Abstract
Secreted protein acidic and rich in cysteine (SPARC)/osteonectin is expressed in different tissues during remodeling and repair, suggesting a function in regeneration. Several gene expression studies indicated that SPARC was expressed in response to muscle damage. Studies on myoblasts further indicated a function of SPARC in skeletal muscle. We therefore found it of interest to study SPARC expression in human skeletal muscle during development and in biopsies from Duchenne and Becker muscular dystrophy and congenital muscular dystrophy, congenital myopathy, inclusion body myositis, and polymyositis patients to analyze SPARC expression in a selected range of inherited and idiopathic muscle wasting diseases. SPARC-positive cells were observed both in fetal and neonatal muscle, and in addition, fetal myofibers were observed to express SPARC at the age of 15–16 weeks. SPARC protein was detected in the majority of analyzed muscle biopsies (23 of 24), mainly in mononuclear cells of which few were pax7 positive. Myotubes and regenerating myofibers also expressed SPARC. The expression-degree seemed to reflect the severity of the lesion. In accordance with these in vivo findings, primary human-derived satellite cells were found to express SPARC both during proliferation and differentiation in vitro. In conclusion, this study shows SPARC expression both during muscle development and in regenerating muscle. The expression is detected both in satellite cells/myoblasts and in myotubes and muscle fibers, indicating a role for SPARC in the skeletal muscle compartment. (J Histochem Cytochem 57:29–39, 2009)
Keywords: skeletal muscle, secreted protein acidic and rich in cysteine/osteonectin/BM-40, congenital myopathy, muscular dystrophy, myogenesis, satellite cell
Adult muscle has a remarkable capacity to restore itself. Damage to the skeletal muscle triggers a cascade of degeneration and regeneration events (Yan et al. 2003; Charge and Rudnicki 2004) of which the satellite cell, residing between the sarcolemma and the basement membrane of muscle fibers (Mauro 1961) is a major participant. Activation and proliferation of this muscle specific stem cell gives rise to myoblasts, which eventually fuse with damaged fibers or each other, thereby forming multinucleated myotybes that mature into fibers (Schultz et al. 1985; Seale and Rudnicki 2000; Kitzmann and Fernandez 2001; Horsley and Pavlath 2004; Shi and Garry 2006). These processes are controlled by signals provided by growth factors (Mourkioti and Rosenthal 2005; Musaro 2005), cytokines (Pelosi et al. 2007), and the extracellular matrix (ECM), which is both essential and instrumental in regeneration of skeletal muscle (Maley et al. 1995; Casar et al. 2004a). During muscle regeneration many ECM proteins are upregulated (e.g., proteoglycans, biglycan, laminin-α4, and integrin-α6) and through these the ECM directly influences adhesion, proliferation, differentiation, and migration of myoblasts and other cells involved in the processes (Caceres et al. 2000; Sorokin et al. 2000; Lewis et al. 2001; Henriquez et al. 2002; Porter et al. 2002; Casar et al. 2004b).
Secreted protein acidic and rich in cysteine (SPARC), also known as osteonectin and BM-40, is a multifunctional matricellular protein of 43 kDa associated with the ECM and expressed abundantly in basal lamina. SPARC specifically binds several ECM molecules including collagens I to V and is involved in modulation of cell–matrix interactions where SPARC can participate in the organization of both connective tissue and the basal lamina. SPARC also regulates the production and deposition of several ECM proteins but does not contribute significantly to the structural integrity of the ECM (Lane and Sage 1994; Bradshaw and Sage 2001; Brekken and Sage 2001).
Expression of SPARC has been associated with a number of biological functions including cancer biology, fibrosis, and wound healing/injury. Tumor growth has been reported to be increased in SPARC-null mice, and it was suggested this was caused by changes both in deposition and organization of the ECM (Brekken et al. 2003; Puolakkainen et al. 2004). However, SPARC has also been associated with neoplastic progression of human melanoma (Ledda et al. 1997a) and SPARC has been shown to induce migration of tumor cells and increase tumor angiogenesis in vivo (Kunigal et al. 2006), whereas antisense inhibition of SPARC inhibits invasiveness and thus tumorigenicity of human melanoma cells (Ledda et al. 1997b). However, SPARC has been shown to induce deadhesion, and SPARC-null mice show both enhanced wound closure and increased cellular invasion of subcutaneous sponges, suggesting that SPARC is an inhibitor of migration (Bradshaw et al. 2001,2002), which is in contrast to the suggested function in malign tumors.
SPARC-null mice also show decreased size of collagen fibrils (Bradshaw et al. 2003b) and decreased pulmonary fibrosis (Strandjord et al. 1999), whereas SPARC expression is correlated with fibrotic disorders (Kuhn and Mason 1995; Pichler et al. 1996).
SPARC is expressed in several organs during embryonic development and has been observed in areas of bone and muscle formation (Holland et al. 1987; Sage et al. 1989a; Mothe and Brown 2001). Eradication of the SPARC homolog from Xenopus laevis embryos (Purcell et al. 1993) and overexpression of the SPARC homolog in Caenorhabditis elegans (Schwarzbauer and Spencer 1993) results in defects during development and affects the motility, suggesting that a normal regulated expression of SPARC is necessary for a normal development of the musculoskeletal system in invertebrates. SPARC-null mice seem to develop normally without any muscle defects instead these mice display severe cataracts (Gilmour et al. 1998) and compromised bone mass formation and remodeling but with increased tendency to form adipocytes (Delany et al. 2003). This indicates that an alternative, compensatory mechanism could exist in vertebrates or SPARC plays different roles depending on the species.
In vitro studies have shown that SPARC gene expression is upregulated during myoblast differentiation in C2C12 cells and inhibition of SPARC in these cells prevents differentiation (Cho et al. 2000). Moreover, addition of SPARC protein to MM14 myoblasts promotes differentiation of these cells (Motamed et al. 2003).
SPARC as a regulator of cell/ECM interaction during development and in response to tissue injury is observed in different organs, e.g., in the gut (Lussier et al. 2001) and during liver fibrosis (Blazejewski et al. 1997). A possible role for SPARC in muscle regeneration is indicated by several microarray studies on gene expression in Duchenne muscular dystrophy (DMD) and α-sarcoglycan deficiency (limb girdle muscular dystrophy type 2D) (Chen et al. 2000; Haslett et al. 2002; Noguchi et al. 2003). Furthermore, a study in porcine muscle showed upregulation of SPARC during regeneration after induced injury (Ferre et al. 2007).
Based on the observed effects of SPARC on proliferation, migration, and differentiation and its presence in diseased muscle, the aim of this study was to determine whether SPARC is generally involved in human myogenic processes. We studied the extent and sites of SPARC expression during fetal myogenesis, in normal and diseased skeletal muscle, and during differentiation of primary isolated satellite cells.
Materials and Methods
Muscle Biopsies
Muscle biopsies were obtained from patients diagnosed with Duchenne muscular dystrophy (n=4), Becker muscular dystrophy (BMD; n=3), congenital myopathy (n=9), congenital muscular dystrophy (n=4), and inflammatory myopathy (inclusion body myositis and polymyositis, n=4). Normal human quadriceps muscle biopsies were obtained from men with a suspicion of musculoskeletal disorders but where the biopsy showed no muscle or nerve pathology (age, 20–24 years; n=3). All biopsies were obtained and used after informed consent according to the guidelines of and permission from the Regional Ethics Committee for Southern Denmark 15879.
Fetal and Neonatal Tissue
Fetal and neonatal tissue was obtained during autopsy of aborted fetuses 15 (n=2), 16 (n=1), 20 (n=4), 22 (n=2), and 23 (n=1) weeks of age or of premature infant deaths (neonatal, 3 months; n=2). None of the biopsies showed musculoskeletal or central nervous system (CNS) pathology, and all were from the tissue archive from the Department of Clinical Pathology, Odense University Hospital. All biopsies were obtained and used according to the guidelines of and permission from the Regional Ethics Committee for Southern Denmark 15879.
Isolation and Culture of Human Satellite Cells
Mononuclear muscle cells were isolated separately from each healthy muscle biopsy (n=3). Briefly, muscles were dissected to remove connective and adipose tissue. Remaining muscle was minced and enzymatically treated for 40–60 min (37C) with 0.3% collagenase II (Worthington; Medinova Scientific, Glostrup, Denmark). Myofiber-associated and interstitial cells were released by gentle trituration of the digested muscle tissue, and a single cell suspension was obtained by serially filtering the cell samples through 100- and 40-μm cell strainers. To reduce the number of fibroblasts, preplating was performed for 20 min at 37C, and non-adherent cells were harvested and expanded in culture for further analysis.
Isolated cells were seeded on ECM gel (Sigma-Aldrich; Brøndby, Denmark)–coated dishes and initially cultured in growth medium consisting of a 1:1 mixture of DMEM/25 mM HEPES (Invitrogen; Taastrup, Denmark) and DMEM/glutamax (Invitrogen) supplemented with antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin) and 10% FCS (Invitrogen). Cells were cultured at 37C with 5% CO2 in a humidified chamber, and after the first passage, the serum concentration was reduced to 2%, and 2% Ultroser G (Sigma-Aldrich) was added to keep the cells in a proliferative state. The medium was changed every 3–4 days. Subculturing and preplating (15 min) was performed 12–14 d after initial plating and repeated when cells reached 60–70% confluence.
For differentiation studies, proliferating cells were cultured to ∼90% confluence in growth medium before insulin (25 ρM) was added to the medium to induce myofiber formation. Medium was hereafter changed every other day.
Immunocytochemistry and IHC
All muscle biopsies and samples were fixated overnight in 4% normal buffered formaldehyde (NBF), embedded in paraffin, sectioned at 4 μm, and mounted on glass slides. Tissue sections were deparaffinized by 10-min immersion in xylene, followed by 10-min rehydration in 99% ethanol. The tissue sections were incubated with 0.5% H2O2 in methanol to block endogenous peroxidase activity. Antigen retrieval was performed by heating slides at 100C in TEG buffer (10 mM Tris/0.5 mM EGTA, pH 9.0) for 15 min. The tissue sections were incubated after antigen retrieval with mouse anti-human SPARC 1:100 (NCL-O-NECTIN; clone 15G12, NovoCastra, Newcastle Upon Tyne, UK) for 60 min and detected using the EnVision+ system (Dako; Glostrup, Denmark). Nuclei were counterstained with Mayer's hematoxylin. For double staining of tissue sections, mouse anti-chicken Pax7 (Developmental Studies Hybridoma Bank; Iowa City, IA) was added 1:200 overnight at 4C after the antigen retrieval step followed by detection using the EnVision+ system, and mouse-anti-human SPARC antibody was added and detected using the EnVision+ system.
Human myoblasts were cultured on ECM gel–coated coverslips and induced to differentiate. At given time points, coverslips (n=12) were harvested, gently washed (3×) in TBS (pH 7.4), and mounted on glass slides. To detect SPARC, cells were fixated in 4% normal-buffered formaldehyde for 15 min, followed by 10 min in 96% ethanol and a heat-induced antigen retrieval step. Here, glass coverslips with short-term fixated cells were heated in TEG buffer at 95C for 15 min. Cells were incubated with mouse anti-human SPARC as described for tissue sections. For staining of cells with neural cell adhesion molecule (NCAM) and desmin, cells were fixated for 10 min in 100% acetone, followed by addition of mouse-anti-NCAM (Leu19; BD Biosciences, Brondby, Denmark) 1:50 or mouse-anti-desmin (Dako) 1:25 for 60 min. All primary antibodies were detected with the EnVision+ system.
For all antibody stainings both on tissue sections and cells, negative controls with omission of the primary antibody were performed.
Western Blotting
Proliferating human muscle-derived cells (p5) were gently detached and washed twice in ice-cold PBS (pH 7.4). Total protein extracts were prepared by briefly sonicating cells on ice in RIPA lysis buffer (Sigma-Aldrich) containing a mixture of protease inhibitors (Complete mini; Roche, Hvidovre, Denmark). The lysate was centrifuged (15 min, 12,000 × g, 4C), and the supernatant was stored at −80C until use.
For Western blot analysis, protein samples were separated by SDS-PAGE (NuPAGE 4-12% gels; Invitrogen) and transferred onto PVDF membranes. Membranes were blocked in PBS/0.05% Tween 20/0.5%BSA for 15 min at room temperature and incubated overnight at 4C with mouse anti-human SPARC antibody (NCL-O-NECTIN; clone 15G12, NovoCastra) diluted 1:50 in PBS/0.05% Tween 20 (washing buffer). After wash (3×), membranes were incubated for 1 hr at room temperature with rabbit anti-mouse Ig (Dako) diluted 1:1000 in washing buffer, washed three times, and visualized using standard protocol for 3-amino-9-ethylcarbazole color development.
RT-PCR
Proliferating human myoblasts were cultured to ∼90% confluence and induced to differentiate as described above. At given time points (after 4, 5, 7, 9, 10, and 13 days in culture), total RNA was isolated from each cell sample using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. Using the SuperScript III First-Strand Synthesis System (Invitrogen), each cDNA sample was generated from 400 ng of total RNA using random hexamer primers: SPARC forward, 5′-GAGGTGACTGAGGTATCTGTGGGA-3′; SPARC reverse, 5′-GGTCAGCTCAGAGTCCAGGCAAGG-3′; Gapdh forward, 5′-GTCGTATTGGGCGCCTGGTCAC-3′; Gapdh reverse, 5′-TGATGACAAGCTTCCCGTTCTC-3′; 18s rRNA forward, 5′-CTGCAGTTAAAAAGCTCGTAGTTG 3′; 18s rRNA reverse, 5′-AACCGCGGTCCTATTCCATTATT-3′.
PCR reactions were run in 20 μl volume: 20 ng cDNA sample, 2 μl Taq 10× PCR buffer (Sigma-Aldrich), 0.2 μl Taq DNA polymerase (Sigma-Aldrich), 200 μM dNTP (Sigma-Aldrich), and 10 pmol of each primer. To the SPARC PCR reaction 1.5 M betaine was added as an enhancing agent to facilitate strand separation by equalizing the melting temperature of the individual base pairs in the template DNA (Sigma-Aldrich). Cycling conditions were initial denaturing at 94C for 30 sec, followed by 35 cycles of 94C for 30 sec, 60C for 30 sec, 72C for 30 sec, and final elongation at 72C for 7 min.
PCR products were separated on a 1% agarose gel and visualized by ethidium bromide staining. All PCR products were extracted from the gel using a PCR clean up and gel extraction kit (NucleoSpin; Macherey-Nagel, AH Diagnostics, Aarhus, Denmark) and sequenced for verification using the BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems; Foster City, CA) (data not shown).
SPARC mRNA expression at each time point was quantified using Quantity One Software (BioRad Laboratories; Copenhagen, Denmark) and normalized to the mRNA expression of Gapdh and 18s rRNA. The use of Gapdh mRNA and 18s rRNA was validated by a geNorm analysis of reference gene stability (Vandesompele et al. 2002). The normalized data for SPARC mRNA were calculated as fold expression to day 4 in culture (proliferation conditions; n=2).
Results
SPARC Expression During Normal Skeletal Muscle Development
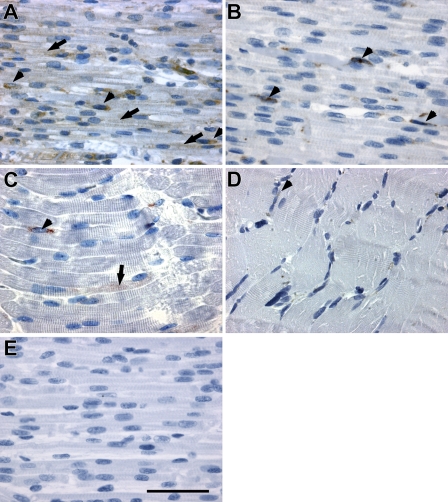
In skeletal muscle from fetuses 15 weeks of age, SPARC was present both in the forming myotubes (Figure 1A, arrows) and in the mononuclear cells located along the myotubes (Figure 1A, arrowheads). However, 5–8 weeks later, at a fetal age of 20–23 weeks, SPARC expression was almost restricted to the mononuclear cells (Figure 1B, arrowheads). This change corresponded to the transition from myotubes with central nuclei to muscle fibers with peripherally situated nuclei. In neonatal muscle, SPARC expression was observed in a few mononuclear cells located adjacent to myofibers (Figure 1C, arrowhead), and a slight expression in sparse myofiber could still be detected (Figure 1C, arrow). In normal adult skeletal muscle, SPARC was expressed by a few mononuclear cells. (Figure 1D, arrowhead). Thus, SPARC seems to be highly expressed initially during fetal muscle development, whereas the expression decreases as the muscles mature.
Figure 1.
Protein expression and localization of secreted protein acidic and rich in cysteine (SPARC) in skeletal muscle during human embryonic development. SPARC protein was expressed in myotubes and in mononuclear cells located adjacent to the myotubes in fetuses 15–16 weeks of age. (A) Tissue from week 15 is shown. The expression in fibers decreased with development and was mainly restricted to mononuclear cells at fetal age of 20–23 weeks. (B) Tissue from week 22 is shown. (C) SPARC expression in mononuclear cells was still present in neonatal muscle, and a few myofibers had scarce expression of SPARC. (D) SPARC expression in normal adult muscle was weakly detectable in mononuclear cells, possibly satellite cells. (E) A negative control image of fetal muscle from week 15 is shown to validate the specificity of the IHC staining for SPARC protein. Arrows indicate SPARC expression in myotubes/muscle fibers, and arrowheads indicate SPARC expression in mononuclear cells. Bar = 50 μm.
The SPARC-positive mononuclear cells were found both adjacent to fibers (Figure 1) and in the connective tissue in the fetal samples (data not shown). Throughout the period studied from fetal week 15 to postnatal month 3, the staining intensity of the individual cells appeared uniform, whereas the number of stained cells decreased, and the expression in the myotubes decreased. In addition, SPARC expression was observed in endothelial cells throughout development (data not shown).
SPARC in Primary Isolated Human Satellite Cells
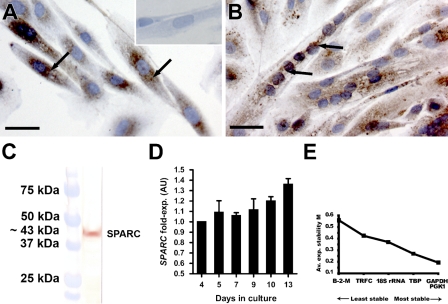
Primary isolated human satellite cells (hSCs) were expanded in vitro and induced to form myotubes by addition of insulin. The myogenic origin of the hSCs after isolation was confirmed by immunocytochemical (ICC) staining for NCAM (CD56) and desmin. NCAM is known as a satellite cell marker both in human and mouse muscle (Illa et al. 1992; Dubois et al. 1994), and desmin is expressed by activated satellite cells (myoblasts) and in myotubes (Creuzet et al. 1998; Stewart et al. 2003) (data not shown). We observed that SPARC was expressed at both protein and mRNA levels during proliferation and differentiation of hSCs (Figure 2). ICC showed SPARC expression in proliferating, mononuclear cells (Figure 2A), as well as in myotubes (Figure 2B). SPARC protein appeared to be located in a granular pattern around the nuclei in mononuclear cells (Figure 2A, arrows), whereas the staining pattern appeared less confined around the nuclei with distribution in the entire cytoplasm of the myotubes (Figure 2B, arrows). Western blot of the isolated proteins from proliferating cells confirmed that the used antibody recognized a band of ∼43 kDa, corresponding to SPARC protein size (Figure 2C).
Figure 2.
SPARC is expressed in primary derived human satellite cells (hSCs). (A) Proliferating hSCs expressed SPARC protein in a granular pattern located around the nuclei (black arrows). (A, inset) Negative control confirming the specificity of IHC staining for SPARC protein. (B) Differentiated hSCs forming myotubes still expressed SPARC protein but with a more generally cytoplasmic located distribution (black arrows). (C) Western blot on total protein extraction from confluent, proliferating hSCs. The band corresponds to a protein of ∼43 kDa equal to SPARC protein size. (D) SPARC gene expression was studied in hSCs in vitro using semiquantitative RT-PCR. Cells were harvested after 4, 5, 7, 9, 10, and 13 days in culture during proliferation, differentiation, and fusion of the cells. SPARC mRNA was quantified and normalized to Gapdh mRNA and 18s rRNA expression and calculated as fold expression at day 4. SPARC mRNA expression initiated already at a low cell confluence (∼50%, day 4) and remained steady during proliferation until 100% confluence (days 4–7). The expression increased during differentiation and fusion into myotubes (days 9–13). The RT-PCR experiment was performed in duplicate. (E) The use of Gapdh mRNA and 18s rRNA as reference genes has been validated using geNorm analysis, where all the tested reference genes are ranked according to their individual stability within the hSCs (Vandesompele et al. 2002). Bar = 20 μm.
SPARC mRNA expression level was analyzed using semiquantitative RT-PCR, and, already at low cell confluence (∼50%, day 4), the hSCs were observed to express SPARC mRNA. During proliferation (days 4–7), the SPARC mRNA level remained constant. At 100% confluence (day 7), the cells were induced to differentiate by addition of insulin to the medium, and during differentiation and formation of myotubes of the hSCs, SPARC mRNA expression increased (days 9–13).
These results show that SPARC is expressed by hSCs, and the expression seems to be regulated during proliferation and fusion, thus suggesting a role for SPARC in these processes.
SPARC in Myopathies
The involvement of SPARC in muscle formation and regeneration was further studied by analyzing SPARC protein expression and localization in selected inherited and idiopathic muscle disorders.
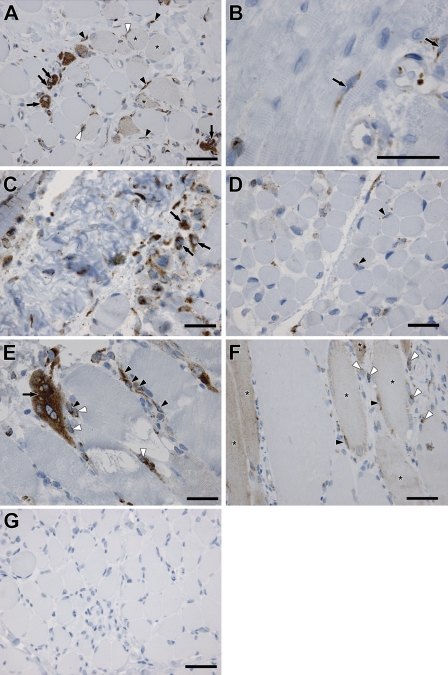
We analyzed biopsies from DMD, which is caused by mutation(s) in the dystrophin gene (Hoffman et al. 1987), and the milder form, BMD (Engel and Ozawa 2004). We detected an intense expression of SPARC protein in the cytoplasm of newly formed, centro-nucleated myotubes in biopsies from patients with DMD (Figure 3A, arrows). SPARC protein was also localized in the cytoplasm of mononuclear cells located in the interstitium/connective tissue of the endomysium surrounding regenerating fibers and myotubes (Figure 3A, black arrowheads) and in mononuclear cells adjacent to regenerating myofibers (Figure 3A, white arrowheads). The localization in cells in close contact with myofibers correlates with SPARC expression in activated satellite cells repairing injured fibers. A less intense staining pattern was detected by fibers of a larger size with peripherally located nuclei (Figure 3A, asterisks) compared with newly formed myotubes with intense SPARC staining, suggesting a decrease in SPARC expression as the myotubes mature during regeneration. Most of the muscle biopsies from the patients with congenital myopathy that were studied did not present SPARC protein staining in regenerating fibers or myotubes but only in mononuclear cells (Figure 3B, black arrows). The congenital myopathies constitute a heterogeneous class of muscle disorders present at birth, which all display characteristic structural and histological abnormalities within the muscle (Laing 2007). These disorders are relatively rare and slowly progressive, thus not exhibiting the severity of disorders such as DMD. Biopsies from 4- and 1-month-old patients with congenital muscular dystrophy (Figures 3C and 3D, respectively) exhibited SPARC protein expression both in myotubes (Figure 3C, black arrows) and in mononuclear cells (Figure 3D, black arrowheads). Congenital muscular dystrophy is also a heterogeneous group of muscle disorders present at birth; however, they do not exhibit the presence of structural features as the congenital myopathies. Instead, these disorders are mostly caused by disruption of genes involved in interactions with the muscle and the extracellular matrix (Schessl et al. 2006).
Figure 3.
Expression of SPARC in human muscle wasting disorders. SPARC-positive mononuclear cells were observed in all myopathic muscle biopsies (inherited and acquired myopathies) analyzed except from one congenital myopathy patient (data not shown). Muscle fibers and myotubes expressing SPARC were found in all myopathies analyzed and were most profound in patients with (A; black arrows) Duchennes muscular dystrophy (DMD) and (E,F; black arrow) inflammatory myopathies [E: polymyositis (PM), F: inclusion body myositis (IBM)]. However, most of the biopsies from the group of patients with (B) congenital myopathy (CM) did not show any SPARC in the fibers. Instead SPARC was expressed in mononuclear cells (black arrows). These samples with SPARC-negative fibers were biopsies from less severe congenital myopathies and constituted one third of the total muscle biopsies analyzed in this study. (C,D) SPARC expression in congenital muscular dystrophy (CMD) in two patients 4 and 1 months of age, respectively. Black arrows, SPARC-positive myotubes; black arrowheads, SPARC-positive mononuclear cells. (G) A negative control image of DMD muscle to validate the specificity of the IHC staining for SPARC protein in the patient biopsies. Black arrowheads (A,E,F) indicate SPARC-positive cells in the endomysium, white arrowheads (A,E,F) indicate SPARC-positive cells adjacent to myofibers, and asterisks (A,F) mark less intense SPARC staining in myofibers with peripherally located nuclei. Bar = 50 μm.
The inflammatory myopathies inclusion body myositis (Figure 3F), which is a sporadic age-related neurodegenerative, inflammatory muscle disease with resemblance to Alzheimers (Askanas and Engel 2007), and the related polymyositis (Figure 3E), both with unknown causes, generally showed more extensive SPARC expression than the congenital myopathies and congenital muscular dystrophies. SPARC expression and localization resembled the observation from DMD and BMD patients. Specifically, in polymyositis, a very intense staining pattern of SPARC was observed in regenerating myofibers with centrally located nuclei (Figure 3E, black arrow) and in mononuclear cells in the connective tissue endomysium (Figure 3E, black arrowheads), some of which were in close connection with the fibers (Figure 3E, white arrowheads). SPARC expression in inclusion body myositis patients was detected in fibers (Figure 3F, asterisks), in mononuclear cells directly adjacent to the fibers (Figure 3F, black arrowheads), and in (polymorph)-mononuclear cells (Figure 3F, white arrowheads) within the endomysium, showing that SPARC is expressed by different cell types within the damaged muscle.
The intensity of SPARC expression in all biopsies analyzed seems to correlate, with the severity of the disease with DMD and the inflammatory myopathies being the most severe forms, thus connecting SPARC with muscle injury and regeneration.
SPARC-positive spindle-shaped cells were seen in the endomysium of all samples as described. Similar cells were also observed in the surrounding connective tissue and in the adipose tissue (data not shown). In addition, many vessels in damaged areas were SPARC positive. These observations indicate that SPARC is involved in the regeneration of muscle in both inherited and idiopathic human muscle diseases and is expressed in a manner that depends on the severity of the lesion.
SPARC Expression in Mononuclear Cells
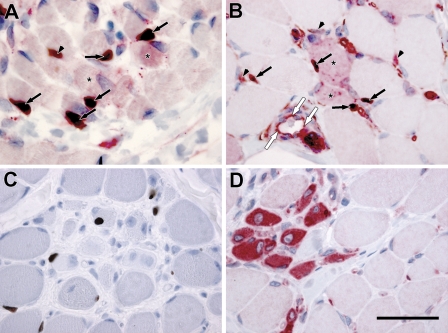
Both the in vitro experiments and the observation of SPARC-positive cells adjacent to and in close contact with muscle fibers in both inherited and idiopathic muscle diseases point to the existence of SPARC-expressing satellite cells. To explore this further, we performed individual and double stainings with SPARC and the satellite cell marker pax7 (Seale et al. 2000) in both fetal (15 weeks; Figure 4A) and dystrophic muscle (DMD; Figures 4B–4D). Double-labeled cells were found in both types of tissue (Figures 4A and 4B, arrows), showing the existence of SPARC-positive myogenic cells in vivo. However, pax7-negative cells expressing SPARC were also present both adjacent to muscle fibers (Figures 4A and 4B, arrowheads) in the interstitium and in the endothelial cells lining the blood vessels (Figure 4B, white arrows), indicating that several different cell populations in the myogenic environment express SPARC during myogenesis and regeneration of injured muscle.
Figure 4.
SPARC and pax7 colocalization in fetal skeletal and dystrophic muscle. (A) Skeletal muscle from a fetus 15 weeks of age exhibits colocalization of SPARC (red staining surrounding nuclei) and pax7 (black nuclear staining) protein in some mononuclear cells (arrows), but not all mononuclear cells expressing SPARC is positive for pax7 (arrowheads). Fibers also express SPARC protein (asterisk). (B) In DMD, some mononuclear cells (black arrows) coexpress SPARC (red staining around nuclei) and pax7 (black nuclear staining). Mononuclear cells only expressing SPARC is also observed adjacent to myofibers (arrowheads) and in endothelial cells lining a blood vessel (white arrows). SPARC-positive fibers are observed in connection with double-stained mononuclear cells (asterisk). Single stainings of pax7 (C) and SPARC (D) of DMD muscle are included as controls for the double stainings because they serve as both negative and positive controls for each of the two antibodies in the double labeling. Bar = 50 μm.
Discussion
In this study, we showed that SPARC is highly expressed in skeletal muscle during fetal development, with the expression decreasing as muscle maturation proceeds. This indicates that SPARC could be a regulatory factor in formation of fetal human muscle as reported for C. elegans (Schwarzbauer et al. 1994) and X. laevis (Purcell et al. 1993; Schwarzbauer and Spencer 1993). The expression and localization of SPARC protein was age dependent, thus being downregulated in fibers during the transition from myotubes to mature muscle fibers. During regeneration of injured fibers in muscle diseases, SPARC was re-expressed in a manner similar to the expression detected during embryonic muscle development, where newly formed tubes stained extensively, whereas normal fibers were negative. Furthermore, SPARC was only detected in a few mononuclear cells in the adult, suggesting a recapitulation of the developmental expression pattern during repair. The expression pattern with repetition of fetal development during regeneration is seen for other proteins, e.g., GLUT3 (Gaster et al. 2002) and NCAM (Figarella-Branger et al. 1990; Lyons et al. 1992; Fazeli et al. 1996). Our observation that the expression reflects the severity of damage and the significant upregulation of gene expression in dystrophies reported by others (Chen et al. 2000; Haslett et al. 2002; Noguchi et al. 2003) supports this repetition of the myogenic processes.
Besides being present in multinuclear myotubes and fibers, we found SPARC in mononuclear cells in the endomysium in both fetal and adult muscle. Chen et al. (2000) also described SPARC expression in the endomysium but did not provide details about localization. Experiments in vitro have shown that the satellite cell–derived mouse myoblast line C2C12 expresses SPARC (Cho et al. 2000). We were also able to show SPARC expression in primary isolated human satellite cells both at the mRNA and protein level. Double staining for SPARC and pax7 in muscle biopsies confirmed the existence of satellite cell–derived SPARC expressing myogenic cells in fetal and regenerating skeletal muscle, but pax7-negative/SPARC-positive mononuclear cells were observed in the endomysium as well. Thus, SPARC has several histological locations in muscle because it is observed in fibers and in satellite cells and along with vessels, adipose tissue, and fibrous tissue. This indicates a very complex and delicate temporal and spatial regulation of the SPARC milieu in the ECM in support of the developmental and regenerative processes in the muscle. The increase in SPARC expression at the start of maturation of C2C12 myoblasts (Cho et al. 2000) and in the primary isolated hSCs observed in this study during both proliferation and differentiation reflects this.
Considering the function of SPARC in muscle, it has been shown in the C2C12 (Cho et al. 2000) and the MM14 (Motamed et al. 2003) myoblast cell lines that SPARC promotes myoblast differentiation in tissue culture. In the MM14 myoblast experiment, a murine muscle cell line almost exclusively expressing fibroblast growth factor (FGF) receptor 1, the colony size was reduced in the presence of SPARC, and the inhibitory effect of SPARC has been shown in many other cell systems as well (Basu et al. 1999; Brekken and Sage 2000; Bradshaw and Sage 2001; Motamed et al. 2003). This inhibitory action on proliferation is probably mediated by effects downstream of FGF receptor 1 (Motamed et al. 2003) and through effects on the insulin-like growth factor 1 receptor pathways (Basu et al. 1999). Both of these receptors and their ligands are known to be important in the propagation of resting myogenic cells to muscle fibers (Soulet et al. 1994; Martelly et al. 2000; Motamed et al. 2003; Foulstone et al. 2004). The combined effect of inducing differentiation and inhibiting proliferation correlates with a function of SPARC in the terminal part of muscle formation and regeneration. This is also in agreement with the observation that SPARC inhibits cell migration (Sage et al. 1989b; Bradshaw et al. 2002,2003b; Puolakkainen et al. 2005). However, SPARC has been suggested as a marker for invasive meningiomas (Rempel et al. 1999) and has been shown to induce migration of glioblastoma cell lines (Kunigal et al. 2006). This effect of SPARC on mobility is also a possible explanation for its effects on myotube formation, because cells have to migrate to align and fuse to form multinucleated tubes (Horsley and Pavlath 2004). However, a study on SPARC-null mice showed increased fibroblast invasion in subcutaneous sponges (Bradshaw et al. 2001), and the modulatory actions of SPARC on migration still remains elusive.
We detected SPARC expression not only in myotubes, regenerating fibers, and satellite cells, but also in other types of mononuclear cells. The polymorphonuclear cells observed to express SPARC in inclusion body myositis could be inflammatory cells in addition to fibroblasts within the connective tissue of the endomysium. SPARC has been suggested to modulate immune cells in a study showing that, in a SPARC-deficient environment, dendritic cell migration and T-cell priming is increased (Sangaletti et al. 2005), and exogenous SPARC enhanced the production of matrix metalloproteases in monocytes (Shankavaram et al. 1997). Thus, the presence of SPARC in the myogenic environment could, in addition to aiding in the repair processes, also modulate the inflammatory response, possibly by modulating the connective tissue turnover by monocytes/macrophages.
As suggested, SPARC could also be expressed by fibrotic cells in the endomysium and, because SPARC has been highly implicated in fibrotic disorders (Strandjord et al. 1999; Savani et al. 2000; Socha et al. 2007), a suggested role for SPARC in these cells could be to participate in the scar reaction occurring during muscle repair.
SPARC directly binds several ECM-associated components including collagens I–V, and this allows SPARC to influence both fibrous tissue and basal lamina organization. Moreover, SPARC also regulates ECM deposition (Lane and Sage 1994; Bradshaw and Sage 2001). The observation in human muscular disorders that the degree of SPARC protein expression seems to reflect severity could indicate that, in the more severe forms, an increased ECM reorganization is occurring. In DMD, the dystrophin deficiency results in decreased sarcolemmal stability and myofiber degeneration followed by satellite cell mobilization and repair (Charge and Rudnicki 2004; Dhawan and Rando 2005). Because the satellite cells have the same genetic defect, this only results in a vicious cycle of regeneration and degeneration until the satellite cell pool is exhausted (Heslop et al. 2000; Jejurikar and Kuzon 2003) during this disease progression, and the muscle tissue is eventually replaced by connective tissue and fat. However, a recent study has also suggested that the satellite cells are directly implicated in the fibrotic deposition reactions in dystrophic muscle by switching into a more pro-fibrotic phenotype. This was especially obvious from aged satellite cells (Alexakis et al. 2007); thus, the prominent expression of SPARC in DMD and to a lesser extent in BMD could be a reflection of the increased fibrotic and adipogenic processes. SPARC could also directly influence the fibrotic reaction by increased ECM deposition. Moreover, increased SPARC expression has been associated with adipose tissue hyperplasia and obesity (Tartare-Deckert et al. 2001; Chavey et al. 2006), and even though SPARC-null mice were found to show increased formation of adipocytes (Delany et al. 2003) and increased size of the adipocytes (Bradshaw et al. 2003a), a potential role for SPARC in the diseased muscle could be to modulate or even enhance the adipogenic processes.
In addition to being upregulated in diseased muscle, SPARC has also been detected in regeneration of porcine muscle after induced injury (Ferre et al. 2007); thus, SPARC seems to be generally involved in muscle formation both during growth and repair.
From our study, SPARC seems to have a spatial and temporal presentation as a regulatory factor in myogenesis and skeletal muscle regeneration. Because SPARC protein is localized in a variety of structures and cells, SPARC could play a multifunctional role within the myogenic environment both during growth and repair. However, the exact roles for SPARC still need further clarification.
Acknowledgments
This work was supported by the Danish Muscular Dystrophy Foundation and Danish Stem Cell Research Center.
We thank Lone Christiansen (Odense University Hospital) for excellent technical assistance.
References
- Alexakis C, Partridge T, Bou-Gharios G (2007) Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol 293:C661–669 [DOI] [PubMed] [Google Scholar]
- Askanas V, Engel WK (2007) Inclusion-body myositis, a multifactorial muscle disease associated with aging: current concepts of pathogenesis. Curr Opin Rheumatol 19:550–559 [DOI] [PubMed] [Google Scholar]
- Basu A, Rodeck U, Prendergast GC, Howe CC (1999) Loss of insulin-like growth factor I receptor-dependent expression of p107 and cyclin A in cells that lack the extracellular matrix protein secreted protein acidic and rich in cysteine. Cell Growth Differ 10:721–728 [PubMed] [Google Scholar]
- Blazejewski S, Le BB, Boussarie L, Blanc JF, Malaval L, Okubo K, Saric J, et al. (1997) Osteonectin (SPARC) expression in human liver and in cultured human liver myofibroblasts. Am J Pathol 151:651–657 [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Graves DC, Motamed K, Sage EH (2003a) SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci USA 100:6045–6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene SE (2003b) SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol 120:949–955 [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Reed MJ, Carbon JG, Pinney E, Brekken RA, Sage EH (2001) Increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges in SPARC-null mice. Wound Repair Regen 9:522–530 [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Reed MJ, Sage EH (2002) SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem 50:1–10 [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Sage EH (2001) SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH (2003) Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest 111:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekken RA, Sage EH (2000) SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol 19:569–580 [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sage EH (2001) SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 19:816–827 [DOI] [PubMed] [Google Scholar]
- Caceres S, Cuellar C, Casar JC, Garrido J, Schaefer L, Kresse H, Brandan E (2000) Synthesis of proteoglycans is augmented in dystrophic mdx mouse skeletal muscle. Eur J Cell Biol 79:173–181 [DOI] [PubMed] [Google Scholar]
- Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E (2004a) Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J Cell Sci 117:73–84 [DOI] [PubMed] [Google Scholar]
- Casar JC, McKechnie BA, Fallon JR, Young MF, Brandan E (2004b) Transient up-regulation of biglycan during skeletal muscle regeneration: delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev Biol 268:358–371 [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238 [DOI] [PubMed] [Google Scholar]
- Chavey C, Boucher J, Monthouel-Kartmann MN, Sage EH, Castan-Laurell I, Valet P, Tartare-Deckert S, et al (2006) Regulation of secreted protein acidic and rich in cysteine during adipose conversion and adipose tissue hyperplasia. Obesity (Silver Spring) 14:1890–1897 [DOI] [PubMed] [Google Scholar]
- Chen YW, Zhao P, Borup R, Hoffman EP (2000) Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol 151:1321–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WJ, Kim EJ, Lee SJ, Kim HD, Shin HJ, Lim WK (2000) Involvement of SPARC in in vitro differentiation of skeletal myoblasts. Biochem Biophys Res Commun 271:630–634 [DOI] [PubMed] [Google Scholar]
- Creuzet S, Lescaudron L, Li Z, Fontaine-Perus J (1998) MyoD, myogenin, and desmin-nls-lacZ transgene emphasize the distinct pat-terns of satellite cell activation in growth and regeneration. Exp Cell Res 243:241–253 [DOI] [PubMed] [Google Scholar]
- Delany AM, Kalajzic I, Bradshaw AD, Sage EH, Canalis E (2003) Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology 144:2588–2596 [DOI] [PubMed] [Google Scholar]
- Dhawan J, Rando TA (2005) Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15:666–673 [DOI] [PubMed] [Google Scholar]
- Dubois C, Figarella-Branger D, Pastoret C, Rampini C, Karpati G, Rougon G (1994) Expression of NCAM and its polysialylated isoforms during mdx mouse muscle regeneration and in vitro myogenesis. Neuromuscul Disord 4:171–182 [DOI] [PubMed] [Google Scholar]
- Engel A, Ozawa E (2004) Dystrophinopathies. In: Engel AG, Franzini-Armstrong C, eds. Myology. New York, McGraw-Hill, 961–1025
- Fazeli S, Wells DJ, Hobbs C, Walsh FS (1996) Altered secondary myogenesis in transgenic animals expressing the neural cell adhesion molecule under the control of a skeletal muscle alpha-actin promoter. J Cell Biol 135:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre PJ, Liaubet L, Concordet D, SanCristobal M, Uro-Coste E, Tosser-Klopp G, Bonnet A, et al. (2007) Longitudinal analysis of gene expression in porcine skeletal muscle after post-injection local injury. Pharm Res 24:1480–1489 [DOI] [PubMed] [Google Scholar]
- Figarella-Branger D, Nedelec J, Pellissier JF, Boucraut J, Bianco N, Rougon G (1990) Expression of various isoforms of neural cell adhesive molecules and their highly polysialylated counterparts in diseased human muscles. J Neurol Sci 98:21–36 [DOI] [PubMed] [Google Scholar]
- Foulstone EJ, Huser C, Crown AL, Holly JM, Stewart CE (2004) Differential signalling mechanisms predisposing primary human skeletal muscle cells to altered proliferation and differentiation: roles of IGF-I and TNFalpha. Exp Cell Res 294:223–235 [DOI] [PubMed] [Google Scholar]
- Gaster M, Beck-Nielsen H, Schroder HD (2002) Regenerating human muscle fibres express GLUT3 protein. Pflugers Arch 445:105–114 [DOI] [PubMed] [Google Scholar]
- Gilmour DT, Lyon GJ, Carlton MB, Sanes JR, Cunningham JM, Anderson JR, Hogan BL, et al. (1998) Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. EMBO J 17:1860–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, et al. (2002) Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci USA 99:15000–15005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez JP, Casar JC, Fuentealba L, Carey DJ, Brandan E (2002) Extracellular matrix histone H1 binds to perlecan, is present in regenerating skeletal muscle and stimulates myoblast proliferation. J Cell Sci 115:2041–2051 [DOI] [PubMed] [Google Scholar]
- Heslop L, Morgan JE, Partridge TA (2000) Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci 113:2299–2308 [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51:919–928 [DOI] [PubMed] [Google Scholar]
- Holland PW, Harper SJ, McVey JH, Hogan BL (1987) In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol 105:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK (2004) Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs 176:67–78 [DOI] [PubMed] [Google Scholar]
- Illa I, Leon-Monzon M, Dalakas MC (1992) Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol 31:46–52 [DOI] [PubMed] [Google Scholar]
- Jejurikar SS, Kuzon WM Jr (2003) Satellite cell depletion in degenerative skeletal muscle. Apoptosis 8:573–578 [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Fernandez A (2001) Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell Mol Life Sci 58:571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, Mason RJ (1995) Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol 147:1759–1769 [PMC free article] [PubMed] [Google Scholar]
- Kunigal S, Gondi CS, Gujrati M, Lakka SS, Dinh DH, Olivero WC, Rao JS (2006) SPARC-induced migration of glioblastoma cell lines via uPA-uPAR signaling and activation of small GTPase RhoA. Int J Oncol 29:1349–1357 [PMC free article] [PubMed] [Google Scholar]
- Laing NG (2007) Congenital myopathies. Curr Opin Neurol 20:583–589 [DOI] [PubMed] [Google Scholar]
- Lane TF, Sage EH (1994) The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J 8:163–173 [PubMed] [Google Scholar]
- Ledda F, Bravo AI, Adris S, Bover L, Mordoh J, Podhajcer OL (1997a) The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J Invest Dermatol 108:210–214 [DOI] [PubMed] [Google Scholar]
- Ledda MF, Adris S, Bravo AI, Kairiyama C, Bover L, Chernajovsky Y, Mordoh J, et al. (1997b) Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med 3:171–176 [DOI] [PubMed] [Google Scholar]
- Lewis MP, Machell JR, Hunt NP, Sinanan AC, Tippett HL (2001) The extracellular matrix of muscle–implications for manipulation of the craniofacial musculature. Eur J Oral Sci 109:209–221 [DOI] [PubMed] [Google Scholar]
- Lussier C, Sodek J, Beaulieu JF (2001) Expression of SPARC/osteonectin/BM4O in the human gut: predominance in the stroma of the remodeling distal intestine. J Cell Biochem 81:463–476 [PubMed] [Google Scholar]
- Lyons GE, Moore R, Yahara O, Buckingham ME, Walsh FS (1992) Expression of NCAM isoforms during skeletal myogenesis in the mouse embryo. Dev Dyn 194:94–104 [DOI] [PubMed] [Google Scholar]
- Maley MA, Davies MJ, Grounds MD (1995) Extracellular matrix, growth factors, genetics: their influence on cell proliferation and myotube formation in primary cultures of adult mouse skeletal muscle. Exp Cell Res 219:169–179 [DOI] [PubMed] [Google Scholar]
- Martelly I, Soulet L, Bonnavaud S, Cebrian J, Gautron J, Barritault D (2000) Differential expression of FGF receptors and of myogenic regulatory factors in primary cultures of satellite cells originating from fast (EDL) and slow (Soleus) twitch rat muscles. Cell Mol Biol (Noisy-le-Grand) 46:1239–1248 [PubMed] [Google Scholar]
- Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamed K, Blake DJ, Angello JC, Allen BL, Rapraeger AC, Hauschka SD, Sage EH (2003) Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: a role for protein kinase A. J Cell Biochem 90:408–423 [DOI] [PubMed] [Google Scholar]
- Mothe AJ, Brown IR (2001) Differential mRNA expression of the related extracellular matrix glycoproteins SC1 and SPARC in the rat embryonic nervous system and skeletal structure. Brain Res 892:27–41 [DOI] [PubMed] [Google Scholar]
- Mourkioti F, Rosenthal N (2005) IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol 26:535–542 [DOI] [PubMed] [Google Scholar]
- Musaro A (2005) Growth factor enhancement of muscle regeneration: a central role of IGF-1. Arch Ital Biol 143:243–248 [PubMed] [Google Scholar]
- Noguchi S, Tsukahara T, Fujita M, Kurokawa R, Tachikawa M, Toda T, Tsujimoto A, et al. (2003) cDNA microarray analysis of individual Duchenne muscular dystrophy patients. Hum Mol Genet 12:595–600 [PubMed] [Google Scholar]
- Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, et al. (2007) Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J 21:1393–1402 [DOI] [PubMed] [Google Scholar]
- Pichler RH, Hugo C, Shankland SJ, Reed MJ, Bassuk JA, Andoh TF, Lombardi DM, et al. (1996) SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney Int 50:1978–1989 [DOI] [PubMed] [Google Scholar]
- Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, et al. (2002) A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet 11:263–272 [DOI] [PubMed] [Google Scholar]
- Puolakkainen PA, Bradshaw AD, Brekken RA, Reed MJ, Kyriakides T, Funk SE, Gooden MD, et al. (2005) SPARC-thrombospondin-2-double-null mice exhibit enhanced cutaneous wound healing and increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges. J Histochem Cytochem 53:571–581 [DOI] [PubMed] [Google Scholar]
- Puolakkainen PA, Brekken RA, Muneer S, Sage EH (2004) Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res 2:215–224 [PubMed] [Google Scholar]
- Purcell L, Gruia-Gray J, Scanga S, Ringuette M (1993) Developmental anomalies of Xenopus embryos following microinjection of SPARC antibodies. J Exp Zool 265:153–164 [DOI] [PubMed] [Google Scholar]
- Rempel SA, Ge S, Gutierrez JA (1999) SPARC: a potential diagnostic marker of invasive meningiomas. Clin Cancer Res 5:237–241 [PubMed] [Google Scholar]
- Sage H, Vernon RB, Decker J, Funk S, Iruela-Arispe ML (1989a) Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem 37:819–829 [DOI] [PubMed] [Google Scholar]
- Sage H, Vernon RB, Funk SE, Everitt EA, Angello J (1989b) SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol 109:341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaletti S, Gioiosa L, Guiducci C, Rotta G, Rescigno M, Stoppacciaro A, Chiodoni C, et al. (2005) Accelerated dendritic-cell migration and T-cell priming in SPARC-deficient mice. J Cell Sci 118:3685–3694 [DOI] [PubMed] [Google Scholar]
- Savani RC, Zhou Z, Arguiri E, Wang S, Vu D, Howe CC, DeLisser HM (2000) Bleomycin-induced pulmonary injury in mice deficient in SPARC. Am J Physiol Lung Cell Mol Physiol 279:L743–750 [DOI] [PubMed] [Google Scholar]
- Schessl J, Zou Y, Bonnemann CG (2006) Congenital muscular dystrophies and the extracellular matrix. Semin Pediatr Neurol 13:80–89 [DOI] [PubMed] [Google Scholar]
- Schultz E, Jaryszak DL, Valliere CR (1985) Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 8:217–222 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE, Musset-Bilal F, Ryan CS (1994) Extracellular calcium-binding protein SPARC/osteonectin in Caenorhabditis elegans. Methods Enzymol 245:257–270 [DOI] [PubMed] [Google Scholar]
- Schwarzbauer JE, Spencer CS (1993) The Caenorhabditis elegans homologue of the extracellular calcium binding protein SPARC/osteonectin affects nematode body morphology and mobility. Mol Biol Cell 4:941–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Rudnicki MA (2000) A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol 218:115–124 [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786 [DOI] [PubMed] [Google Scholar]
- Shankavaram UT, DeWitt DL, Funk SE, Sage EH, Wahl LM (1997) Regulation of human monocyte matrix metalloproteinases by SPARC. J Cell Physiol 173:327–334 [DOI] [PubMed] [Google Scholar]
- Shi X, Garry DJ (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev 20:1692–1708 [DOI] [PubMed] [Google Scholar]
- Socha MJ, Manhiani M, Said N, Imig JD, Motamed K (2007) Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. Am J Pathol 171:1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin LM, Maley MA, Moch H, von der Mark H, von der Mark K, Cadalbert L, Karosi S, et al. (2000) Laminin alpha4 and integrin alpha6 are upregulated in regenerating dy/dy skeletal muscle: comparative expression of laminin and integrin isoforms in muscles regenerating after crush injury. Exp Cell Res 256:500–514 [DOI] [PubMed] [Google Scholar]
- Soulet L, Chevet E, Lemaitre G, Blanquaert F, Meddahi A, Barritault D (1994) FGFs and their receptors, in vitro and in vivo studies: new FGF receptor in the brain, FGF-1 in muscle, and the use of functional analogues of low-affinity heparin-binding growth factor receptors in tissue repair. Mol Reprod Dev 39:49–54 [DOI] [PubMed] [Google Scholar]
- Stewart JD, Masi TL, Cumming AE, Molnar GM, Wentworth BM, Sampath K, McPherson JM, et al. (2003) Characterization of proliferating human skeletal muscle-derived cells in vitro: differential modulation of myoblast markers by TGF-beta2. J Cell Physiol 196:70–78 [DOI] [PubMed] [Google Scholar]
- Strandjord TP, Madtes DK, Weiss DJ, Sage EH (1999) Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. Am J Physiol 277:L628–635 [DOI] [PubMed] [Google Scholar]
- Tartare-Deckert S, Chavey C, Monthouel MN, Gautier N, Van OE (2001) The matricellular protein SPARC/osteonectin as a newly identified factor up-regulated in obesity. J Biol Chem 276:22231–22237 [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, et al. (2003) Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278:8826–8836 [DOI] [PubMed] [Google Scholar]