution of 1mm × 1mm (FOV=256). T2-weighted functional scans (TR=2s; TE=30ms) were acquired using an interleaved ascending EPI sequence, consisting of 36 axial slices of 3mm thickness (1mm gap) with an in-plane resolution of 1.675mm × 1.675mm. The localizer scan consisted of 224 functional volumes, while the two experimental scans consisted of 168 functional volumes each.
Data Analysis
The first volume of each functional scan was discarded to allow for scanner equilibration. All MRI data were analyzed using BrainVoyager QX Version 1.8. Preprocessing of functional scans consisted of corrections for slice scan time acquisition, head motion (trilinear interpolation), and temporal filtering with a high pass filter in order to remove frequencies less than 3 cycles/time course. Subjects showing excessive head motion (>2° translation or rotation in any direction) were excluded from further analyses. Functional runs were individually co-registered to their respective anatomical scan, using the first retained functional volume to generate the co-registration matrix.
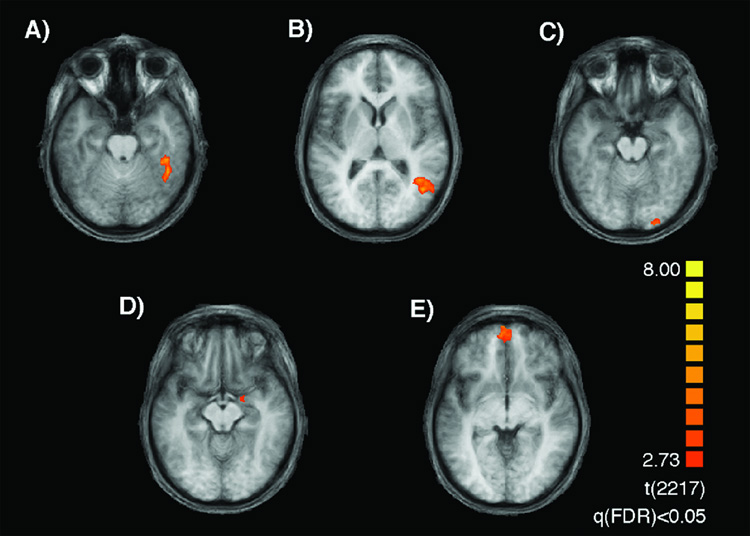
The localizer time course was analyzed via a multi subject GLM, with object (O) and faces (F) (expressive and neutral) as predictors. A direct contrast between faces and objects was performed to localize face selective voxel clusters. Bilateral clusters located on the temporal portion of the fusiform gyrus were designated as the fusiform face area (FFA), and bilateral clusters on the posterolateral superior temporal gyrus as the superior temporal sulcus (STS). In addition to the FFA and STS, in which we specifically test our a priori hypotheses concerning their roles in the processing of facial attractiveness, we also considered clusters located on the lateral surface of the occipital lobe (the occipital face area; OFA), the orbitofrontal cortex (OFC), and the amygdala (AMG) for explorative analyses. Functional regions-of-interest (ROIs) were defined in the average brain, and activity within these individual ROIs was subjected to group analyses. In Table 2 we report peak activity, cluster size, and MNI coordinates (Montreal Neurological Institute, Montreal, Canada) for each ROI. (Original analyses were performed at single subject level to account for the well-known variability in the localization of the face areas across individuals. These analyses, however, were then replaced by the group average analyses under explicit request of one reviewer as a Conditio sine qua non for publishing the manuscript. Fortunately, the findings were similar in both cases).
Table 2.
Peak activity, cluster size, and MNI coordinates of the fusiform face area (FFA), the superior temporal sulcus (STS), the occipital face area (OFA), the amygdala (AMG) and the orbitotofrontal cortex (OFC) for both the right (R) and left (L) hemisphere, as localized in the group average. Note that the coordinates of the peak activity in the OFC are located in the left hemisphere but the activity extended to the right one, thus forming a single cluster.
| ROI | Voxels | Peak t-value | X | Y | Z |
|---|---|---|---|---|---|
| R-FFA | 1196 | 6.38 | 39 | −52 | −20 |
| L-FFA | 14 | 4.1 | −36 | −40 | −26 |
| R-STS | 1834 | 6.66 | 39 | −54 | 5 |
| L-STS | 161 | 4.74 | −45 | −66 | 7 |
| R-OFA | 224 | 5.08 | 24 | −93 | −22 |
| L-OFA | 110 | 2.99 | −24 | −93 | −26 |
| R-AMG | 107 | 4.45 | 18 | −4 | −10 |
| L-AMG | 110 | 4.37 | −18 | −1 | −13 |
| R/L-OFC | 7743 | 6.83 | −6 | 58 | −4 |
Behavioural responses were recorded during the two experimental runs, and face images were uniquely classified for each subject based on their own response (Attractive, Average, Unattractive) during the explicit task. As a primary analysis, we examined task effects within each ROI by analyzing functional activity at the block level. This was achieved using a standard multi-study GLM with separate predictors for Explicit and Implicit blocks. Then, in a secondary analysis, we considered rating effects within each ROI by analyzing functional activity at the event level. This was achieved using a multi-study deconvolution analysis with 6 predictors (Attractive-Explicit, Average-Explicit, Unattractive-Explicit, Attractive-Implicit, Average-Implicit, Unattractive-Implicit). For each predictor, the β-weight was estimated at stimulus onset and again 9 more times (every 2s) resulting in an unbiased estimate of the hemodynamic response. Summed percent signal change values (corrected for serial correlations) for the full hemodynamic response were used for contrasts between the three levels of facial attractiveness (Attractive>Average, Attractive>Unattractive, Average>Unattractive). Separate contrasts examining task and ratings effects were performed within each ROI. All contrasts examining task and rating effects were paired t-tests with significance adjusted to p<0.001 in order to account for the performance of multiple contrasts (4 contrast by 9 ROIs)
RESULTS
Neuropsychological study
Explicit rating of attractiveness (Figure 4)
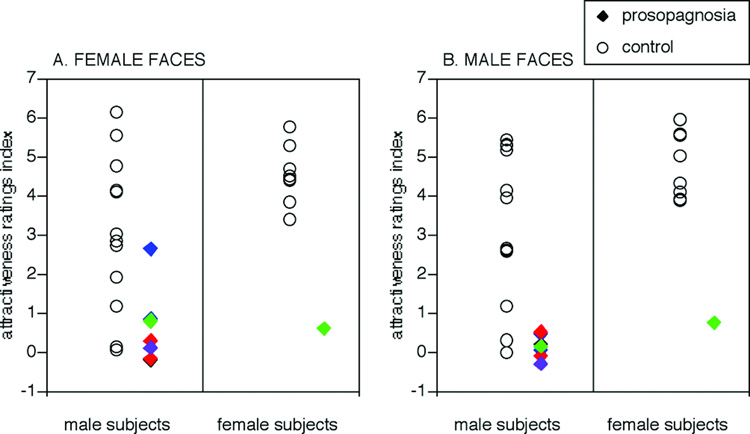
Figure 4.
Explicit rating of attractiveness. The ratings index for each subject refers to the mean score given to beautiful faces minus the mean score given to non-attractive faces. Ratings of male and female subjects for male and female face stimuli are shown separately. Rings show data of control subjects, solid diamonds show that of prosopagnosic subjects. Colours refer to lesion category: red = bilateral occipitotemporal lesions (subjects 010 and 011), blue = unilateral right occipitotemporal lesions (subjects 005 and 009), green = bilateral anterior temporal lesions (subjects 008 and 015), black = childhood onset prosopagnosia (subject 001), purple = unilateral left occipitotemporal lesion (subject 014).
In this study we asked three questions. (i) Did the prosopagnosic group differ from the control group in the ability to perceive facial beauty? We first performed a statistical analysis limited to the healthy control participants in order to replicate previous findings (Aharon et al., 2001) and assess the validity of our current findings. The ANOVA of Aesthetic-class (average, beautiful) × Observer-gender (female, male) × Stimulus-gender (same as observer, opposite of observer) with attractiveness rating as the repeated measure revealed a significant main effect of Aesthetic-class (F(1,1495) = 1449, p<0.0001), confirming that our healthy participants largely concurred on ratings of facial beauty with the subjects whose judgements were used to create the two aesthetic categories. The analysis also revealed a significant interaction effect between Observer-gender and Aestheticclass (F(1,1495) = 58.8, p<0.0001), replicating a previous finding (Aharon et al., 2001). Post hoc comparisons showed a significant difference between how female and male subjects rated average faces (t = 2.50, p<0.014) but not between their ratings of beautiful faces (t = 1.43, p = 0.15). On average, females rated average faces as 3.04 and beautiful faces as 7.70, while males rated them respectively as 4.03 and 7.13.
To assess the ability of the prosopagnosic group to perceive facial attractiveness, we then performed an ANOVA of Group (prosopagnosics, healthy controls) × Aesthetic-class (average, beautiful) × Observer-gender (female, male) × Stimulus-gender (same as observer, opposite of observer) with attractiveness rating as the repeated measure. We found a significant main effect of Aesthetic-class (F(1,2121) = 378, p<0.0001) and a significant interaction effect between Aesthetic-class and Observer-gender (F(1,2121) = 16.9, p<0.0001). Post hoc comparisons revealed that females gave a larger range of mean ratings (4.26 for average faces versus 6.93 for beautiful faces) than male subjects (4.64 for average faces versus 6.37 for beautiful faces). Most importantly, for our purpose, the ANOVA revealed a highly significant interaction effect between Group and Aesthetic-class (F(1,2121) = 219, p < 0.0001). Post hoc comparisons revealed that, while control subjects gave significantly different mean ratings (t = 42.9, p<0.0001) for average (3.54) versus beautiful faces (7.41), prosopagnosic subjects gave more similar mean ratings for average faces (5.36) and beautiful faces (5.89), a small difference that was statistically significant (t = 2.53, p<0.01). Put another way, prosopagnosics did not rate average faces as unattractive as controls did (t = 3.39, p<0.0007), nor did they rate beautiful faces as attractive as controls did (t = 5.39, p<0.0001).
(ii) Did any individual prosopagnosic subjects show residual appreciation of facial attraction? First, for each subject we determined whether their scores for average versus beautiful faces differed significantly. With this approach, four subjects showed some residual ability to discern facial attractiveness: subject 005 (t=2.17, p<0.037) and subject 009 (t=4.99, p<0.001), both with unilateral right occipitotemporal lesions; and subjects 008 (t=3.48, p<0.002) and 015 (t=2.42, p<0.03), both with bilateral anterior temporal lesions.
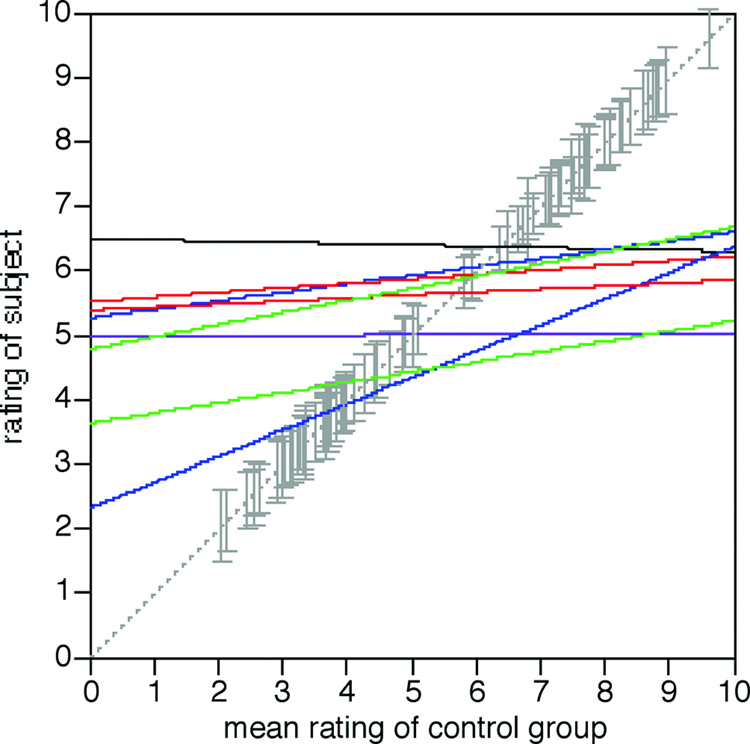
In a second approach, we calculated for each prosopagnosic subject the correlation of the ratings they gave for each face with the mean rating given by controls for that face (Figure 5). For the prosopagnosic group as a whole, the average correlation coefficient was 0.25 (sd = 0.20), which was not significantly different from zero, indicating that the aesthetic judgments of the rosopagnosic group were not correlated with those of the control group. The data of individual subjects showed significant correlations for the same four subjects above: subjects 005 (r=0.30, F(1,78)= 7.58, p<0.008) and 009 (r=0.53, F(1,78) = 30.1, p<0.0001) with unilateral right occipitotemporal lesions; and subjects 008 (r = 0.43, F(1,78) = 17.4, p<0.0001) and 015 (r= 0.37, F(1,78) = 12.6, p<0.0007) with bilateral anterior temporal lesions. (To give a sense of the variability in the healthy controls, the mean correlation of each control subject against the group mean was r = 0.84 (sd = 0.13)).
Figure 5.
Correlation analysis of explicit ratings. For each subject, the score s/he gave for a specific face is plotted against the mean score given by the control group for that face. Solid lines show the linear regressions through the data for each individual subject. Colours refer to lesion category: red = bilateral occipitotemporal lesions (subjects 010 and 011), blue = unilateral right occipitotemporal lesions (subjects 005 and 009), green = bilateral anterior temporal lesions (subjects 008 and 015), black = childhood onset prosopagnosia (subject 001), purple = unilateral left occipitotemporal lesion (subject 014). Only the green and blue lines show a correlation coefficient significantly different from zero. The dashed grey line shows where a perfect correlation would lie, if the subject’s judgments were in full agreement with the control group. The grey vertical error bars mark the location of the mean control attractiveness rating of each of the 40 faces, with the size of the bars indicating the standard error of the scores of the control group.
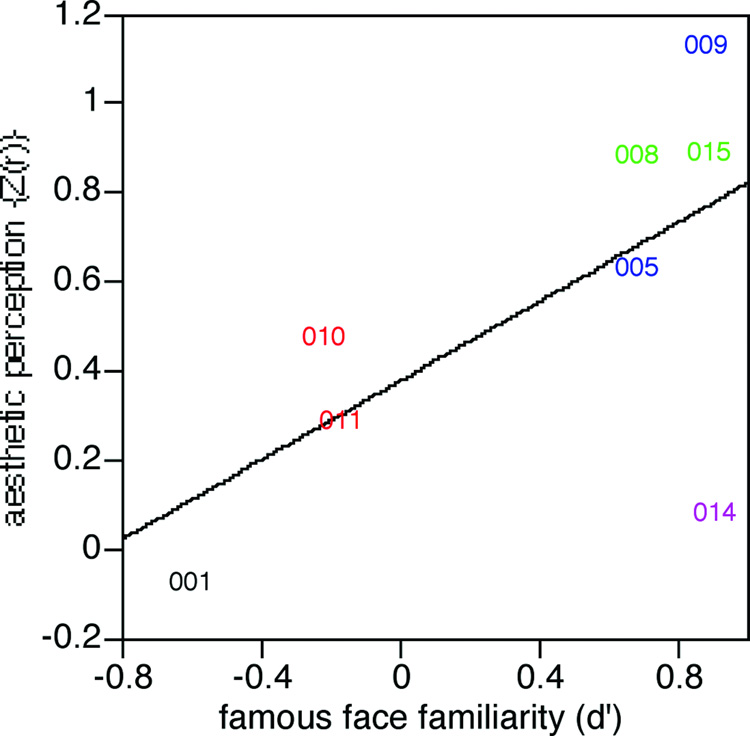
(iii) Was the processing of facial aesthetics related to the processing of facial identity? We examined whether this correlative index of the ability to perceive facial attractiveness (the Fisher’s Z(r) transform of the correlation coefficient of a subject’s ratings versus the mean ratings of control subjects) was related to the severity of their prosopagnosia, as indexed by their d’ in the test of familiarity for famous faces (Table 1). This resulted in a correlation coefficient of 0.64 with a trend to statistical significance (F(1,6)=4.13, p<0.089). The plotting of these data (Figure 6) showed that subject 014, a left-handed subject with an atypical early-onset unilateral left occipitotemporal lesion, was an anomalous outlier. If his data are omitted, the data shows a very high correlation coefficient between aesthetic judgment and facial recognition of 0.94 in the remaining subjects (F(1,5)=35.3, p<0.002). Even if we also omit the data of subject 001, another subject who differed from the others in that he had no visible lesion and also had early-onset prosopagnosia, a significant correlation of 0.89 remains for subjects with bilateral or right-sided lesions (F(1,4) = 15.1, p<0.018).
Figure 6.
The relation between ratings of attractiveness and familiarity for famous faces. The correlation coefficient Fisher’s Z(r) obtained in the correlation analysis for attractiveness ratings is plotted against the discriminative power (d’) obtained in the famous faces test. The figure shows a trend that becomes highly significant (r = .94) when the data of the outlying subject 014 (who had an anomalous left occipitotemporal lesion) are omitted from the analysis. As in the prior figures, colours refer to lesion category: red = bilateral occipitotemporal lesions (subjects 010 and 011), blue = unilateral right occipitotemporal lesions (subjects 005 and 009), green = bilateral anterior temporal lesions (subjects 008 and 015), black = childhood onset prosopagnosia (subject 001), purple = unilateral left occipitotemporal lesion (subject 014).
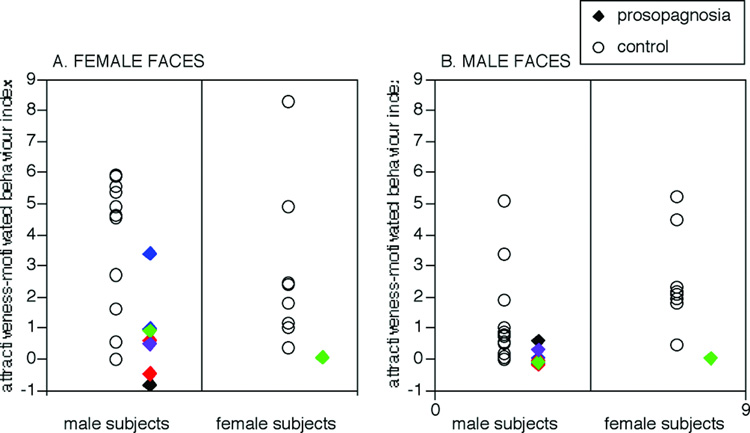
Attractiveness-motivated behaviour (Figure 7)
Figure 7.
Attractiveness-motivated behaviour. The index for each subject is the mean score for beautiful faces minus the mean score for plain faces. Rating of male and female subjects for male and female faces are shown separately. Rings refer to the control subjects, solid diamonds refer to the prosopagnosic subjects. Colours refer to lesion category: red = bilateral occipitotemporal lesions (subjects 010 and 011), blue = unilateral right occipitotemporal lesions (subjects 005 and 009), green = bilateral anterior temporal lesions (subjects 008 and 015), black = childhood onset prosopagnosia (subject 001), purple = unilateral left occipitotemporal lesion (subject 014).
As for the previous task, we first analyzed the performance of the healthy control participants to assess validity and replicate prior findings (Aharon et al., 2001). We performed an ANOVA of Aesthetic-class (average, beautiful) × Observer-gender (female, male) × Stimulus-gender (same as observer, opposite of observer) with exposure time as the repeated measure. The results revealed a significant main effect of Aesthetic-class (F(1,1495) = 696, p<0.0001) and Stimulus-gender (F(1,1495) = 43.5, p<0.0001): post hoc comparisons for these main effects showed that participants looked at beautiful faces (7.78 s) longer than average faces (5.16 s), and looked at faces of the opposite gender (6.8 s) longer than faces of the same gender (6.15 s). The ANOVA revealed also a significant interaction effect between Aesthetic-class and Stimulus-gender (F(1,1495) = 32.1, p<0.0001): while there was no difference between how long subjects looked at average faces of the same (5.12 s) or opposite (5.21 s) gender, they looked longer at beautiful faces of the opposite gender (8.39 s) than at beautiful faces of the same gender (7.17 s) (t=8.67, p<0.0001). The interaction effect between Observer-gender and Stimulus-gender F(1,1495) = 39.6, p<0.0001) resulted significant as well: post hoc comparisons revealed that while males (6.91 s) and females (6.69 s) did not differ in how long they viewed faces of the opposite sex, males (5.63 s) were less inclined than females (6.67 s) to look at faces of the same sex (t = 2.05, p<0.04). Finally, the analysis revealed a significant three-way interaction effect between Aesthetic-class, Observer-gender and Stimulus-gender (F(1495) = 47.1, p<0.0001): post hoc comparisons showed that the gender of the face did not influence the key-press behaviour for average faces for either males (same gender: 4.90 s; different gender: 4.93 s) or females (same gender: 5.34 s; different gender: 5.56 s). However, while females looked longer at beautiful faces regardless of gender (same gender: 7.80 s: different gender: 7.66 s), males were less willing to look at beautiful male faces (6.16. s) than they were for beautiful female faces (8.40 s). (t = 13.85, p<0.0001). Altogether, these findings replicate the data reported previously (Aharon et al., 2001) and extend this to female subjects, who were not included in the previous study.
To compare the performance of prosopagnosic subjects and healthy controls, we performed an ANOVA of Group (prosopagnosics, healthy controls) × Aesthetic-class (average, beautiful) × Observer-gender (female, male )× Stimulus-gender (same of observer, different of observer) with exposition time as repeated measure. The results showed the same main effects of aesthetic class (F(1,2121) = 147, p<0.0001), and face gender (F(1,2121) = 33.2, p<0.0001), the same 2-way interactions of Aesthetic-class and Stimulus-gender (F(1,2121) = 9.21, p<0.002) and Observer-gender and Stimulus-gender (F(1,2121) = 23.4, p<.0001), and the same 3-way interaction between Aesthetic-class, Observer-gender and Stimulus-gender (F(1,2121) = 13.5, p<0.0002) that were described above. The key finding in this analysis was a significant interaction effect between Group and Aesthetic-class (F(1,2121) = 102, p < .0001): post-hoc comparisons revealed that while controls showed a highly significant difference in how long they looked at average (5.14 s) versus beautiful faces (7.74 s) (t = 27.9, p<.0001), prosopagnosic subjects did not (average: 7.32 s; beautiful: 7.68 s).
We examined the performance of individual prosopagnosic subjects by examining whether there was a significant difference in their key-press behaviour for beautiful versus average faces. No subject who failed to show an ability to judge facial attractiveness on the first task showed a significant effect of aesthetic class on their key-press behaviour. Of the four subjects who had shown some residual discrimination of attractiveness in the first task, only two subjects, subjects 005 (t=2.83, p<0.008) and 009 (t=3.45, p<0.002), who had unilateral right occipitotemporal lesions, showed a significant difference between looking at average versus beautiful faces. The other two subjects, subjects 008 and 015, who had anterior temporal lesions, failed to show a significant impact of facial attraction on key-press behaviour.
Neuroimaging study
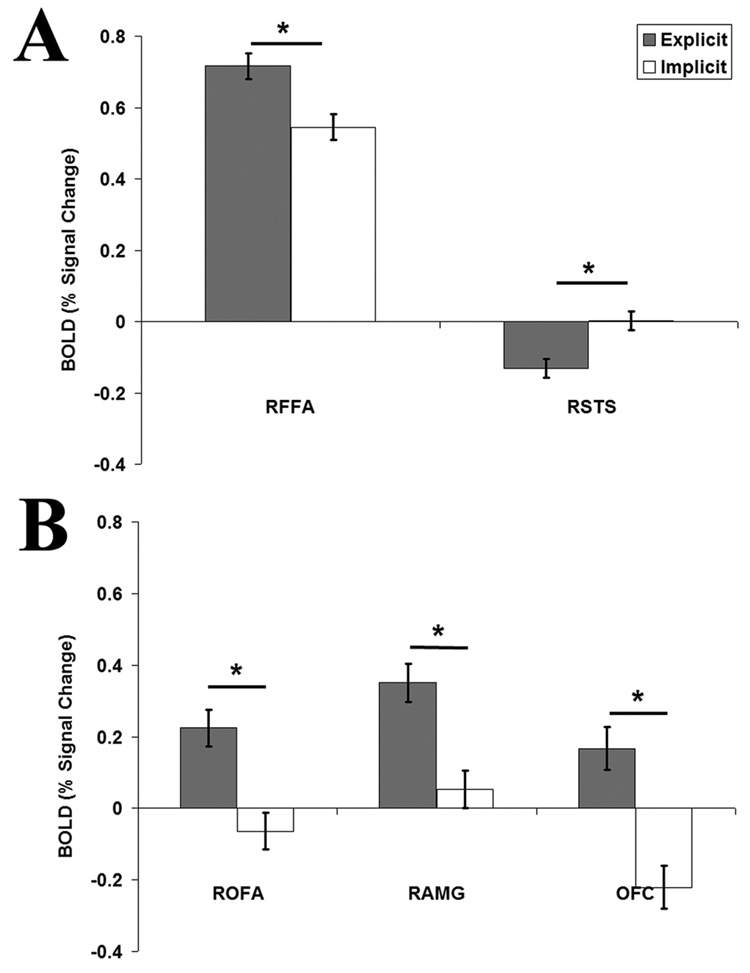
We found a statistically significant effect of Task in the right FFA (t=4.55, p<0.00001), revealing more activity during the Explicit rating of attractiveness than during the Implicit task (Figure 8A). The opposite pattern was observed in the right STS (t=-4.96, p<0.000001; Figure 8A) with more activity during the Implicit than the Explicit task. No significant effect of task was detected in either the left FFA (t=2.63, p>0.001) or the left STS (t=−0.89, p=0.37). There were not observable ratings effects within either the FFA or STS (p>0.15), suggesting an equal involvement of these regions in the processing of faces no matter the degree of their facial attractiveness.
Figure 8.
Results of the analysis of task effects throughout the localized ROIs. (A) Significant, but opposite, task effects are observed in the right fusiform face area (RFFA) and right superior temporal sulcus (RSTS). (B) Significant task effects, similar to those seen in the right FFA, are also observed in the right occipital face area (ROFA), right amygdala (RAMG) and the orbitotofrontal cortex (OFC).
Although not the primary aim of our study, we also looked at the effects of task and rating in the OFA, the OFC, and the amygdala. In the OFA, we found a significant effect of Task in both the left (t=3.45, p<0.001) and right (t=5.43, p<0.000001) hemisphere (Figure 8B). Similarly, a significant task effect task was found bilaterally in the left (t=6.26, p<0.000001) and right (t=5.51, p<0.000001) amygdala, and within the OFC (t=6.21, p<0.000001; Figure 8B). In all these regions there was more activity during the Explicit rating of attractiveness than during the Implicit task, a pattern also observed in the left and right FFA. Again we did not detect ratings effects in any of these regions (p>0.15).
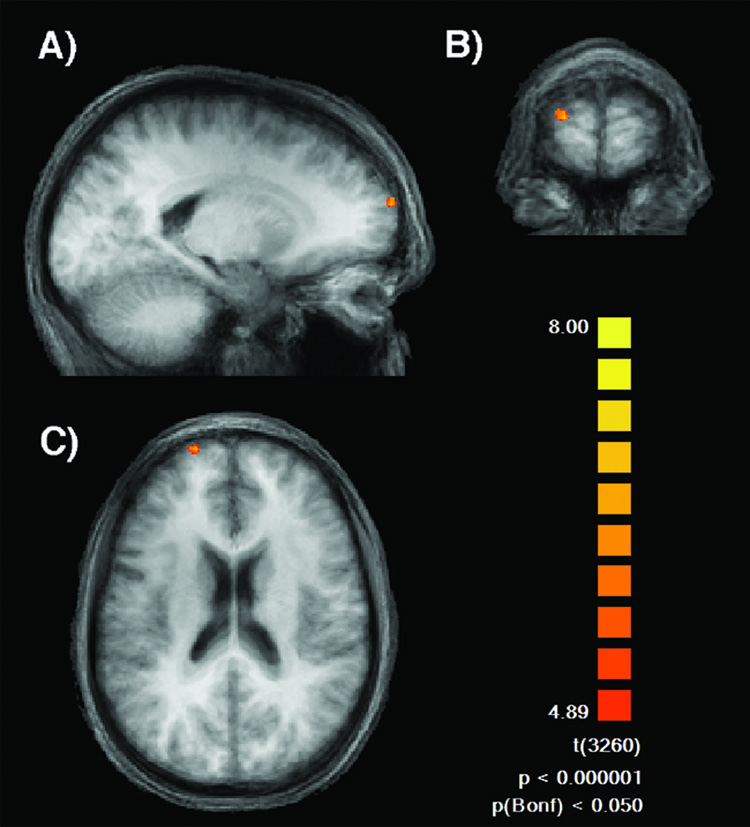
Finally, to examine potential ratings effects elsewhere in the brain, we performed a whole brain analysis contrasting attractive with unattractive faces. The results of this direct contrast revealed selective neural activity in the superior medial region of the frontal cortex (Brodmann Area 10) in the left hemisphere (X=−18, Y=66, Z=24; Peak t−value=7.78; Cluster-size=88 voxels) (Fig. 9).
Figure 9.
Results of the direct contrast between attractive and unattractive faces. Significantly more activity for attractive faces was detected in the medial region of the left frontal cortex ( X= −18, Y=66, Z= 24; Peak t-value=7.78) as shown in (A) sagittal, (B) coronal and (C) horizontal view.
DISCUSSION
Current concepts of face perception are ambiguous as to whether the processing of facial attractiveness involves the superior temporal sulcus, in the lateral occipitotemporal cortex (based on its status as a social signal) or the fusiform face area, in the medial occipitotemporal cortex (based on its likely derivation from temporally invariant facial structure), or both. The aim of our two studies was to provide converging evidence from both lesion and fMRI data on whether the medial occipitotemporal regions involved in processing identity also contribute to processing facial attractiveness. If the superior temporal sulcus is contributing alone in the processing of facial attractiveness, then we would expect sparing of attractiveness judgments in many prosopagnosic subjects (particularly those with functional sparing of expression perception and anatomic sparing of the superior temporal sulcus), a lack of correlation of attractiveness perception with identity recognition in this population, and lack of involvement of the fusiform face area in the fMRI signal detected while processing attractiveness. On the other hand, if the medial occipitotemporal cortex contributes to encoding facial attributes of attractiveness, then judgments of attractiveness should be impaired in nearly all prosopagnosic subjects, attractiveness perception should be correlated with the severity of identity processing deficits, and fMRI signals should reveal effects of the explicit processing of facial attractiveness in the fusiform face area. Our findings converge upon a conclusion that supports the latter case.
In the behavioural study we asked prosopagnosic subjects and healthy controls to look at beautiful and average faces, and (i) provide an explicit judgment of facial attractiveness and (ii) perform an attractiveness-motivated task. Our results showed that the difference in ratings given to beautiful versus average faces was reduced in the prosopagnosic group compared to the healthy control group. Similarly, during the attractiveness-motivated behaviour task healthy participants showed a greater desire to look at beautiful faces than average faces, while the prosopagnosic group did not show this preference. The analysis of individual subjects showed that a weak residual ability to perceive attractiveness was found only in subjects with unilateral right occipitotemporal or bilateral anterior temporal brain regions. The two patients with unilateral right occipitotemporal damage also were the only subjects to show some residual attractiveness-motivated behaviour, whereas the residual attractiveness perception in the two subjects with bilateral anterior temporal damage failed to translate into significant attractiveness-motivated behaviour. The weak residual ability of processing attractiveness in our patients with unilateral lesions is consistent with a recent study by Fairhall and Ishai (Fairhall and Ishai, 2007) revealing effective connectivity between the FFA and the orbitofrontal cortex. According to these findings, our patients may exhibit some residual ability of processing attractiveness due to the integrity of their orbitofrontal cortex.
Despite evidence of such a weak residual ability, our findings clearly show that prosopagnosic subjects, whose core deficit is an impairment in processing facial identity, are also uniformly impaired in perceiving facial attractiveness. As with any lesion study, however, our subjects vary in their functional deficits and anatomic damage. That is, some subjects appear to have difficulty processing expression as well as identity (e.g. subjects 005, 010, 011), but others do not (e.g. subjects 001, 008, 009, 015). Some have lesions that extend to the superior temporal sulcus (e.g. subject 011), but others do not (subjects 005, 008, 015, 010, 009). Yet, others have posterior lesions that may compromise the occipital face area as well as the fusiform gyrus (e.g. subjects 009, 010), but others do not (subjects 005, 008, 015). This variability naturally limits the ability to ascribe their deficit to a single anatomic structure, or to exclude a potential role for expression-related processes, and the superior temporal sulcus, in the perception of attractiveness (which was not the aim of our neuropsychological study). However, the facts that (1) these patients who are uniformly impaired in processing identity by definition, while varying in their ability to process facial expression, are also uniformly impaired in processing attractiveness, and that (2) the severity of their identification deficit is correlated with the severity of their deficit in processing attractiveness, supports a conclusion that the processes involved in extracting identity from facial structure also contribute to processing facial attributes that are relevant for judgments of facial attractiveness.
While current data suggest that facial attractiveness cannot be reduced to a single principle (Armstrong, 2004), the three most important factors may be averageness (faces high in average trait values for the population), symmetry, and sexual dimorphism (faces high in traits that emerge as a result of sexual maturity) (Rhodes, 2006). These properties are most likely to emerge from analysis of dynamically invariant properties of facial structure, which current models propose to be processed by ventral occipitotemporal structures such as the fusiform face area (Haxby et al., 2000), areas that are also implicated in processing identity. Our prior data show that those of our subjects with medial occipitotemporal damage (including the fusiform gyri) show impairments in perceiving structural properties of faces (Barton et al., 2002a, Barton, 2007), properties that may also be relevant to aesthetic judgments (Rhodes, 2006).
Our previous studies also showed that the identification of faces is more severely affected when medial occipitotemporal lesions are bilateral (Barton, 2007); similarly, our data show some residual attractiveness perception in the two subjects with unilateral right occipitotemporal lesions but no attractiveness perception in the two with bilateral lesions (subjects 010 and 011). However, the lesion of subject 011 also appears to involve the vicinity of the superior temporal sulcus (Figure 2), and if this structure also contributes to aesthetic processing (Winston et al., 2007), this may be an additional factor accounting for the severity of his aesthetic deficit. The lesion of subject 010 does not involve the superior temporal sulcus, but may compromise the occipital face area (Figure 1, Figure 2). In this regard, a contrast between his data and that of subject 009 is of interest: although both have lesions extending to the vicinity of the occipital face area, subject 009, with a unilateral lesion, shows some residual perception of attractiveness, while subject 010, with bilateral lesions, does not.
Some residual perception of attractiveness was also possible in the two subjects with bilateral anterior damage not extending to the fusiform gyri. These subjects have better preserved - though not entirely normal - perception of facial structure (Barton et al., 2002a, Barton et al., 2003, Fox et al., 2007), suggesting that they have less problems with structural encoding than with associating perception to facial memories. Their better perception of facial attractiveness may thus also reflect some preservation of structural encoding. It is also possible that their reduced appreciation of facial beauty reflects damage to anterior structures that may be involved in evaluative judgments, such as the amygdala (Winston et al., 2007), rather than in structural encoding. Lesions to such structures may also account for their failure to show motivation of behaviour by attractiveness despite some residual ability to perceive it.
The processing of facial attractiveness in prosopagnosic subjects has recently been investigated in subjects with congenital prosopagnosia (Sadr et al., 2004, Le Grand et al., 2006), with results that have led to mixed conclusions. One group suggested that the processing of facial attractiveness could be dissociated from the processing of facial identity (Sadr et al., 2004), while the other group concluded that impaired face perception negatively affects the development of the “tools” needed for aesthetic judgments during an early critical period (Geldart et al., 1999, Cooper and Maurer, 2002, Le Grand et al., 2006). While providing data about potential behavioural correlations between identity and aesthetic facial processing, studies of congenital prosopagnosia cannot provide information about the relation between structure and function, since these subjects do not have visible lesions on neuroimaging (the same argument applies to patients in which brain lesions cannot be identified, as in the case of subject 001 in our study). On the other hand, subjects with acquired prosopagnosia can have a variety of lesions of various sizes and affecting various structures (Barton, 2003), as demonstrated in our cohort, limiting the ability to make firm anatomic deductions from a single subject. By studying a series of subjects, our study showed that both anterior temporal and medial occipitotemporal structures (including the fusiform gyrus) likely contribute to the processing of facial attractiveness, and that regions in the left hemisphere may have a minor contribution as well.
To provide converging evidence, we performed an fMRI study in healthy participants. Within the right fusiform face area, a region implicated in processing facial identity (Haxby et al., 2000), we found a task effect, with increased BOLD activity when subjects were explicitly rating facial attractiveness than when they were performing the implicit task (Fig. 8A). This suggests that task-directed processing during judgments of facial attractiveness increases utilization of resources within the fusiform face area. Thus, while we do not see differential modulation of activity by the perceived attractiveness of the face, we do see evidence that the fusiform face area is contributing to the processing of facial attractiveness. This finding supports the link between identity and attractiveness processing we observed in the neuropsychological impairments of our prosopagnosic cohort.
While we suggest that the fusiform face area contributes to the processing of facial attractiveness, we do not propose that this region is the site in which such processing occurs. Although current neuroimaging-based models promote the fusiform face area as a key structure in processing facial identity, there is also neuropsychological evidence that identity perception involves additional regions (Barton, 2003, Steeves et al., 2006). Indeed, recent fMRI studies report patients, with congenital or acquired prosopagnosia, in whom an FFA can still be localized (Marotta et al., 2001, Rossion et al., 2003, Avidan et al., 2005). Preserved FFA activity associated with face recognition impairments suggests that damage elsewhere in the face processing network is sufficient to disrupt this perceptual process. Our neuropsychological data suggest that this is also true of the perception of facial aesthetics. Critically, subjects with anterior temporal lesions sparing the right fusiform gyrus (e.g. subject 015) were also impaired in perceiving facial attractiveness. While perceiving the spatial structure of faces is severely impaired in prosopagnosia by lesions in the vicinity of the fusiform face area (Barton et al., 2002b), anterior temporal lesions may also cause subtle impairments in the perception of face shape (Barton, 2003, Fox et al., 2007). Hence, the encoding of temporally-invariant facial structure may involve a number of regions in medial occipital and temporal cortex, in both right and left hemispheres, and damage to any of these regions may contribute to deficits in perceiving either identity or attractiveness.
In addition to the task effects observed in the right fusiform face area, we also observed a task effect in the right superior temporal sulcus; in this case, however, the results revealed increased neural activity when attractiveness was processed implicitly rather than explicitly. The superior temporal sulcus has been reported to show task effects (opposite to what we observed here) in the processing of facial attractiveness, with increased activity when judging attractiveness rather than the age of a face (Winston et al., 2007). That report, however, describes a group analysis overlaid on the whole brain, resulting in four distinct clusters within the right superior temporal sulcus which each showed this task effect. Winston and colleagues (2007) speculated that task effects within the superior temporal sulcus may be related to a role in social evaluation, possibly through “representation of those aspects that are important for attractiveness judgments”. In our study, we used a functional localizer to ensure that, within the superior temporal sulcus, our analysis only included voxels selective for face processing. Thus we confirm that this specific region, hypothesized to be involved in the representation of dynamic attributes of faces (Haxby et al., 2000), also contributes to the processing of facial attractiveness but in an implicit manner. Therefore, it is reasonable to hypothesize that within the superior temporal gyrus, several regions may differentially contribute to the processing of facial attractiveness.
Previous neuroimaging studies investigating facial attractiveness have also documented neural activity in a variety of other cortical regions. An early study with positron emission tomography revealed activity in the left medial frontal cortex and left frontotemporal junction while participants were shown beautiful faces as compared to unattractive ones (Nakamura et al., 1998). In accordance with these findings, in our study, the contrast between attractive and unattractive faces resulted in selective neural activity located on the medial region of the left frontal cortex (Fig. 9). These findings are consistent with the involvement of left frontal regions in affective behaviours, wherein these regions are directly involved in the processing of pleasant and unpleasant emotions (Lane et al., 1997) In addition, other fMRI studies have shown that the passive viewing of beautiful faces compared to average ones activated reward-related regions such as the orbitofrontal cortex and the nucleus accumbens in the ventral striatum (Aharon et al., 2001, O'Doherty et al., 2003), as well as the right amygdala (Winston et al., 2007). Moreover, recent studies showed that face perception and attractiveness are modulated by sexual preferences, no matter the sexual orientation of the individuals, resulting in increased neural activity within the thalamus and the medial region of the orbitofrontal cortex (Kranz and Ishai, 2006, Ishai, 2007). These findings confirm a distributed neural network involved in face processing (Haxby et al., 2000, Ishai et al., 2005) and extend such a network to the selective brain regions underlying reward mechanisms associated with attractive facial features (Kranz and Ishai, 2006, Ishai, 2007). Our evidence also supports a distributed network for processing facial attractiveness by revealing task effects (i.e.-increased activity during the explicit processing of attractiveness) bilaterally within the FFA, OFA, amygdala and in the orbitofrontal cortex. However, the main goal of our study was to use a region of interest approach to ask a more specific question regarding the contribution of two face-selective regions (the fusiform face area and the superior temporal sulcus) in the percept of attractiveness. Herein, we report the first evidence that explicit processing of facial attractiveness also modulates activity within the fusiform face area.
To conclude, in the present study we have provided evidence that (1) subjects with prosopagnosia, an impairment in processing facial identity most often due to right or bilateral medial occipitotemporal lesions, are impaired in processing facial attractiveness, (2) impairments in processing attractiveness correlate with the degree of impairment in processing identity in these subjects, and (3) activity within the fusiform face area is greater during the explicit rating of facial attractiveness than when performing an implicit task, irrespective of the perceived degree of facial attractiveness. These converging neuropsychological and neuroimaging findings suggest that the inferior temporal cortex, including the fusiform face area, does in fact contribute to the perception of facial attractiveness by processing information that may be a relevant output for higher cognitive processing of attractiveness.
Figure 3.
Results of the group analysis contrasting activity when viewing faces and objects. The localized (A) right fusiform face area (FFA), (B) right superior temporal sulcus (STS), (C) right occipital face area (OFA), (D) right amygdala (AMG) and (E) orbitotofrontal cortex (OFC), are overlaid on horizontal sections of the averaged anatomical brain. Note that the single region designated as OFC includes bilateral regions of the orbitofrontal cortex. For coordinates and peak t-value see Table 2.
Acknowledgments
This work was supported by operating grants from the NIMH (RO1-MH069898) and CIHR (MOP-77615). GI is supported by the Alzheimer Society of Canada and Michael Smith Foundation for Health Research, CJF by a Canadian Institutes of Health Research Canada Graduate Scholar Doctoral Award and a Michael Smith Foundation for Health Research Senior Graduate Studentship, CTW by American Academy of Neurology SIGN and Thomas Dohm student scholarships, and JJSB by a Canada Research Chair and Michael Smith Foundation for Health Research Senior Scholar Award. We thank Rebecca Hefter for assistance with data collection. Data were first presented at the Vision Sciences Society meeting in Sarasota, May 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Armstrong J. The secret power of beauty: why happiness is in the eye of the beholder. Allen Lane, London. 2004 [Google Scholar]
- Avidan G, Hasson U, Malach R, Behrmann M. Detailed exploration of face-related processing in congenital prosopagnosia: 2. Functional neuroimaging findings. J Cogn Neurosci. 2005;17:1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Cild Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Barton J, Cherkasova M, O'Connor M. Covert recognition in acquired and developmental prosopagnosia. Neurology. 2001;57:1161–1167. doi: 10.1212/wnl.57.7.1161. [DOI] [PubMed] [Google Scholar]
- Barton J, Press D, Keenan J, O'Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002a;58:71–78. doi: 10.1212/wnl.58.1.71. [DOI] [PubMed] [Google Scholar]
- Barton J, Zhao J, Keenan J. Perception of global facial geometry in the inversion effect and prosopagnosia. Neuropsychologia. 2003;41:1703–1711. doi: 10.1016/s0028-3932(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Barton JJ. Disorders of face perception and recognition. Neurol Clin. 2003;21:521–548. doi: 10.1016/s0733-8619(02)00106-8. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Cherkasova MV. Impaired spatial coding within objects but not between objects in prosopagnosia. Neurology. 2005;65:270–274. doi: 10.1212/01.wnl.0000168901.98616.1c. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Cherkasova MV, Hefter R. The covert priming effect of faces in prosopagnosia. Neurology. 2004a;63:2062–2068. doi: 10.1212/01.wnl.0000145772.77040.24. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Cherkasova MV, Press DZ, Intriligator JM, O'Connor M. Perceptual functions in prosopagnosia. Perception. 2004b;33:939–956. doi: 10.1068/p5243. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Press DZ, Keenan JP, O'Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002b;58:71–78. doi: 10.1212/wnl.58.1.71. [DOI] [PubMed] [Google Scholar]
- Barton JJS. Structure and function in acquired prosospagnosia: lessons from a series of ten patients with brain damage. J Neuropsychology. 2007 doi: 10.1348/174866407x214172. in press. [DOI] [PubMed] [Google Scholar]
- Benton AL, Van Allen MW. Prosopagnosia and facial discrimination. J Neurol Sci. 1972;15:167–172. doi: 10.1016/0022-510x(72)90004-4. [DOI] [PubMed] [Google Scholar]
- Bouvier SE, Engel SA. Behavioral deficits and cortical damage loci in cerebral achromatopsia. Cereb Cortex. 2006;16:183–191. doi: 10.1093/cercor/bhi096. [DOI] [PubMed] [Google Scholar]
- Bowers D, Bauer RM, Coslett HB, Heilman KM. Processing of faces by patients with unilateral hemisphere lesions. I. Dissociation between judgments of facial affect and facial identity. Brain Cogn. 1985;4:258–272. doi: 10.1016/0278-2626(85)90020-x. [DOI] [PubMed] [Google Scholar]
- Cooper PA, Maurer D. Influence of vsual familiarity on developmental changes in judgements of attractiveness; Annual meeting of the International Society for the Study of Behavioural Development, August; Ottawa, Canada. 2002. Aug, [Google Scholar]
- Damasio A, Damasio H, van Hoessen G. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology. 1982;32:331–341. doi: 10.1212/wnl.32.4.331. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Frissen I, Barton J, Hadjikhani N. A modulatory role for facial expressions in prosopagnosia. Proc Natl Acad Sci U S A. 2003;100:13105–13110. doi: 10.1073/pnas.1735530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Pourtois G, Vroomen J, Bachoud-Levi AC. Covert processing of faces in prosopagnosia is restricted to facial expressions: evidence from cross-modal bias. Brain Cogn. 2000;44:425–444. doi: 10.1006/brcg.1999.1203. [DOI] [PubMed] [Google Scholar]
- Dion K, Berscheid E, Walster E. What is beautiful is good. J Pers Soc Psychol. 1972;24:285–290. doi: 10.1037/h0033731. [DOI] [PubMed] [Google Scholar]
- Dipboye RL, Formkin HL, Wiback K. Relative importance of applicant sex, attractiveness, and scholastic standing in evaluation of job applicant resumes. Journal of Applied Psychology. 1975;60:39–43. [Google Scholar]
- Etcoff NL, Freeman R, Cave KR. Can we lose memories for faces? Content specificity and awareness in a prosopagnosic. Journal of Cognitive Neuroscience. 1991;3:25–41. doi: 10.1162/jocn.1991.3.1.25. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Levinson KL, Klein KL. Face perception and within-category discrimination in prosopagnosia. Neuropsychologia. 1995a;33:661–674. doi: 10.1016/0028-3932(95)00002-k. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain HM, Tanaka JR. The inverted face inversion effect in prosopagnosia: evidence for mandatory, face-specific perceptual mechanisms. Vision Res. 1995b;35:2089–2093. doi: 10.1016/0042-6989(94)00273-o. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Malcolm GL, Barton JJS. Preserved face perception is correlated with normal fusiform face area activity in associative prosopagnosia. Neurology. 2007;68 suppl 1:A387. [Google Scholar]
- Frieze IH, Ison JE, Russell J. Attractiveness and income for men and women in management. Journal of Applied Social Psychology. 1991;21:1039–1057. [Google Scholar]
- Geldart S, Maurer D, Henderson H. Effects of the height of the internal features of faces on adults' aesthetic ratings and 5-month-olds' looking times. Perception. 1999;28:839–850. doi: 10.1068/p2943. [DOI] [PubMed] [Google Scholar]
- George N, Dolan RJ, Fink GR, Baylis GC, Russell C, Driver J. Contrast polarity and face recognition in the human fusiform gyrus. Nat Neurosci. 1999;2:574–580. doi: 10.1038/9230. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, de Gelder B. Neural basis of prosopagnosia: an fMRI study. Hum Brain Mapp. 2002;16:176–182. doi: 10.1002/hbm.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AM, Aguirre GK. Prosopagnosia. Curr Biol. 2007;17:R7–R8. doi: 10.1016/j.cub.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Ishai A. Sex, beauty and the orbitofrontal cortex. Int J Psychophysiol. 2007;63:181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Ishaiv A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci U S A. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Hamilton SE, Bernstein JH. The perception of curvature can be selectively disrupted in prosopagnosia. Brain Cogn. 1995;27:36–58. doi: 10.1006/brcg.1995.1003. [DOI] [PubMed] [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Curr Biol. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Le Grand R, Cooper PA, Mondloch CJ, Lewis TL, Sagiv N, de Gelder B, Maurer D. What aspects of face processing are impaired in developmental prosopagnosia? Brain Cogn. 2006;61:139–158. doi: 10.1016/j.bandc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Marotta JJ, Genovese CR, Behrmann M. A functional MRI study of face recognition in patients with prosopagnosia. Neuroreport. 2001;12:1581–1587. doi: 10.1097/00001756-200106130-00014. [DOI] [PubMed] [Google Scholar]
- Meadows JC. The anatomical basis of prosopagnosia. J Neurol Neurosurg Psychiatry. 1974;37:489–501. doi: 10.1136/jnnp.37.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Nagumo S, Ito K, Sugiura M, Kato T, Nakamura A, Hatano K, Kubota K, Fukuda H, Kojima S. Neuroanatomical correlates of the assessment of facial attractiveness. Neuroreport. 1998;9:753–757. doi: 10.1097/00001756-199803090-00035. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Pashos A, Niemitz C. Results of an explorative empirical study on human mating in Germany: handsome men, not high-status men, succeed in courtship. Anthropol Anz. 2003;61:331–341. [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annu Rev Psychol. 2006;57:199–226. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- Sadr J, Duchaine B, Nakayama K. The Percetion of Facial Attractiveness in Prosopagnosia. Vision Sciences Society. 2004;4:249–250. [Google Scholar]
- Sergent J, Ohta S, MacDonald B. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain. 1992;115(Pt 1):15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Steeves JK, Culham JC, Duchaine BC, Pratesi CC, Valyear KF, Schindler I, Humphrey GK, Milner AD, Goodale MA. The fusiform face area is not sufficient for face recognition: evidence from a patient with dense prosopagnosia and no occipital face area. Neuropsychologia. 2006;44:594–609. doi: 10.1016/j.neuropsychologia.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Gangestad SW, Comer R. Human female orgasm and mate fluctuating asymmetry. Animal Behaviour. 1995;50:1601–1615. [Google Scholar]
- Warrington E. Warrington recognition memory test. Western Psychological Services: Los Angeles. 1984 [Google Scholar]
- Winston JS, O'Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]