Abstract
Ferroportin (FPN), the only iron exporter identified to date, participates in iron release from enterocytes and macrophages, regulating its absorption and recycling. We used a murine model of experimental hemolytic anemia to study adaptive changes in the localization of FPN in duodenum, liver, and spleen. FPN was assessed by IHC in healthy and anemic mice using rabbit anti-mouse FPN polyclonal antibodies. Goat-labeled polymer-horseradish peroxidase anti-rabbit Envision+System (DAB) was used as secondary antibody. Tissue iron was studied by Prussian blue iron staining. Anemia evolution and erythropoietic recovery was assessed using conventional hematological tests. Healthy mice showed mainly supranuclear expression of FPN in enterocytes and a weak basolateral expression, whereas in anemic mice, the expression was detected mainly at the basolateral membrane (days 4 and 5). Red pulp macrophages of healthy mice showed FPN-hemosiderin colocalization. In the liver of healthy mice, FPN was mainly cytoplasmic, whereas in anemic mice, it was redistributed to the cell membrane. Our findings clearly show that anemia induces adaptive changes in FPN expression, contributing to anemia restoration by increasing available iron. FPN expression in the membrane is the main pathway of iron release. Our data indicate that iron homeostasis in vivo is maintained through the coordinated expression of this iron exporter in both intestinal and phagocytic cells. (J Histochem Cytochem 57:9–16, 2009)
Keywords: ferroportin, anemia, iron, enterocytes, macrophages
Knowledge of iron metabolism has been greatly advanced by the identification and characterization of transmembrane iron transport proteins involved in the acquisition, transportation, and recycling of iron (Knutson and Wessling-Resnick (2003); Anderson and Frazer 2005). The first mammalian iron transporter to be identified was divalent metal transporter 1 (DMT1; also called divalent cation transporter 1, Nramp2, and Slc11a2), responsible for the uptake of dietary iron (Mackenzie and Garrick 2005). Another key protein involved in iron homeostasis is ferroportin (FPN; also known as Ireg1, or metal transporter protein 1, MTP1), which transports iron across the basolateral membrane of enterocytes into the bloodstream (McKie and Barlow, 2004).
Ferroportin is a 62-kDa iron export protein with 9 or 10 predicted transmembrane regions reported independently by three groups (Abboud and Haile 2000; Donovan et al. 2000; McKie et al. 2000). This multispanning membrane channel is found not only in duodenal enterocytes, but also in all cell types exporting iron into plasma: macrophages of the reticuloendothelial system, placental trophoblasts, and cells of the central nervous system (Donovan et al. 2000; Burdo et al. 2001; McKie and Barlow, 2004).
Like ferritin, FPN mRNA contains a functional iron responsive element (IRE) in its 5′-untranslated region (UTR), indicating that translation increases when iron is abundant (Lymboussaki et al. 2003). However, some studies have reported tissue-specific differences in gene regulation, and further study is therefore needed to better define the role of IRE–iron regulatory protein interactions in the control of FPN levels (Wessling-Resnick 2006).
There is also evidence implicating the involvement of another FPN regulator: a circulating peptide, hepcidin, seems to regulate iron export from both enterocytes and macrophages into the bloodstream, presumably through modulation of FPN protein levels (Atanasiu et al. 2006; Ganz and Nemeth 2006a). Hepcidin is produced under iron-loading and inflammatory conditions to suppress iron absorption, its synthesis decreasing in response to iron deficiency or enhanced erythropoiesis to promote iron uptake (Nicolas et al. 2002). Direct functional evidence of FPN regulation by hepcidin was provided by collaborative work from the laboratories of Ganz and Kaplan (Nemeth et al. 2004). These researchers showed that hepcidin regulates FPN protein levels by inducing its internalization and lysosomal degradation, supporting the hypothesis that FPN may be the receptor for the major iron regulator hepcidin (Nemeth et al. 2004).
On the basis of the above, it may be postulated that FPN is one of the iron metabolism proteins responding to regulatory signals from iron stores and/or erythroid regulators (Yeh et al. 2003). Consequently, these two systemic factors and other local signals determine the rate at which iron is absorbed by influencing the expression of key proteins in duodenal enterocytes and in other cell types involved in iron metabolism (Latunde-Dada et al. 2004).
Little is known to date about the in vivo regulation of FPN in response to changes in body iron stores. Phenylhydrazine (PHZ)-induced anemia is an experimental situation in which iron stores are mobilized, and erythroid demand is increased (Roque et al. in press). During anemia, bone marrow needs are met by the release of iron from stores and eventually by increasing intestinal iron absorption. Iron homeostasis can therefore be expected to be associated with changes in the expression of key proteins like FPN to restore the anemic state.
Although several studies have shown FPN expression in healthy mouse tissues, no definitive data have been published on its expression in anemia in vivo nor on tissue behavior from the time of onset to restoration of anemia. Identifying the subcellular localization of FPN in anemia is therefore crucial to defining the processes involved in its regulation. With a view to gaining new insight into the regulation of iron homeostasis, we used a murine model of experimental hemolytic anemia to study adaptive changes of FPN expression in duodenum, liver, and spleen.
Materials and Methods
Animals
Adult female mice (CF1) were bred at the animal facility of the Universidad Nacional del Sur. The animals were kept in cages at controlled room temperature and humidity for 10 days before the start of the study and were fed throughout on a standard diet with access to water ad libitum, under standard conditions: a 12-hr light-dark period. The initial body weight of each mouse in the group was similar (30 ± 3 g), and daily measurements showed that the weight did not change during the study. The procedures followed are in line with the Guide for the Care and Use of Laboratory Animals. Before the initiation of this study, the protocol was approved by the Advisory Committee on Animal Use of the Universidad Nacional del Sur.
Experimental Design
Adult mice were divided into two groups (16 mice per group): (a) healthy mice receiving saline solution intraperitoneally (0.9% NaCl) on days 0 and 2 and (b) anemic mice receiving PHZ (Sigma Chemical; St. Louis, MO) intraperitoneally on days 0 and 2, dissolved in 0.5 ml saline solution. The PHZ concentration injected was 60 mg/kg of body weight. Mice were subjected to blood controls on day 0, before PHZ or saline solution administration. Animals were anesthetized with diethyl ether for blood collection to determine hematological parameters on days 3, 4, and 5. Erythropoietic recovery was assessed using conventional hematological tests. Samples were collected throughout the study from the retro-orbital venous plexus (25 μl each).
Tissue Preparation
The animals were anesthetized for euthanasia by diethyl ether inhalation on day 0, 3, 4, or 5 (n=4, for each time point). The spleen, liver, and duodenum from both healthy and anemic mice were removed under sterile conditions by abdominal incision and fixed in 10% buffered formalin. Tissues were embedded in paraffin, and sections of 4 μm were obtained.
Antibodies
Primary antibodies against mouse ferroportin were raised against a peptide corresponding to the C terminus of ferroportin (GPDEKEVTDENQPNTS), kindly provided by Bruno Galy, from the European Molecular Biology Laboratory (EMBL), Heidelberg, Germany. The characterization of the FPN antibody was developed by McKie et al. (2000) using protein extracts from Hela cells transfected with a mouse ferroportin cDNA and reported by Galy et al. (2005). Phycoerythrin (PE)-coupled F4/80 monoclonal antibodies that recognize a murine macrophage-restricted cell surface glycoprotein were used to test cell morphology of tissue macrophages (kindly provided by Rachel Golub of the Pasteur Institute, Paris, France).
IHC
The sections were processed for IHC according to the peroxidase-antiperoxidase method (PAP). Before PAP treatment, sections were deparaffinized and dipped in an aqueous 3% solution of hydrogen peroxide for 15 min to inhibit any endogenous peroxidase activity. After washing in PBS (pH 7.0), sections were incubated for 1 hr at room temperature with rabbit polyclonal antiserum against mouse FPN (diluted 1:500 with PBS, pH 7.0). After washing, sections were incubated for 30 min with peroxidase-labeled polymer-horseradish peroxidase (HRP) conjugated to goat anti-rabbit immunoglobulins (Envision + System-HRP-DAB; Dako, Carpinteria, CA). Staining was completed by incubation with 3,3′diaminobenzidine chromogen solution (chromogen solution is part of Envision kit). The sections were counterstained with Harris's hematoxylin, dehydrated, and mounted. Negative controls included incubation with PBS without the primary antibody. Frozen sections of spleen, liver, and duodenum were obtained for further controls and processed for IHC as described above. Immunostaining was analyzed using a BX51 microscope (Olympus; Tokyo, Japan), equipped with ×10, ×20, and ×40 dry objectives and a ×100 oil immersion objective. Digital images were acquired with an Olympus C7070 camera.
Prussian Blue Iron Staining
Deparaffinized tissue sections were incubated in 2% HCl containing 10% potassium ferrocyanide for 15 min, washed, and counterstained with nuclear red before visualization. Some of the spleen and liver sections were treated with anti-FPN antibody using the PAP technique followed by Prussian blue iron staining to determine the presence of hemosiderin deposits in FPN-positive cells. These sections were counterstained with nuclear red.
Statistical Analysis
In all mouse experiments, at least four mice were tested individually. Data were analyzed by one-way ANOVA followed by the Tukey multiple comparison test. The level of statistical significance was set at p<0.05. All values are expressed as mean ± SD.
Results
FPN Expression in Healthy Mice
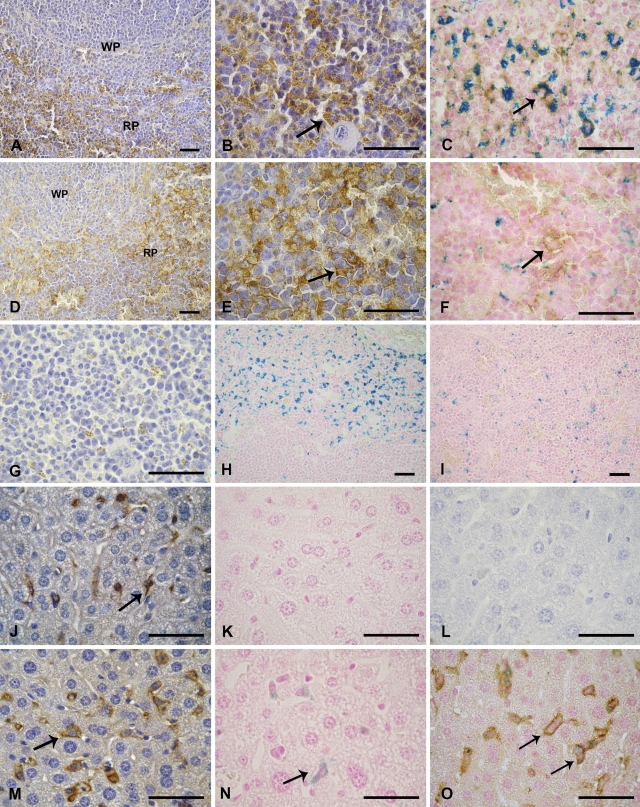
FPN expression was observed in healthy mouse duodenum, liver, and spleen; the highest level of expression in our mouse strain (CF1) occurred in the latter. Spleen FPN expression was particularly marked in red pulp macrophages surrounding nodules of white pulp. Immunoreactivity was observed both cytoplasmically and on the plasma membrane. FPN expression was not detected in white pulp (Figures 1A and 1B). Cell morphology of splenic macrophages was tested by direct immunofluorescence using PE-coupled anti-F4/80 antibodies (data not shown).
Figure 1.
Immunolocalization of ferroportin (FPN) in spleen and liver of healthy and anemic mice. (A) Splenic tissue of healthy mice showing FPN on red pulp (RP) and white pulp (WP). (B) Red pulp of healthy mice showing FPN with intracellular localization (arrow). (C) Red pulp of healthy mice showing colocalization of FPN and hemosiderin (arrow). (D) Splenic tissue of anemic mice showing FPN on red pulp (RP) and white pulp (WP). (E) Red pulp of anemic mice showing FPN (arrow). (F) Red pulp of anemic mice showing FPN on cell membrane (arrow) and hemosiderin. (G) Negative control of spleen. (H) Splenic tissue of healthy mice showing iron deposits on red pulp. (I) Splenic tissue of anemic mice showing little iron deposits. (J) Hepatic tissue of healthy mice showing cytoplasmic FPN on Kupffer cells (arrow). (K) Hepatic tissue of healthy mice with Prussian blue iron staining. (L) Negative control of liver. (M) Hepatic tissue of anemic mice showing FPN on cell membrane of Kupffer cells (arrow). (N) Hepatic tissue of anemic mice with iron deposits on Kupffer cells (arrow). (O) Hepatic tissue of anemic mice showing FPN on cell membrane of Kupffer cells and with cytoplasmic iron (arrows). Spleen and liver sections prepared as described in Materials and Methods were stained with polyclonal rabbit anti-FPN antibody. Bar = 20 μm.
FPN expression in the liver was assessed to determine its distribution in this tissue. For this purpose, FPN immunostaining was limited to a few discrete cells—identified as Kupffer cells on the basis of their cell morphology—where expression was found to be mainly cytoplasmic with modest expression at the plasma membrane (Figure 1J). The pattern of FPN expression in macrophages along the tissue was diffuse and not associated with vascular sites. Hepatocytes, hepatic stellate cells (HSCs), and sinusoidal endothelial cells (SECs) showed no immunostaining. Cell morphology of Kupffer cells was tested by direct immunofluorescence using PE-coupled anti-F4/80 antibodies (data not shown).
In summary, in both reticuloendothelial tissues, the subcellular localization of FPN was mainly cytoplasmic. These data indicate that macrophages of the reticuloendothelial system (RES) are the predominant FPN-positive cells in these tissues.
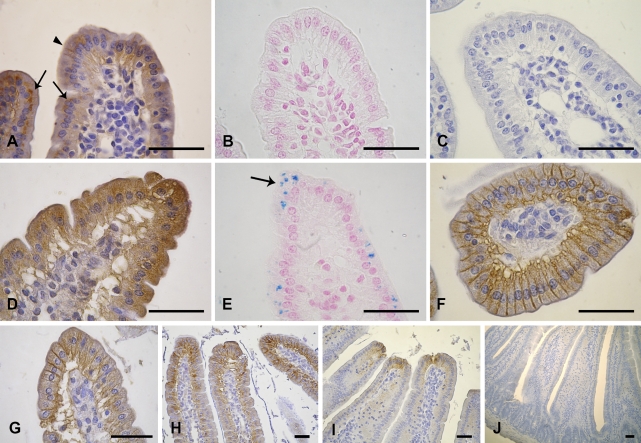
Duodenal FPN was detected mainly inside enterocytes, localized immediately above the nucleus on the apical side (Figure 2A). However, slight FPN expression was also observed along the basolateral membrane throughout villous enterocytes (Figure 2A).
Figure 2.
Immunolocalization of FPN in duodenum of healthy and anemic mice. (A) Duodenum of healthy mice showing supranuclear (arrows) and basolateral FPN (arrowhead). (B) Duodenum of healthy mice with Prussian blue iron staining. (C) Negative control of duodenum. (D) Duodenum of anemic mice (day 3) showing FPN with intracellular localization. (E) Duodenum of anemic mice (day 3) with iron pigments (arrow). (F) Transversal section of duodenum of anemic mice (day 4) showing FPN on basolateral membrane. (G) Duodenum of anemic mice (day 4) showing FPN on basolateral membrane. (H) Duodenum of anemic mice (day 4) showing FPN on basolateral membrane along the villi. (I) Duodenum of anemic mice (day 5) showing FPN on basolateral membrane at the top of the villi. (J) Crypts of duodenum of anemic mice (day 5) without FPN expression. Intestinal sections prepared as described in Materials and Methods were stained with polyclonal rabbit anti-FPN antibody. Bar = 20 μm.
FPN immunoreactions in frozen sections of spleen, liver, and duodenum showed the same pattern of distribution observed with paraffin sections, allowing us to discard artifacts caused by formalin fixation (data not shown).
FPN Expression in Anemic Mice
In anemic mice, by IHC, we observed both cytoplasmic and cell membrane FPN expression on red pulp macrophages (Figures 1D and 1E). To confirm cell membrane localization of FPN, IHC followed by Prussian blue was performed (Figure 1F).
In the liver of anemic mice, we observed not only an increase in the number of positive FPN cells but also a redistribution of FPN, leading to a relative accumulation on the cell surface of Kupffer cells (Figure 1M). On day 3, FPN expression was cytoplasmic, similar to that observed in healthy mice, but with incipient expression on the Kupffer cell membrane (data not shown). However, FPN expression was more marked on the day of acute hemolysis (day 4) and was clearly identified on cell borders (Figures 1M and 1O). On day 5, immunostaining showed no significant variations with respect to day 4 (data not shown).
IHC studies of anemic mouse duodenum also showed changes in FPN localization with respect to that observed in healthy mice. A dramatic increase in FPN expression was noted on day 3, predominantly along the basolateral surface of enterocytes and cytoplasmically, confirming its role as iron exporter (Figure 2D).
This finding was corroborated on the day of acute hemolysis (day 4), when FPN expression was observed mainly along the basolateral membranes of enterocytes (Figures 2F–2H). Similar immunostaining was detected on day 5. Furthermore, on day 5, iron exporter expression was also found at the basal pole of villus enterocytes in mature absorptive cells of duodenum (Figure 2I). No immunoreaction was seen in the crypts (Figure 2J).
FPN immunoreactions in frozen sections of spleen, liver, and duodenum showed the same pattern of distribution observed with paraffin sections, allowing us to discard artifacts caused by formalin fixation (data not shown).
Iron Deposits in Healthy and Anemic Mice
To examine whether the changes in the expression and immunolocalization of FPN are related to iron deposits, Prussian blue iron staining was performed on spleen, liver, and duodenum from healthy and anemic mice.
An abundance of the iron pigment hemosiderin was found in the macrophage cytoplasm of the splenic red pulp of healthy mice, indicating that red pulp is likely the main tissue participating in iron storage in our mouse strain (Figure 1H). No iron staining was observed in splenic white pulp. PHZ treatment was found to cause a dramatic decrease in splenic hemosiderin (Figure 1I).
To study the colocalization of FPN and its relationship with iron deposits, we applied a technique permitting the detection of double-positive cells using the iron transporter and hemosiderin. We found numerous FPN-positive cells in healthy mice that also contained hemosiderin, suggesting a colocalization of FPN with hemosiderin (Figure 1C).
There was no evidence of iron deposits in hepatic macrophages, showing that iron is not stored in the liver of healthy mice (Figure 1K). In anemic mice, however, we observed a slight increase in iron deposition in Kupffer cells on day 4 (Figure 1N). By means of the double-staining method, we were able to confirm not only the intracellular localization of iron deposition in Kupffer cells but also the immunolocalization of FPN in the plasma membrane of these cells (Figure 1O).
Prussian blue iron staining was performed to study the presence of iron in the duodenum of healthy mice and mainly along anemia evolution. Enterocytes from healthy mice showed no iron pigments (Figure 2B). Surprisingly, we noted iron in absorbing enterocytes at the onset of hemolysis, which was day 3 (Figure 2E). Intraepithelial macrophages were observed only in connective tissue (data not shown). We observed enterocytes that were positive for Perl's Prussian blue, especially along the duodenal villi, but not in crypts. There was no visible iron staining on days 4 and 5, suggesting that the process of iron absorption occurs immediately after the onset of anemia (data not shown).
Hematological Data in Healthy and Anemic Mice
Hematological data in healthy [day 0: hematocrit (HCT), 48.0 ± 0.5%; hemoglobin (Hb), 15.4 ± 0.75 g/dl] and anemic mice (days 3 and 4: HCT, 33.5 ± 1.5%; Hb, 10.0 ± 0.8 g/dl) were measured by standard methods. On day 5, a reverse trend toward control values was observed (HCT, 38.0 ± 1.8%; Hb, 11.3 ± 0.6 g/dl).
Discussion
This study reports subcellular FPN localization in healthy and anemic mice. To our knowledge, this is the first study to show adaptive changes in FPN expression during the development of hemolytic anemia from onset to restoration.
Duodenal FPN expression along the basolateral surface of enterocytes is the expected pattern for a molecule involved in iron metabolism in healthy mice, because this localization is appropriate for the uptake of dietary iron to satisfy the erythropoietic needs of the whole organism (Canonne-Hergaux et al. 2006; Laftah et al. 2006). Our results additionally show the supranuclear presence of FPN in healthy mice, suggesting a different role for FPN as reported in a previous study also showing its intracellular expression in healthy mice (Oates and Thomas 2005). These authors explain the supranuclear position of FPN in terms of a possible role inside the cell in moving iron into an organelle or some vesicle during iron absorption. From here, the iron would be transported toward the membrane for its incorporation into the bloodstream when there is a lack of iron. The basolateral and supranuclear FPN localization observed by us could indicate a double role for FPN: vesicular transport and iron export. In seeking to explain the supranuclear position of FPN, a novel contribution of our study, which has not been reported to date, is elucidation of the subcellular distribution of FPN during the onset and restoration of hemolytic anemia. In this context, the most relevant results are the marked intracellular localization seen 1 day after the last injection of PHZ (day 3) and the redistribution to basolateral membrane seen on days 4 and 5, this being the main adaptive response allowing increased dietary iron absorption to restore the anemic state.
Interestingly, iron distribution along the duodenum concurred with changes observed in FPN localization during anemia. Surprisingly, 1 day after the last PHZ injection (day 3), we observed iron pigments inside the enterocytes when FPN expression was mainly cytoplasmic, and the number of such iron pigments decreased on days 4 and 5. This iron could not have the appearance of iron destined for absorption because few enterocytes are involved. However, because no iron pigments were observed in enterocytes on days 4 and 5, we assume that intracellular iron seen on day 3 was absorbed by intestinal cells. A logical explanation for this behavior is that FPN accumulates into the basolateral membrane of enterocytes, thus permitting the entry of iron into the bloodstream from absorbing enterocytes and decreasing the amount remaining inside the cell.
As described in several studies, differential FPN modulation in healthy and anemic mice may be associated with local and systemic regulators (Frazer et al. 2003; Ganz and Nemeth 2006b; Wessling-Resnick 2006). In the former case, the regulation depends mainly on intracellular iron levels in mature enterocytes, and in the latter, regulation depends on systemic signals regulated by body iron requirements. In hemolytic anemia, iron demand is notably enhanced, altering iron absorption through increased FPN expression. Although the precise nature of these signals is still not clear, hepcidin is postulated to be the molecular signal that communicates iron demand to the duodenum (Ganz and Nemeth 2006a; Darshan and Anderson 2007). Furthermore, it is well known that experimentally induced anemia induces a decrease in hepcidin levels, allowing the expected iron absorption across the small intestine through FPN membrane expression (Nicolas et al. 2002). In anemia, the internalization of FPN could be prevented, leading to its accumulation on the cell surface because of the reduced hepcidin levels.
It is known that expansion of the absorptive surface is a precise and adaptive mechanism for increasing iron absorption in hemolytic anemia across the duodenal brush border (Latunde-Dada et al. 2004). Furthermore, enterocytes have a limited lifespan, so after several days, they senesce and are always restored, most of the mature enterocytes being concentrated in the pole of the villi (Donovan et al. 2005). In agreement with this, we found duodenal expression of FPN limited to the top of mature absorptive epithelial cells of the villus and on the basolateral surface. This finding corresponds to the day when begin the restoration of anemic state (day 5).
As previously shown, high levels of FPN were also detected in splenic and hepatic macrophages of healthy mice, consistent with the role of this cell in iron recycling from senescent erythrocytes (Delaby et al. 2005; Knutson et al. 2005). Splenic and hepatic FPN expression in healthy mice showed a pronounced intracellular localization as clearly observed in enterocytes. The intracellular pool of FPN possibly acts as a reservoir from which surface protein can be withdrawn as needed. This intracellular localization suggests vesicular trafficking of the protein between the cytosol and the cell surface (Delaby et al. 2005). Knutson and Wessling-Resnick (2003) have suggested that intracellular distribution of FPN in macrophages mediates the intracellular redistribution of iron released by heme catabolism.
It is well known that hepatocytes play a central role in iron metabolism (Zhang et al. 2004). However, in healthy mouse liver, FPN seems to be expressed mainly in Kupffer cells, the resident macrophages of the liver that also play a role in iron homeostasis (Graham et al. 2007). The findings of previous studies coincide with this IHC localization in liver for FPN (Abboud and Haile 2000; Canonne-Hergaux et al. 2006). However, other authors have reported FPN expression in hepatocytes and in HSCs, suggesting a specific role of these cell types in iron metabolism (Zhang et al. 2004). We cannot exclude the possibility that hepatocytes express FPN, although probably in our experimental conditions (i.e., sensitive staining procedure, antibody), FPN expression was not visible in these cells.
Interestingly, in anemic mice, FPN changed its cytoplasmic subcellular localization in Kupffer cells to cell surface localization. On day 4 (acute hemolysis), the expression was seen clearly on the cell borders. This can be explained by the fact that increases in erythropoietic activity occur concomitantly with increases in Hb catabolism, this in turn generating increased amounts of iron that is later released from Kupffer cells (Latunde-Dada et al. 2006). Furthermore, small amounts of FPN may be continually recycling between the plasma membrane and intracellular compartments; as in the duodenum, in anemia, this FPN accumulates on the cell membrane for iron export.
Changes in the subcellular localization of FPN are thus in line with the specific role of this iron recycler and exporter when erythroid demand increases. Furthermore, we detected on day 4 a small increment in iron deposits. Assuming FPN to be involved in iron export from Kupffer cells and taking into account that FPN activity is enhanced on the cell membrane of anemic mice, the presence of stored iron and FPN on the cell membrane could be a normal homeostatic response when the influx of iron into Kupffer cells exceeds their capacity to export it (Delaby et al. 2005; Latunde-Dada et al. 2006).
Although our findings make apparent the colocalization of FPN and hemosiderin, this was difficult to achieve with classical IHC methods. We overcame this difficulty by using the double-staining technique, enabling us to clearly establish the cytoplasmic colocalization of the two proteins. Our findings are corroborated by the work of Abboud and Haile (2000).
In anemic mice, FPN expression observed on the cell borders of splenic macrophages suggests a redistribution of FPN to the cell membrane. This finding, and the observed decrease in iron deposits, could indicate that FPN was the iron release pathway used to restore hemolytic anemia.
In agreement with the findings of Canonne-Hergaux et al. (2006), we detected an expanded erythroid compartment in anemia that hampers definition of changes in FPN expression using IHC techniques. A further impediment is loss of the classical tissue organization of splenic compartments induced by PHZ treatment. Further study is therefore needed to clarify this subject in splenic tissue.
We conclude that the subcellular localization of FPN in healthy mice differs from that in anemic mice. In the former case, it may mediate iron export through the use of an intracellular compartment, acting as an iron concentrator or as a reservoir, and in the latter case, FPN is stored within the cell until it is needed for iron export, at which time it is recruited to the membrane.
Our data suggest that iron homeostasis is maintained through the coordinated expression of FPN, the sole iron exporter identified to date, in both intestinal and phagocytic cells.
In summary, this in vivo study focused on adaptive changes undergone by FPN during hemolytic anemia, thereby deepening our understanding of the complex mechanisms involved in maintaining iron homeostasis.
Acknowledgments
This research was supported by the Secretaría General de Ciencia y Tecnología de la Universidad Nacional del Sur (Grant 24/B116) and the Agencia de Promoción Científica y Tecnológica (Grant-908). M.C.D. and T.V. are Research Fellows of the Consejo Nacional de Investigaciones Científicas y Técnicas.
We thank Drs. Matthias Hentze and Bruno Galy from the European Molecular Biology Laboratory for providing FPN antibody reagents and Dr. Rachel Golub from Pasteur Institute, Paris, France, for providing F4/80 antibody. The authors gratefully acknowledge Christian Gatti for technical assistance and helpful advice in IHC studies.
References
- Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275:19906–19912 [DOI] [PubMed] [Google Scholar]
- Anderson GJ, Frazer DM (2005) Recent advances in intestinal iron transport. Curr Gastroenterol Rep 7:365–372 [DOI] [PubMed] [Google Scholar]
- Atanasiu V, Manolescu B, Stoian I (2006) Hepcidin-central regulator of iron metabolism. Eur J Haematol 78:1–10 [DOI] [PubMed] [Google Scholar]
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, et al. (2001) Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res 66:1198–1207 [DOI] [PubMed] [Google Scholar]
- Canonne-Hergaux F, Donovan A, Delaby C, Wang H, Gros P (2006) Comparative studies of duodenal and macrophage ferroportin proteins. Am J Physiol Gastrointest Liver Physiol 290:156–163 [DOI] [PubMed] [Google Scholar]
- Darshan D, Anderson GJ (2007) Liver-gut in the regulation of iron homeostasis. World J Gastroenterol 13:4737–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F (2005) Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood 106:3979–3984 [DOI] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, et al. (2000) Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403:776–781 [DOI] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC (2005) The iron exporter ferroportin/SLc40a1 is essential for iron homeostasis. Cell Metab 1:191–200 [DOI] [PubMed] [Google Scholar]
- Frazer DM, Wilkins SJ, Becker EM, Murphy TL, Vulpe CD, McKie AT, Anderson GJ (2003) A rapid decrease in the expression of DMT1 and Dcytb but not Ireg1 or hephaestin explains the mucosal block phenomenon of iron absorption. Gut 52:340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy B, Ferring D, Minana B, Bell O, Janser HG, Muckenthaler M, Schümann K, et al. (2005) Altered body iron distribution and microcytosis in mice deficient in iron regulatory protein 2 (IRP2). Blood 106:2580–2589 [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E (2006a) Iron imports IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol 290:199–203 [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E (2006b) Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta 1763:690–699 [DOI] [PubMed] [Google Scholar]
- Graham RM, Chua AC, Herbison CE, Olynuk JK, Trinder D (2007) Liver iron transport. World J Gastroenterol 13:4725–4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M (2005) Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA 102:1324–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson MD, Wessling-Resnick M (2003) Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Molec 38:61–88 [DOI] [PubMed] [Google Scholar]
- Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, Tselepis C (2006) Tumour necrosis factor α causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J 397:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latunde-Dada GO, McKie AT, Simpson RJ (2006) Animals models with enhanced erythropoiesis and iron absorption. Biochim Biophys Acta 1762:414–423 [DOI] [PubMed] [Google Scholar]
- Latunde-Dada GO, Vulpe CD, Anderson GJ, Simpson RJ, McKie AT (2004) Tissue specific changes in iron metabolism genes in mice following phenylhydrazine-induced haemolysis. Biochim Biophys Acta 1690:169–176 [DOI] [PubMed] [Google Scholar]
- Lymboussaki A, Pignatti E, Montosi G, Garuti C, Haile DJ, Pietrangelo A (2003) The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J Hepatol 39:710–715 [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Garrick MD (2005) Iron imports. II. Iron uptake at the apical membrane in the intestine. Am J Physiol Gastrointest Liver Physiol 289:981–986 [DOI] [PubMed] [Google Scholar]
- McKie AT, Barlow DJ (2004) The SLC40 basolateral iron transporter family (IREG1/FERROPORTIN/MTP1). Eur J Phys 447:801–806 [DOI] [PubMed] [Google Scholar]
- McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, et al. (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5:299–309 [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, McVey Ward D, Ganz T, et al. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093 [DOI] [PubMed] [Google Scholar]
- Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, et al. (2002) The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia and inflammation. J Clin Invest 110:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates PS, Thomas C (2005) Ferroportin is expressed on the mucous granule membrane of a subpopulation of goblet cells in the duodenum of the rat. Histol Histopathol 20:681–687 [DOI] [PubMed] [Google Scholar]
- Roque M, DAnna C, Gatti C, Veuthey T (In Press) Hematological and morphological analysis of the erythropoietic regenerative response in phenylhydrazine-induced hemolytic anemia in mice. Scand J Lab Anim Sci
- Wessling-Resnick M (2006) Iron imports. III. Transfer of iron from the mucosa into circulation. Am J Physiol Gastrointest Liver Physiol 290:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh K, Yeh M, Glass J (2003) Hepcidin regulation of ferroportin 1 expression in the liver and intestine of the rat. Am J Physiol Gastrointest Liver Physiol 286:385–394 [DOI] [PubMed] [Google Scholar]
- Zhang A, Xiong S, Tsukamoto H, Enns CA (2004) Localization of iron metabolism-related mRNAs in rat liver indicate that HFE is expressed predominantly in hepatocytes. Blood 103:1509–1514 [DOI] [PubMed] [Google Scholar]