Abstract
This review article describes the pathways and mechanisms of endocytosis and post-endocytic sorting of the EGF receptor (EGFR/ErbB1) and other members of the ErbB family. Growth factor binding to EGFR accelerates its internalization through clathrin-coated pits which is followed by the efficient lysosomal targeting of internalized receptors and results in receptor down-regulation. The role of EGFR interaction with the Grb2 adaptor protein and Cbl ubiquitin ligase, and receptor ubiquitination in the clathrin-dependent internalization and sorting of EGFR in multivesicular endosomes is discussed. Activation and phosphorylation of ErbB2, ErbB3 and ErbB4 also results in their ubiquitination. However, these ErbBs are internalized and targeted to lysosomes less efficiently than EGFR. When overexpressed endocytosis-impaired ErbBs may inhibit the internalization and degradation of EGFR.
Keywords: EGF, Receptor, Endocytosis, Clathrin, Ubiquitination, Degradation, Endosome, Lysosome
Introduction
The discovery of EGF and its receptor was immediately followed by the investigation of the pathways and mechanisms of EGFR endocytosis. Such an interest in understanding EGFR endocytic trafficking has been driven by the recognition of the important role that this trafficking has in the regulation of signaling processes triggered by receptor tyrosine kinases (RTKs). In addition, the availability of EGF, antibodies to EGFR and other experimental tools has been and still remains to be the key factor that helps to sustain the ever increasing number of publications on endocytosis of this receptor.
The first comprehensive study of the EGF endocytosis, in which many of the key concepts of internalization and lysosomal degradation of EGF have been established, was published by Carpenter and Cohen [1]. This and other early studies by Cohen’s group remain the basis of the current understanding of EGFR endocytosis. EGFR endocytosis is one of the most well characterized models for studying the morphology, kinetics and mechanisms of endocytic pathways, and is a prototypic model for the endocytosis of other RTKs. Studies of endocytosis of other ErbBs have been trailing the EGFR research because the natural ligands to ErbB3 and ErbB4 were discovered much later than EGF, and because the experimental tools to study these receptors and ErbB2 are only now becoming available.
EGFR is also the most popular model used to study the crosstalks between endocytosis and signaling. After internalization, EGF and EGFR are efficiently degraded, which results in the dramatic decrease in the half-life (t1/2) of the EGFR protein [2]. Accelerated internalization and degradation of activated EGFR lead to the decreased number of receptors at the cell surface, a phenomenon referred to as EGF-induced down-regulation of EGFR. Thus, the process of EGFR down-regulation and degradation is the major negative feedback regulatory mechanism that controls the intensity and duration of receptor signaling [3]. On the other hand, EGF-receptor complexes remain to be active in endosomes and continue to signal after internalization. Therefore, endocytosis has both “positive” and “negative” effects on the signaling network. The complex role of endocytosis in the regulation of EGFR signaling has been discussed in detail in several recent review articles [4–7]. Hence this chapter will focus on the most recent advances in understanding the molecular mechanisms of the endocytic trafficking of EGFR and other ErbBs.
Turnover and trafficking of ErbBs in the absence of activation
In cultured cells expressing low or moderate levels of EGFR (<200,000/cell), receptors turn over with t1/2 in the range of 6–10 h, whereas in cells overexpressing EGFR, such as human epidermoid carcinoma A-431, t1/2 could be 24 h or longer [2,8,9]. The turnover rate of ErbB2 expressed in NR6 cells was similar to that rate of unstimulated EGFR [10]. The t1/2 of endogenous ErbB3 in MCF-7 cells was 2.4 h [11]. ErbB4 expressed in COS cells had a half-life of about 5–7 h [12]. The general trend is that the basal turnover rates of unstimulated ErbBs reciprocally correlate with their expression levels, presumably due to the saturability of the internalization and degradation steps of trafficking.
As other transmembrane proteins, ErbBs are co-translationally translocated through the endoplasmic reticulum (ER) membrane, transported to the Golgi apparatus, where the extracellular domain acquires N-linked glycosylation, and from where the receptors are finally delivered to the plasma membrane [13,14]. In polarized cells, newly synthesized ErbBs are preferentially sorted to the basolateral membrane, although a pool of EGFR and ErbB2 could be detected at the apical surface in some polarized cells [15,16]. Analysis of ErbB2 sorting in MDCK cells revealed that binding of ErbB2 to the PDZ domain of LIN-7 protein is important for the retention of ErbB2 at the basolateral membrane whereas another interaction between the kinase domain of ErbB2 and LIN-7 is necessary for targeting the newly synthesized ErbB2 to the basolateral surface [15]. The evolutionary conserved complex consisting of LIN-2, LIN-7 and LIN-10 proteins is also required for the basolateral localization of LET-23/EGFR in vulva precursor cells of C. elegans [17,18]. EGFR substrate protein 8 (EPS8) was found to associate with LIN-2 to retain EGFR at the basolateral membrane in these cells [19].
At steady-state cell growth conditions the bulk of cellular ErbB proteins are located in the plasma membrane. In most cells, EGFR is constitutively internalized at the rate comparable to the rate of basal membrane recycling (internalization rate constant ke ~ 0.02–0.05 min−1) [20–22]. After internalization, inactive ErbB receptors are mainly recycled back to the cell surface [5]. Because the constitutive recycling rate (rate constant kr ≥ 0.2 min−1) is several times higher than the basic internalization rate, the distribution of EGFR is determined by the ratio of the internalization and recycling rates resulting in the predominant localization of EGFR at the cell surface and a small endosomal pool. Similarly low rates of the constitutive internalization and rapid recycling of ErbB2, which leads to the predominantly surface exposed ErbB2, were also reported [23].
Ligand-induced endocytosis of EGFR
ErbBs are activated by ligand-induced dimerization. There are more than 20 ligands of EGFR, ErbB3 and ErbB4, whereas ErbB2 does not have its own soluble ligand and is activated through heterodimerization with other ligand-occupied ErbBs [24]. The ligand-induced endocytic trafficking of activated EGFR is the best characterized among ErbBs. Therefore, in the following sections we will describe endocytosis and post-endocytic sorting of activated EGFR as the basic model, and subsequently discuss the mechanisms specific to the regulated endocytosis of other ErbBs.
Pathways and kinetics of EGFR internalization: clathrin-dependent versus clathrin-independent mechanisms
Binding of EGF to EGFR results in acceleration of receptor internalization [20]. Several lines of the experimental evidence support the view that this acceleration is due to endocytosis of EGF-receptor complexes through clathrin-coated pits. Firstly, EGF and ligand-activated EGFR were found concentrated in coated pits and vesicles [25–30]. Secondly, the specific rates of EGF internalization are within the range of these rates measured for other receptors that are internalized by means of clathrin-mediated endocytosis (CME), such as transferrin receptors (ke ~ 0.2–0.4 min−1) [22,31]. Thirdly, overexpression of dominant-negative mutants of proteins essential for CME, for example the dynamin K44A mutant, inhibited EGFR internalization [32]. Finally, RNA interference (RNAi) analysis, in which depletion of clathrin heavy chain or dynamin has been shown to inhibit EGFR endocytosis, strongly argues that CME is the major pathway of EGFR internalization [33,34].
CME is the fastest and highly regulated pathway of internalization of integral membrane proteins. Interestingly, high internalization rates of EGFR, that are characteristic of CME, were observed only when EGF was used in low, physiological concentrations (≥1–2 ng/ml), whereas the apparent rate of EGF uptake was decreased with increasing EGF concentrations [35]. Based on such a saturability of the internalization process, Wiley et al. proposed that the rapid internalization pathway has limited capacity and is overwhelmed in the presence of high concentrations of EGF-receptor complexes at the cell surface [31]. Under these conditions, most of these complexes are internalized with a slow kinetics, minimizing the contribution of the rapid pathway in the overall uptake of EGF. More recently, it was shown that the uptake of high EGF concentrations (high receptor occupancy) was only minimally affected by overexpression of the K44A dynamin mutant, whereas the same mutant efficiently blocked internalization of EGF at low concentrations [36]. Moreover, knock-down of the clathrin heavy chain by small interfering RNA (siRNA) did not significantly affect EGF internalization when EGF was used at high concentrations [37]. These data imply that under physiological conditions (low ligand concentrations and moderate expression levels of EGFR), EGFR is internalized mostly by CME, whereas under conditions of receptor overexpression and/or high ligand concentrations, clathrin-independent internalization determines the overall apparent rate of the EGFR uptake into the cell. In some cells expressing low or moderate levels of endogenous EGFR, however, the CME has the capacity to internalize EGFR stimulated with high EGF concentrations [31,35,38].
Clathrin-independent endocytosis of EGFR was first demonstrated in early studies using A-431 cells. EGF treatment of these cells causes extensive plasma membrane ruffling and formation of micro- and macropinocytic vesicles containing labeled EGF and lacking the clathrin coat [39,40]. Such EGF-induced pinocytosis was attributed to the extraordinary high levels of EGFR in A-431 cells. Later, internalization of EGFR by large vesicular structures lacking clathrin in cells treated with high concentrations of EGF was also observed by fluorescence microscopy in COS cells that express a much lesser level of EGFR [41]. Recently, clathrin-independent internalization of EGFR via the vesicular-tubular endocytic compartment originated from the plasma membrane dorsal ruffles was observed in several types of cells [42]. This pathway required the activity of the EGFR kinase, PI3 kinase and dynamin [42].
The endocytosis of EGF-receptor complexes involving cholesterol-rich lipid rafts and/or caveolae was also proposed [37]. This cholesterol-dependent internalization was observed under conditions of high EGFR occupancy by EGF in HeLa cells. In contrast, another study in HeLa cells found no role of cholesterol-rich rafts and caveolae in EGFR endocytosis, and suggested that CME is the major internalization pathway under conditions of all occupancies of the surface EGFR in these cells [38]. It is possible that the localization of EGFR in the caveolae and the contribution of the lipid-raft/caveolae endocytic pathways is cell-type-specific and may even vary in different subclones of HeLa cells.
In summary, in addition to CME, EGF-receptor complexes can be internalized with a slow kinetics that is similar to the basal constitutive endocytosis of unoccupied EGFR. In cells where EGFR activation leads to increased membrane dynamics, ruffling and pinocytosis, EGF-receptor complexes can enter the pinocytic vesicles and ruffle-generated endocytic compartments. In some cells, activated EGFR can be taken up by the mechanisms sensitive to cholesterol-disrupting drugs. All these clathrin-independent pathways are significantly slower than CME, although they may have a faster kinetics as compared to the constitutive receptor internalization. The lack of specific inhibitors of clathrin-independent endocytosis makes it difficult to elucidate the mechanisms and evaluate the importance of these pathways in EGFR internalization. More importantly, clathrin-independent pathways are typically observed in experiments when high EGF concentrations are used and a large amount of EGF-receptor complexes are present at the cell surface. It is likely that the contribution of these mechanisms in the endocytosis of EGFR in vivo is minimal.
Mechanisms of EGFR internalization via clathrin-coated pits
Two interdependent questions are important to address: (i) what are the molecular determinants in the EGFR that are responsible for its rapid CME upon receptor activation and (ii) what are the components of the endocytic machinery that mediate EGFR internalization. The studies attempting to address these questions during the last 20 years produced numerous observations which are difficult to reconcile with each other. One explanation for the inconsistency of the data is that high concentrations of EGF were often used which did not favor measurements of the rates of CME. In addition, measurements of the specific internalization rates without the contribution of rapid recycling are technically difficult. Therefore, our discussion of the mechanisms of EGFR internalization will be focused mostly on findings that were made using low EGF concentrations and a correct internalization rate assay.
The role of EGFR dimerization, tyrosine kinase activity, phosphorylation and putative internalization sequence motifs in EGFR endocytosis has been extensively investigated. EGF-induced dimerization of EGFR results in activation of the receptor tyrosine kinase (reviewed in this issue). The role of dimerization apart from the kinase activity is difficult to analyze because of the lack of specific inhibitors of dimerization that do not affect kinase activity. On the other hand, inactivation of the kinase by mutating the catalytic Lys721 or by small-molecule inhibitors resulted in low endocytic rates and inefficient recruitment of the receptors into coated pits [20,28,43–45]. Kinase-negative EGFR mutants and a wild-type EGFR inactivated by kinase inhibitors are internalized and accumulated in endosomes; however, the rate of this internalization is significantly lower than that of CME [20,46,47]. Accumulation of the kinase-negative EGFR in endosomes is especially pronounced when high concentrations of EGF are used, which is likely due to the slow recycling of large complexes such as EGFR dimers/oligomers as compared to the monomeric unoccupied EGFR.
Activation of the receptor kinase results in tyrosine phosphorylation of the carboxyl-terminal domain of EGFR as well as phosphorylation of numerous cytoplasmic substrates. Mutations of several major tyrosine phosphorylation sites in the EGFR partially reduced internalization when these EGFR mutants were expressed in fibroblasts [21,44]. Surprisingly, mutation of the major binding sites of the Grb2 adaptor protein (Tyr1068 and Tyr1086) strongly inhibited EGF internalization in porcine aortic endothelial (PAE) cells [48] (Fig. 1). In agreement with these data, Grb2 has been shown to be important for EGFR internalization (see Section Grb2-dependent pathway of endocytosis below). Interestingly, EGFR mutants lacking Grb2 binding sites due to large deletions of the carboxyl-terminus were rapidly internalized in mouse fibroblasts [21] but internalized very slowly in PAE cells [48]. It is possible that C-terminal truncations (e. g. at residues 1022–1023) uncover cryptic internalization motifs leading to Tyr1068/1086-independent endocytosis of truncated EGFR mutants in some cells. It is also possible that Grb2 can bind to truncated mutants by the means other than pTyr1068/1086 in fibroblasts but not in PAE cells.
Fig. 1.
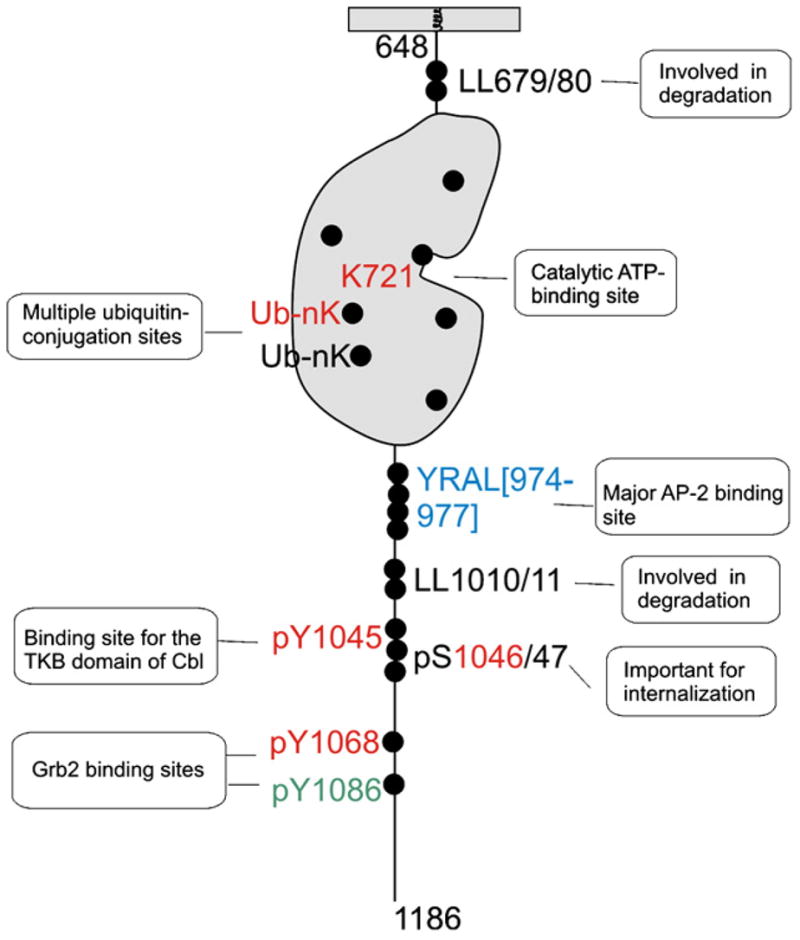
Molecular trafficking signals in EGFR. Schematic representation of the molecular signals in the intracellular part of the EGFR molecule (residues 648–1186) which have been implicated in the endocytic trafficking of the receptor. Sequence motifs and phosphorylation sites/motifs that are not conserved in other ErbBs are shown in “black”, conserved in ErbB2 only — in “blue”, conserved in ErbB3 and/or ErbB4 in addition to ErbB2 — in “red”, and in ErbB3/ErbB4 but not in ErbB2 — in “green”. There are ubiquitin-conjugation sites in the kinase domain that are either conserved in all ErbBs or present only in EGFR.
Serine and threonine phosphorylation of EGFR has also been implicated in the regulation of receptor internalization (Fig. 1). Phosphorylation of the juxtamembrane Thr654 by protein kinase C reduced EGF endocytosis, likely due to the partial inhibition of the kinase activity of the receptor [49]. Phosphorylation of serines 1046 and 1047 was shown to be necessary for EGF internalization [50]. Recently, it was found that endocytosis of unoccupied EGFR can be induced by stress receptor signaling and chemical compounds that activate mitogen-activated protein kinase (MAPK)/p38 [51–53]. It was suggested that p38 induced endocytosis by phosphorylating serines within the region between residues 1002–1020 of the EGFR [53]. However, how exactly this region and pSer1046/47 regulate EGFR internalization is unknown.
The C-terminus of EGFR (downstream of the kinase domain) contains several sequence motifs that are capable of interaction with the clathrin adaptor protein complex 2 (AP-2), a major cargo binding component of coated pits (Fig. 1). Indeed, EGFR was found to directly interact with the μ2 subunit of AP-2 through the Y974RAL motif [54–57]. However, mutations in the YRAL motif did not affect EGFR internalization [56]. Moreover, mutations in the binding interface for this motif in the μ2 protein did not decrease EGFR internalization [58], suggesting that YRAL interaction with AP-2 is not essential for EGFR internalization. Mutations of the NPxY motifs in the EGFR did not reduce EGFR internalization as well [21]. The di-leucine motif (residues Leu1010/1011) was shown to be involved in the tyrosine phosphorylation of the β2 subunit of AP-2, indicative of its possible role in the receptor interaction with AP-2 [59]. However, this LL motif was not necessary for the internalization of the full-length EGFR [36,59]. Furthermore, under certain experimental conditions, depletion of AP-2 by siRNA did not affect EGFR internalization, although there is disagreement among different reports regarding the effect of AP-2 depletion on EGFR endocytosis [30,33,34]. In summary, while EGFR is capable of interaction with AP-2, the role of this interaction remains unknown.
Grb2-dependent pathway of endocytosis
Despite the redundancy of the tyrosine phosphorylation sites in EGFR with regards to their ability to bind Grb2 and other SH2 containing proteins, the low internalization rates observed for Tyr1068/1086 mutant in PAE cells indicated that Grb2 may play an important role in EGFR internalization. The key evidence for the endocytic function of Grb2 was obtained in experiments where siRNA depletion of Grb2 substantially and specifically reduced internalization of EGFR in PAE and HeLa cells (expressing endogenous EGFR) [48]. These experiments were in agreement with the demonstration of the dominant-negative effects of Grb2 mutants on EGFR trafficking in MDCK cells [60]. Furthermore, Grb2–EGFR complexes were found in coated pits, and Grb2 was shown to be necessary for the EGFR recruitment into coated pits (Fig. 2) [29,30,48]. Depletion of Grb2 by siRNA caused a substantial (60–80%) decrease in the EGFR internalization rate in mouse fibroblasts and squamous carcinoma cells (Sorkin A., unpublished observations), strongly suggesting that Grb2-dependent endocytosis is the major pathway of EGFR internalization in many types of cells.
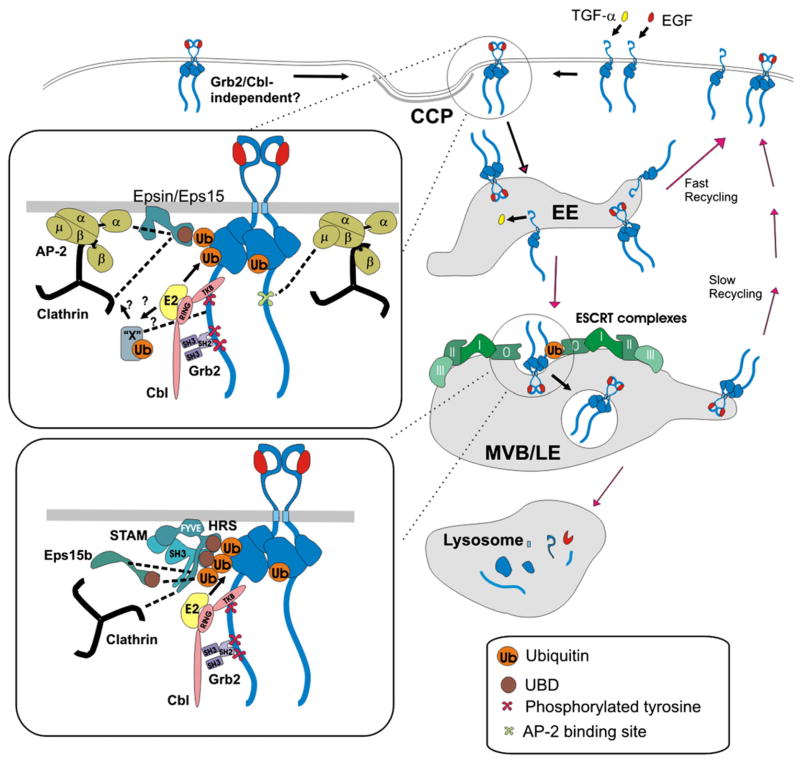
Fig. 2.
A hypothetic model of EGFR endocytosis and intracellular sorting (in the presence of low concentrations of EGF or TGFα). EGF binding to EGFR leads to receptor dimerization and phosphorylation. Grb2–Cbl complex is recruited to C-terminal phosphotyrosine-containing motifs. E2 enzymes (UbcH4/5) are recruited to the RING domain to promote receptor ubiquitination. Ubiquitinated EGFR can be recognized by UBDs of epsin, Eps15 and Eps15R in the plasma membrane and clathrin-coated pits (CCP). These proteins are associated with AP-2 and clathrin heavy chain, a main component of the clathrin triskelion (clathrin). Alternatively, Cbl can mediate ubiquitination of an unknown adaptor protein (“X”) that mediates internalization of EGFR through coated pits by interacting with EGFR and/or clathrin or clathrin-associated proteins. EGFR can directly interact with AP-2 via YRAL and possibly LL motifs. There might be also a Grb2-independent pathway of internalization through coated pits. After internalization EGF-receptor complexes can be rapidly recycled from early endosomes (EE) or remain in these endosomes during their maturation into MVB and late endosomes (LE). In contrast, TGF-α dissociates from EGFR in endosomes, which results in the recycling of the monomeric, inactive receptor. In MVB, ubiquitinated EGFR is engaged into multi-valent interactions with UBDs of the ESCRT-0 complex (HRS and STAM) and associated Eps15b, and then incorporated into internal vesicles of MVB. The concentric ring model of the inward invagination of MVB membrane is depicted. Receptors that are not ubiquitinated can be recycled back to the plasma membrane through the tubular extensions of the limiting membrane of MVB at a slower rate. Recycling of occupied and unoccupied receptors may also involve a late recycling compartment (not shown). MVB fuses with primary lysosomes, which subsequently results in degradation of EGF and EGFR.
Grb2 consists of one SH2 and two SH3 domains. The SH2 domain binds to phosphotyrosine-containing motifs in the EGFR and physically couples proteins that are associated with the SH3 domains to the receptor. One of the major Grb2-interacting proteins, Cbl, has been implicated in the regulation of EGFR internalization and degradation [61] (Fig. 2). There are three members of the Cbl family in mammalian cells, c-Cbl, Cbl-b and Cbl-3. The first two species have extended C-termini with several proline-rich motifs capable of binding to SH3 domains [62]. Cbl proteins are the RING finger containing E3 ubiquitin ligases that mediate ubiquitination of the EGFR by means of the recruitment of E2 ubiquitin conjugating enzymes [63,64]. All three Cbls possess a tyrosine kinase binding (TKB) domain that can directly bind to phosphorylated Tyr1045 of EGFR [63] (Fig. 1). The relative contribution of indirect (Grb2-mediated) and direct (TKB-mediated) interactions of Cbl with EGFR may vary in different cell types.
Several sets of the experimental data support the role of Cbl in the CME of EGFR. Firstly, EGF-induced translocation of c-Cbl to clathrin-coated pits has been demonstrated [65]. Secondly, over-expression of several c-Cbl mutants inhibited EGFR internalization in HeLa, PAE and NIH 3T3 cells [36,66]. Thirdly, chimeric proteins consisting of Grb2 SH2 domain and c-Cbl could rescue EGFR endocytosis in Grb2-depleted cells, confirming the function of Cbl downstream of Grb2 [67]. Fourthly, siRNAs knock-down of both c-Cbl and Cbl-b resulted in partial inhibition of the EGFR internalization [68]. Interestingly, direct Cbl binding to pTyr1045 appears to play a minor, if any, role in the CME of EGFR, whereas Grb2-mediated interaction with EGFR is critical [36]. Altogether, the data obtained in cultured cells strongly argue that Cbls are important for EGFR internalization, and that both the association with Grb2 and the intact RING domain are essential for this Cbl function. In contrast, c-Cbl knock-out did not affect EGF internalization in mouse embryonic fibroblasts [69]. This observation can be explained by the presence of Cbl-b in these cells. Another possibility is that the importance of Cbl for internalization is cell-type-specific.
In Drosophila flies, two isoforms of Cbl, long (D-CblL) and short (D-CblS) are present. A recent study showed that D-CblL facilitates endocytosis, passage through the Rab5 and Rab7 endosomes, and lysosomal degradation of the Gurken (TGFα homolog)-EGFR complex [70]. D-CblS is homologous to mammalian Cbl-3 which lacks SH3 binding and does not bind Grb2. However, D-CblS is able to down-regulate EGFR, perhaps, by directly binding to the receptor and promoting its ubiquitination [71]. The c-Cbl/b-Cbl ortholog in C. elegans, Sli-1, was shown to bind EGFR/LET-23 both directly and indirectly through Grb2/SEM-5 to exert its negative regulatory effect on the receptor [72].
Because Cbl ubiquitinates EGFR, it is logical to hypothesize that Cbl-mediated ubiquitination of EGFR is necessary for receptor internalization. The first indication that this hypothesis is incorrect came from the observation of normal internalization of the EGFR mutant that lacks Tyr1045 and is weakly ubiquitinated [36]. Recently, the ubiquitination sites in EGFR were mapped in the kinase domain of the receptor [68] (Fig. 1). Mutation of these sites did not affect EGFR internalization, confirming that EGFR ubiquitination is not essential for internalization [68,73]. Interestingly, add-back of two major ubiquitination sites to the multi-lysine EGFR mutant (16KR) that displayed partial inhibition of its kinase activity and, therefore, partial inhibition of internalization, restored its internalization, suggesting that ubiquitin moieties are potentially capable of mediating EGFR internalization [68,73]. Furthermore, siRNA knock-down of epsin 1, Eps15 and Eps15R (proteins proposed to be ubiquitin adaptors in coated pits) did not result in the specific inhibition of clathrin-dependent internalization of EGFR [34,37]. In view of the demonstration that EGFR ubiquitination is not essential for internalization, future research should focus on the search of a different type of protein that can mediate internalization of the EGFR–Grb2–Cbl complex. The RING domain of Cbl could be necessary for ubiquitination of another protein or an interaction with a protein other than E2 enzymes (Fig. 2). An example of a potential candidate to mediate Cbl function in endocytosis is intersectin, a protein that is found in clathrin-coated pits, capable of interaction with Cbl and shown to be necessary for internalization and/or degradation of EGFR [74]. Another protein that binds Cbl and has been implicated in EGFR down-regulation is CIN85 [75,76]. However, CIN85 has not been detected in coated pits and may be involved in post-endocytic trafficking of EGFR rather than internalization [77].
Post-endocytic trafficking of EGFR
Pathways through endosomes
Clathrin-coated vesicles containing EGF-receptor complexes rapidly release their coat and fuse with early endosomes, compartments of a heterogeneous morphology consisting of vesicular and tubular membranes and located at the periphery of the cell [78–81] (Fig. 2). The accumulation of EGF and EGFR in early endosomes is evident after 2–5 min of endocytosis at 37 °C. Early endosomes are highly dynamic and tend to rapidly recycle the cargo, or fuse with each other leading to the formation of larger “intermediate” or “sorting” endosomes (reviewed in [82,83]). The pH in early and intermediate endosomes is mildly acidic (6.0–6.5). At this pH EGF-receptor complexes do not substantially dissociate [84]. Consequently, EGFRs in endosomes remain to be dimerized, phosphorylated, associated with Grb2 and Cbl [85–87], and therefore ubiquitinated.
The endosome maturation occurs concomitantly with the sorting of the internalized receptors to different cellular destinations. The maturation process involves fusion of early endosomes and a change in the biochemical composition and morphology of early endosomes to that of multivesicular bodies (MVBs) and late endosomes (Fig. 2). EGF and EGFR begin to accumulate in the intralumenal membranes of MVB that are located in the perinuclear area of the cell after 15–20 min of EGF-induced endocytosis [80,81,88,89]. Serial sectioning electron microscopy demonstrated that internal membranes of MVB represent vesicles that are not connected to the limiting membrane [90]. Therefore, EGFR incorporated into intralumenal membranes cannot be recycled.
During the endosome maturation process, receptors recycle back from endosomes to the cell surface. Since EGF does not significantly dissociate from the receptor, an intact EGF-receptor complex is recycled [91]. Recycling of EGF-receptor complexes occurs through two kinetically and mechanistically distinct pathways (Fig. 2). The rapid pathway involves early endosomes and is efficient at low temperature (16–18 °C) [91]. The second pathway of recycling has a slower kinetics and is completely blocked at low temperature (16–18 °C) [91]. This slow recycling is probably originated from a pool of EGF-receptor complexes that are located in the limiting membrane of MVBs and recycled through the tubular extensions of MVBs, and another pool of EGF-receptor complexes trafficked to the late recycling compartment (Rab11-positive). Significant co-localization of EGFR in the limiting membranes/tubular extensions of MVBs and in recycling endosomes with the constitutively-recycled transferrin receptor supports the above hypothesis [78,84,92,93].
Fusion of MVBs with primary lysosomal vesicles that carry proteolytic enzymes leads to rapid proteolysis of intralumenal components of MVBs containing EGFR [80]. Degradation of EGF and EGFR can be completely blocked by lysosomal inhibitors [1,2]. Inhibitors of proteosome also reduce EGFR degradation [94]. However, the effects of proteosomal inhibitors on EGFR degradation are likely indirect. The proteosomal inhibitors may affect activity of lysosomal enzymes and/or diminish the amount of ubiquitin in the cell. Also, proteosomal degradation controls the turnover of ESCRT proteins and other proteins regulating MVB sorting (see below).
In summary, the model of the endosomal sorting of EGFR based on morphological studies suggests that EGF-receptor complexes can be recycled in a manner similar to unoccupied EGFR and transferrin receptors unless these complexes are trapped in the intralumenal vesicles of MVB (Fig. 2).
Molecular mechanisms of EGFR sorting in MVB
The elucidation of the molecular mechanisms of EGFR sorting in MVB is in large part owed to genetic analysis of the vacuole sorting in yeast that revealed multiple Vps proteins that are components of ESCRT complexes or proteins associated with these complexes (reviewed in [95–97]). The mechanistic model of the EGFR sorting in MVB is presented in Fig. 2. That EGFR ubiquitination is essential for the lysosomal targeting and degradation of the receptor was initially found in experiments with the Y1045F mutant of EGFR [63] and later directly demonstrated using mutations of the EGFR ubiquitination sites [68]. Although EGFR was shown to be poly-and multi-monoubiquitinated, it is unclear whether poly-ubiquitination is necessary for the MVB sorting.
Ubiquitinated EGFR is thought to interact with the ESCRT-0 complex, main components of which are HRS and STAM1/2 proteins, both containing ubiquitin-binding domains (UBDs). A splice variant of Eps15, Eps15b also contains a UBD and is associated with HRS, possibly providing additional means of the recruitment of ubiquitinated EGFR [98]. HRS can also recruit clathrin that forms bilayered coats on endosomes leading to the formation of “HRS microdomains” [99]. The interaction of EGFR with HRS has been shown by co-immunoprecipitation [37]. siRNA depletion of HRS resulted in inhibition of EGFR degradation [100]. In the Drosophila HRS mutant, endosomal membrane invagination and formation of MVB were impaired, leading to the accumulation of activated EGFR in the cells [101].
While a remarkable advance in elucidating the structures of ESCRT-I, -II and -III complexes has been made in recent years, how exactly the ubiquitinated cargo, that is trapped in HRS micro-domains, ends up in internal vesicles of MVB remains unclear. It has been proposed that ubiquitinated cargo is handed from ESCRT-0 to consequently ESCRT-I, -II and then -III complexes, all containing UBDs [97]. Another recent model suggests that ESCRT-I, -II and -III complexes form concentric rings around HRS complexes, which ultimately results in inward membrane invagination [95]. In the former model the ESCRT-0 complex is distal to the epicenter of the membrane invagination, whereas in the concentric ring model this complex is in the epicenter of vesicle formation and must dissociate from the membrane before vesicle scission (Fig. 2). All ESCRTs have lipid binding domains and UBDs that are involved in the interactions with each other. For instance, ESCRT-0 complex facilitates membrane recruitment of ESCRT-I, which in turn facilitates subsequent recruitment of ESCRT-II and -III complexes. Oligomerization of CHMP (a component of ESCRT-III) is believed to result in the formation of a lattice-like structure that is thought to promote invagination of the endosomal membrane [96,97,102,103].
Functional studies confirmed that the components of ESCRT-I and ESCRT-III complexes, TSG101 and hVps24, respectively, are necessary for EGFR degradation [104–108], although the interaction of EGFR with ESCRT-I, -II and -III complexes has not been demonstrated. The requirement of ESCRT-II for EGFR sorting is still a subject of a debate [108,109]. Based on studies in yeast it is proposed that ubiquitinated cargo is deubiquitinated prior to its incorporation into intralumenal vesicles to prevent degradation of ubiquitin [96]. However, EGFR deubiquitination during sorting in MVB has not been demonstrated.
It should be emphasized that preservation of EGF-receptor association, EGFR phosphorylation and association with Cbl (necessary for ubiquitination) in endosomes is essential for the ESCRT-mediated sorting of EGFR to the degradation pathway. Some ligands of EGFR other than EGF have a weaker affinity to the receptor and dissociate from the receptor in the acidic environment of endosomes. For instance, when EGFR is occupied by transforming growth factor α (TGFα), TGFα is released from the receptor in early endosomes, leading to receptor dephosphorylation and recycling back to the plasma membrane [110] (Fig. 2). As a result, TGFα does not cause significant degradation and down-regulation of EGFR.
The ESCRT-dependent model of EGFR sorting to lysosomes is widely accepted. However, two di-leucine motifs of EGFR have been implicated in receptor degradation [59,111] (Fig. 1). The relationships of these motifs and ESCRTs are unknown, and the components of the endocytic machinery interacting with these motifs remain to be elucidated. Furthermore, proteins other than ESCRT complexes, such as sorting nexin 1 and annexin 1, have been specifically implicated in the EGFR degradation [112,113]. The exact role of these proteins in the EGFR sorting process is also unknown.
Besides the endosome/lysosome system, EGF-activated EGFR has been detected in the nuclei of several types of cells (reviewed in Ref. [114]). This EGF-dependent translocation of the full-length EGFR into the nucleus was demonstrated by biochemical fractionation and immunocytochemical methods [115,116]. Nuclear translocation of EGFR was reduced by transient transfection of the dominant-negative dynamin K44A mutant in EGF-stimulated cells, suggesting the role of CME in this pathway [117]. In all of the above mentioned studies the receptor was delivered to the nucleus with a relatively rapid kinetics (minutes). In another recent study, the kinetics of the nuclear appearance of EGFR was shown to be much slower (hours) [118]. This study suggested that EGFR traffics from the plasma membrane to the ER, where it is translocated to the cytosol by the Sec61 complex, and subsequently translocated to the nucleus. Clearly, elucidation of the mechanisms of this novel EGFR trafficking pathway would require further analysis using morphological, including live-cell microscopy, and biochemical methods.
Proteins modulating endocytosis and sorting of EGFR
Whereas Grb2 and Cbl directly and specifically determine the rates of EGFR turnover, internalization and degradation, a number of other proteins have been identified that can have a modulatory effect on the rate of EGF-induced EGFR down-regulation. Interestingly, many of such proteins function by targeting Cbl activity [119]. For example, the Sprouty 2 protein is capable of binding to the RING and TKB domains of Cbl, thus inhibiting Cbl E3 ligase activity and reducing EGFR ubiquitination [120,121]. However, Sprouty 2 also binds Grb2 and several other proteins that signal downstream of EGFR, and it is possible that the mechanisms of Sprouty 2 effects on EGFR are quite convoluted [122]. Analysis of Sprouty function by knock-outs or knock-downs in mammalian cells is difficult because multiple Sprouty proteins, that can be functionally redundant, exist. Whether endogenous Sprouty 2 is involved in the regulation of EGFR endocytosis in mammals is unknown.
An effector and regulator of a small GTPase Cdc42, Cool-1, also binds to Cbl and inhibits its ubiquitination activity in the presence of v-Src, thus inhibiting EGFR degradation [123]. Hominoid-specific oncogenic protein Tbc1d3 appears to affect ubiquitination and degradation of EGFR by interfering with Cbl binding to the receptor [124]. Other proteins that are capable of binding to Cbl and/or EGFR and implicated in the regulation of EGFR internalization and degradation are: Alix [125], c-Abl tyrosine kinase [126], Sts1/TULA2 [127], Lrig-1 [128], and supressors of cytokine signaling SOCS4/5 [129]. The mechanisms of endocytosis-modulating effects of these proteins are not well understood. Similarly, GAPex5, a Rab5 exchange factor, inhibits binding of Cbl to EGFR by an unknown mechanism not mediated by Rab5 [130]. The possible roles of two other Cbl-binding proteins, intersectin and CIN85, were discussed in Section Mechanisms of EGFR internalization via clathrin coated pits. A recent study in Drosophila identifies a new protein Myopic (Mop), which is proposed to be involved in endosome trafficking of EGFR in imaginal discs and S2 cells [131]. Mop is a Drosophila homolog of mammalian tyrosine phosphatase HD-PTP which contains the Bro-1 domain (Bro-1 is associated with ESCRT-III). Whereas Mop functionally interacts with Cbl in the regulation of EGFR degradation, the exact mechanisms of this regulation are unknown.
Several proteins have been recently shown to affect EGFR degradation without affecting Cbl activity. A tyrosine phosphorylation substrate of EGFR, Yme1, has been recently identified by mass-spectroscopy analysis and shown to have an inhibitory effect on EGFR down-regulation [132]. Spartin, a protein mutated in Troyer syndrome (autosomal recessive hereditary spastic paraplegia) patients, was shown to function in EGFR degradation [133]. Depletion of Cdc42-associated tyrosine kinase 1 (ACK1) or over-expression of ACK1 fragments inhibited EGFR degradation [134]. Given that ACK1 is capable of binding to ubiquitinated proteins, the effect of this kinase on EGFR degradation could be related to the regulation of the interactions between ubiquitinated EGFR and the endocytic machinery.
An important family of proteins that regulate EGFR endocytosis and degradation is deubiquitination enzymes (DUBs) [135]. Two DUBs have been implicated in EGFR degradation, the JAMM domain-containing AMSH [136] and UBPY (USP8) [137]. Both AMSH and UBPY are shown to interact with the same site on the STAM protein [138]. UBPY binds components of ESCRT-III [139]. The effects of its siRNA depletion on EGFR degradation varied in different studies [108,137,140,141]. UBPY appears to have wide substrate specificity. Conditional mouse knock-out of USP8/UBPY resulted in the reduction of the expression levels of several RTKs as well as HRS and STAM2, suggesting that this DUB plays a role in the regulation of the turnover of ESCRT complexes [142]. Because the effects of siRNA knock-down of both AMSH and UBPY on EGFR ubiquitination and degradation were either partial or absent in some studies, it is likely that there are other DUBs that can deubiquitinate EGFR in vivo.
Trafficking of activated ErbB2
Because ErbB2 does not have a natural soluble ligand, the early studies of ErbB2 internalization utilized the chimeric proteins consisting of the extracellular domain of EGFR and the intracellular domain of ErbB2 and used labeled EGF to follow internalization of these chimeric receptors [10]. In these studies the EGFR-ErbB2 chimera was internalized several times slower than EGFR despite activation of the ErbB2 kinase and phosphorylation of the ErbB2 intracellular domain. It was concluded that the carboxyl-terminal domain of ErbB2 is internalization-impaired.
Wild-type, full-length ErbB2 can be activated when highly overexpressed or through heterodimerization with other ErbBs. In cells overexpressing ErbB2 it is predominantly localized in the plasma membrane, indicating that overexpression of ErbB2 does not lead to acceleration of its endocytosis and down-regulation [23,24,143–145]. In contrast, heterodimerization of ErbB2 with ligand-occupied EGFR may influence the endocytic trafficking of both ErbB2 and EGFR. EGF treatment resulted in down-regulation of ErbB2 in cells with relatively low levels of ErbB2 expression [146–148]. In cells with high levels of ErbB2, such as many mammary carcinoma cell lines, activation of EGFR did not affect surface expression of ErbB2 and did not accelerate its degradation [24,143,146,147,149]. Moreover, overexpression of ErbB2 had a dominant-negative effect on EGF-induced EGFR down-regulation [143,150]. In some cases, it has been demonstrated that ErbB2 prevented CME of EGFR [149,151]. In other cells, ErbB2 did not slow down EGFR internalization but re-routed internalized EGFR from the degradation to the recycling pathway [147].
The mechanisms by which endocytosis-impaired ErbB2 affects endocytosis of EGFR are not clear. EGFR is phosphorylated on Tyr1068 and ubiquitinated in the presence of the excess of ErbB2 [149]. It is possible that ErbB2 and, therefore, ErbB2-EGFR heterodimers are prevented from efficient internalization by the plasma membrane retention mechanisms. Such mechanisms may involve interactions of ErbB2 with actin-associated proteins in the specialized plasma membrane domains [152] or PDZ-domain proteins like erbin [153,154] and LIN-7 [16]. It has been suggested that the carboxyl-terminal regulatory domain of ErbB2 either contains the molecular signals responsible for the retention of ErbB2 at the cell surface or lacks the signals necessary to support the rapid internalization [10,143]. The “retention” model is supported by the observations of the accelerated endocytosis and down-regulation of ErbB2 upon proteolytic cleavage of the carboxyl-terminus of ErbB2 caused by geldanamycin [155,156]. Against the “retention” model is the inability of overexpressed carboxyl-terminal domain of ErbB2 to relieve the inhibition of endocytosis of ErbB2 and EGFR-ErbB2 heterodimers [149].
It is generally agreed upon that after internalization, ErbB2 and ErbB2-EGFR dimers are not efficiently sorted to lysosomes and mostly recycled back to the plasma membrane [23,147,150]. It is possible that multi-valent interactions between ubiquitinated receptors and the ESCRT-0 complex are necessary for the efficient sorting in MVB, and that EGFR and ErbB2 are not fully ubiquitinated in the heterodimer. Indeed, while activated ErbB2 can recruit Cbl, this recruitment is less efficient as compared to EGFR [157]. It is also possible that heterodimers of ErbB2 with EGFR or ErbB3 dissociate in endosomes due to the release of the ligand in the acidic environment of endosomes [150]. Computational modeling of the trafficking of EGFR and ErbB2 heterodimers predicted that elevated dissociation of ligand in endosomes could not explain the observed trafficking patterns and dynamics of EGFR co-expressed with the excess of ErbB2, while the reduced degradation of EGFR can be explained by the competition of EGFR with the large excess of ErbB2 in MVB for the interactions with ESCRT complexes [158].
An oncogenic mutation in the transmembrane domain of rat ErbB2 (V664E) called Neu leads to activation of Neu kinase and its tyrosine phosphorylation. This ErbB2 variant and a similar mutant of the human ErbB2 display elevated turnover [159,160]. Human activated ErbB2/Neu mutant expressed in CHO cells was constitutively ubiquitinated, and phosphorylation of Tyr1112, homologues to the Cbl-binding site of EGFR, was essential for this ubiquitination and receptor degradation [160]. In Rat-1 cells, the transfected rat Neu mutant was degraded with t1/2 of ~3–4 h, in a manner independent of Cbl [161].
In summary, the ability of activated ErbB2 to be internalized and to impose an inhibitory effect on EGFR internalization appears to depend on the expression levels of ErbB2. ErbB2 clearly has a lower potency to be ubiquitinated and targeted to lysosomes as compared to EGFR.
Similarly to the EGFR, it was proposed that ErbB2 can be translocated from the cell surface to the nucleus and that this trafficking pathway requires ErbB2 kinase activity and can be inhibited by the dynamin mutant, implicating the endocytosis step in this process [162].
Trafficking of ErbB3 and ErbB4
ErbB3 and ErbB4 are activated by a common family of soluble ligands called heregulins or neuregulins. Unlike other ErbBs, ErbB3 has inactive kinase and is tyrosine phosphorylated by the heterodimeric partner. Ligand binding typically causes down-regulation of these receptors [11,12,163], albeit to an extent that is significantly lower than that observed with EGFR down-regulation [164–166]. The neuregulin-induced internalization of ErbB3 and ErbB4 has been observed in various cells, including neurons [12,164,165,167,168]. However, as in the case of ErbB2, the internalization of ErbB3 and ErbB4 is slower than that of EGFR [165,166]. For instance, ErbB3 lacking the entire cytoplasmic domain displayed the same internalization rate as the full-length receptor [164]. In addition, ErbB3 is inefficiently sorted to the degradation pathway, apparently due to the lack of lysosome targeting information in its C-terminus [164,169]. Substitution of the C-terminus of EGFR by the same domain of ErbB3 results in the EGFR mutant with reduced association with Cbl, ubiquitination and down-regulation [169]. It was also suggested that neuregulins do not efficiently target ErbB3 to degradation due to the dissociation of ligand-receptor complexes in endosomes, as it is observed when EGFR is activated by TGFα [169].
It has been recently shown that the steady-state levels of ErbB3 are regulated by a RING domain E3 ubiquitin ligase, Nrgp1 [11,170]. Upon neuregulin 1 binding to ErbB3, the receptor signals to stabilize Nrdp1 via the deubiquitination enzyme UBPY/USP8, thus leading to enhanced ubiquitination and down-regulation of the ErbB3 protein [11].
Growth factor induced ubiquitination of ErbB4 has also been recently demonstrated [12,168]. ErbB4 was shown to bind to and be ubiquitinated by the HECT domain containing the E3 ubiquitin ligase Itch. This ubiquitination results in rapid down-regulation of the neuregulin-activated CYT-2 variant of ErbB4 containing PPxY motif that interacts with WW domains of Itch [12]. In contrast, upon ligand activation the CYT-1 variant of ErbB4 is down-regulated more slowly than CYT-2 [12], and is cleaved in a two-step reaction to release the cytoplasmic fragment that translocates to the nucleus [114].
Thus, trafficking of both ErbB3 and ErbB4 can be regulated by ubiquitination. The amplitude of ligand-induced down-regulation of these receptors is determined mostly by the rates of their degradation rather than the internalization rates.
Conclusions and outstanding questions
Despite an effort of many research groups, many key aspects of the molecular mechanisms of EGFR endocytic trafficking are not understood. It has become clear that the process of EGFR endocytosis is very robust as it relies on several redundant mechanisms, e. g. Grb2-, Cbl- and ubiquitin-dependent and independent, and therefore, is difficult to dissect. Our knowledge about the endocytosis of EGFR under physiological conditions in cell culture and in vivo is especially lapsing. For example, most of the studies of EGFR sorting and degradation were performed using high concentrations of EGF, and it remains to be demonstrated that EGFR ubiquitination and ESCRT complexes are involved in the lysosomal targeting of the receptor activated by physiological concentrations of EGF. The mechanisms of internalization of ErbB2, ErbB3 and ErbB4 are scarcely studied, and further development of quantitative assays is necessary to advance this research. Understanding of the mechanisms underlying the behavior of heterodimers of EGFR with endocytosis-impaired ErbB2–4 is of high importance. However, it is unlikely that this issue could be easily resolved before the endocytic mechanisms of EGFR are better understood.
Acknowledgments
We thank Melissa Adams for her critical reading of the manuscript. This work was supported by NCI grant CA08915 and ACS grant RSG-00-247-04-CSM.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Carpenter G, Cohen S. 125I-Labeled human epidermal growth factor: binding internalization, and degradation in human fibroblasts. J Cell Biol. 1976;71:159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoscheck CM, Carpenter G. “Down-regulation” of EGF receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984;98:1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- 4.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 5.Wiley HS. Trafficking of the ErbB receptors and its influence on signaling. Exp Cell Res. 2003;284:78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 6.Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 7.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007 doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beguinot L, Lyall RM, Willingham MC, Pastan I. Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc Natl Acad Sci U S A. 1984;81:2384–2388. doi: 10.1073/pnas.81.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoscheck CM, Carpenter G. Characterization of the metabolic turnover of epidermal growth factor receptor protein in A-431 cells. J Cell Physiology. 1984;120:296–302. doi: 10.1002/jcp.1041200306. [DOI] [PubMed] [Google Scholar]
- 10.Sorkin A, Di Fiore PP, Carpenter G. The carboxyl terminus of epidermal growth factor receptor/erbB-2 chimerae is internalization impaired. Oncogene. 1993;8:3021–3028. [PubMed] [Google Scholar]
- 11.Cao Z, Wu X, Yen L, Sweeney C, Carraway KL., III Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1. Mol Cell Biol. 2007;27:2180–2188. doi: 10.1128/MCB.01245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundvall M, Korhonen A, Paatero I, Gaudio E, Melino G, Croce CM, Aqeilan RI, Elenius K. Isoform-specific monoubiquitination, endocytosis, and degradation of alternatively spliced ErbB4 isoforms. Proc Natl Acad Sci U S A. 2008;105:4162–4167. doi: 10.1073/pnas.0708333105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todderud G, Carpenter G. Epidermal growth factor: the receptor and its function. BioFactors. 1989;2:11–15. [PubMed] [Google Scholar]
- 14.Cummings RD, Soderquist AM, Carpenter G. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex-type N-linked chains that contain terminal N-acetylgalactosamine residues. J Biol Chem. 1985;260:11944–11952. [PubMed] [Google Scholar]
- 15.Balestreire EM, Apodaca G. Apical epidermal growth factor receptor signaling: regulation of stretch-dependent exocytosis in bladder umbrella cells. Mol Biol Cell. 2007;18:1312–1323. doi: 10.1091/mbc.E06-09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shelly M, Mosesson Y, Citri A, Lavi S, Zwang Y, Melamed-Book N, Aroeti B, Yarden Y. Polar expression of ErbB-2/HER2 in epithelia. Bimodal regulation by Lin-7. Dev Cell. 2003;5:475–486. doi: 10.1016/j.devcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kaech SM, Whitfield CW, Kim SK. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 1998;94:761–771. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitfield CW, Benard C, Barnes T, Hekimi S, Kim SK. Basolateral localization of the Caenorhabditis elegans epidermal growth factor receptor in epithelial cells by the PDZ protein LIN-10. Mol Biol Cell. 1999;10:2087–2100. doi: 10.1091/mbc.10.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stetak A, Hoier EF, Croce A, Cassata G, Di Fiore PP, Hajnal A. Cell fate-specific regulation of EGF receptor trafficking during Caenorhabditis elegans vulval development. Embo J. 2006;25:2347–2357. doi: 10.1038/sj.emboj.7601137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiley HS, Herbst JJ, Walsh BJ, Lauffenberger DA, Rosenfeld MG, Gill GN. The role of tyrosine kinase activity in endocytosis, compartmentalization and down-regulation of the epidermal growth factor receptor. J Biol Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- 21.Chang CP, Lazar CS, Walsh BJ, Komuro M, Collawn JF, Kuhn LA, Tainer JA, Trowbridge IS, Farquhar MG, Rosenfeld MG, Wiley HS, Gill GN. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J Biol Chem. 1993;268:19312–19320. [PubMed] [Google Scholar]
- 22.Resat H, Ewald JA, Dixon DA, Wiley HS. An integrated model of epidermal growth factor receptor trafficking and signal transduction. Biophys J. 2003;85:730–743. doi: 10.1016/s0006-3495(03)74516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15:5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 25.Gorden P, Carpentier JL, Cohen S, Orci L. Epidermal growth factor: morphological demonstration of binding internalization and lydosomal association in human fibroblasts. Proc Nat Acad Sci U S A. 1978;75:5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanover JA, Willingham MC, Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- 27.Carpentier JL, Gorden P, Anderson RGW, Brown MS, Cohen S, Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982;95:73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorkina T, Huang F, Beguinot L, Sorkin A. Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of epidermal growth factor receptor. J Biol Chem. 2002;277:27433–27441. doi: 10.1074/jbc.M201595200. [DOI] [PubMed] [Google Scholar]
- 29.Stang E, Blystad FD, Kazazic M, Bertelsen V, Brodahl T, Raiborg C, Stenmark H, Madshus IH. Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol Biol Cell. 2004 doi: 10.1091/mbc.E04-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannessen LE, Pedersen NM, Pedersen KW, Madshus IH, Stang E. Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and grb2-containing clathrin-coated pits. Mol Cell Biol. 2006;26:389–401. doi: 10.1128/MCB.26.2.389-401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund KA, Opresko LK, Strarbuck C, Walsh BJ, Wiley HS. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J Biol Chem. 1990;265:13723–15713. [PubMed] [Google Scholar]
- 32.Damke H, Gossen M, Freundlieb S, Bujard H, Schmid SL. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 1995;257:209–220. doi: 10.1016/s0076-6879(95)57026-8. [DOI] [PubMed] [Google Scholar]
- 33.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- 35.Wiley HS. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988;107:801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X, Sorkin A. Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic. 2003;4:529–543. doi: 10.1034/j.1600-0854.2003.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- 37.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. From the cover: clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazazic M, Roepstorff K, Johannessen LE, Pedersen NM, van Deurs B, Stang E, Madshus IH. EGF-induced activation of the EGF receptor does not trigger mobilization of caveolae. Traffic. 2006;7:1518–1527. doi: 10.1111/j.1600-0854.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 39.Chinkers M, McKanna JA, Cohen S. Rapid induction of morphological changes in human carcinoma cells A-431 by epidermal growth factor. J Cell Biol. 1979;83:260–265. doi: 10.1083/jcb.83.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haigler HT, McKanna JA, Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A431. J Cell Biol. 1979;81:382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamazaki T, Zaal K, Hailey D, Presley J, Lippincott-Schwartz J, Samelson LE. Role of Grb2 in EGF-stimulated EGFR internalization. J Cell Sci. 2002;115:1791–1802. doi: 10.1242/jcs.115.9.1791. [DOI] [PubMed] [Google Scholar]
- 42.Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 2006;66:3603–3610. doi: 10.1158/0008-5472.CAN-05-2916. [DOI] [PubMed] [Google Scholar]
- 43.Chen WS, Lazar CS, Lund KA, Welsh JB, Chang CP, Walton GM, Der CJ, Wiley HS, Gill GN, Rosenfeld MG. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 1989;59:33–43. doi: 10.1016/0092-8674(89)90867-2. [DOI] [PubMed] [Google Scholar]
- 44.Sorkin A, Waters CM, Overholser KA, Carpenter G. Multiple autophosphorylation site mutations of the epidermal growth factor receptor. J Biol Chem. 1991;266:8355–8362. [PubMed] [Google Scholar]
- 45.Lamaze C, Schmid SL. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honegger AM, Dull TJ, Felder S, Obberghen EV, Bellot F, Szapary D, Schmidt A, Ullrich A, Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987;51:199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Villeneuve G, Wang Z. Control of epidermal growth factor receptor endocytosis by receptor dimerization, rather than receptor kinase activation. EMBO reports. 2005;6:942–948. doi: 10.1038/sj.embor.7400491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang X, Huang F, Marusyk A, Sorkin A. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol Biol Cell. 2003;14:858–870. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lund KA, Lazar CS, Chen WS, Walsh BJ, Welsh JB, Herbst JJ, Walton GM, Rosenfeld MG, Gill GN, Wiley HS. Phosphorylation of the epidermal growth factor receptor at threonine 654 inhibits ligand-induced internalization and down-regulation. J Biol Chem. 1990;265:20517–20523. [PubMed] [Google Scholar]
- 50.Countaway JL, Nairn AC, Davis RJ. Mechanism of desensitization of the epidermal growth factor receptor protein-tyrosine kinase. J Biol Chem. 1992;267:1129–1140. [PubMed] [Google Scholar]
- 51.Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. Embo J. 2006;25:5683–5692. doi: 10.1038/sj.emboj.7601457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vergarajauregui S, San Miguel A, Puertollano R. Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization. Traffic. 2006;7:686–698. doi: 10.1111/j.1600-0854.2006.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. Embo J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sorkin A, Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- 55.Nesterov A, Kurten RC, Gill GN. Association of epidermal growth factor receptors with coated pit adaptins via a tyrosine phosphorylation-regulated mechanism. J Biol Chem. 1995;270:6320–6327. doi: 10.1074/jbc.270.11.6320. [DOI] [PubMed] [Google Scholar]
- 56.Sorkin A, Mazzotti M, Sorkina T, Scotto L, Beguinot L. Epidermal growth factor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J Biol Chem. 1996;271:13377–13384. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- 57.Sorkin A, McKinsey T, Shih W, Kirchhausen T, Carpenter G. Stoichiometric interaction of the epidermal growth factor receptor with the clathrin-associated protein complex AP-2. J Biol Chem. 1995;270:619–625. doi: 10.1074/jbc.270.2.619. [DOI] [PubMed] [Google Scholar]
- 58.Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. Embo J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang F, Jiang X, Sorkin A. Tyrosine phosphorylation of the beta2 subunit of clathrin adaptor complex AP-2 reveals the role of a di-leucine motif in the epidermal growth factor receptor trafficking. J Biol Chem. 2003;278:43411–43417. doi: 10.1074/jbc.M306072200. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Moran MF. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1939. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- 61.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thien CB, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–166. doi: 10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1 [in process citation] Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 64.Umebayashi K, Stenmark H, Yoshimori T. Ubc4/5 and c-Cbl continue to ubiquitinate EGF receptor after internalization to facilitate polyubiquitination and degradation. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-10-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Melker AA, van der Horst G, Calafat J, Jansen H, Borst J. c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J Cell Sci. 2001;114:2167–2178. doi: 10.1242/jcs.114.11.2167. [DOI] [PubMed] [Google Scholar]
- 66.Thien CB, Walker F, Langdon WY. RING finger mutations that abolish c-Cbl-directed polyubiquitination and downregulation of the EGF receptor are insufficient for cell transformation. Mol Cell. 2001;7:355–365. doi: 10.1016/s1097-2765(01)00183-6. [DOI] [PubMed] [Google Scholar]
- 67.Huang F, Sorkin A. Growth factor receptor binding protein 2-mediated recruitment of the RING domain of Cbl to the epidermal growth factor receptor is essential and sufficient to support receptor endocytosis. Mol Biol Cell. 2005;16:1268–1281. doi: 10.1091/mbc.E04-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 69.Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL, Ghosh A, Fernandes N, Zhou P, Mullane-Robinson K, Rao N, Donoghue S, Rogers RA, Bowtell D, Naramura M, Gu H, Band V, Band H. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–28960. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- 70.Chang WL, Liou W, Pen HC, Chou HY, Chang YW, Li WH, Chiang W, Pai LM. The gradient of Gurken, a long-range morphogen, is directly regulated by Cbl-mediated endocytosis. Development. 2008;135:1923–1933. doi: 10.1242/dev.017103. [DOI] [PubMed] [Google Scholar]
- 71.Pai LM, Wang PY, Chen SR, Barcelo G, Chang WL, Nilson L, Schupbach T. Differential effects of Cbl isoforms on Egfr signaling in Drosophila. Mechanisms of development. 2006;123:450–462. doi: 10.1016/j.mod.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Yoon CH, Chang C, Hopper NA, Lesa GM, Sternberg PW. Requirements of multiple domains of SLI-1, a Caenorhabditis elegans homologue of c-Cbl, and an inhibitory tyrosine in LET-23 in regulating vulval differentiation. Mol Biol Cell. 2000;11:4019–4031. doi: 10.1091/mbc.11.11.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci U S A. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin NP, Mohney RP, Dunn S, Das M, Scappini E, O’Bryan JP. Intersectin regulates epidermal growth factor receptor endocytosis, ubiquitylation, and signaling. Mol Pharmacol. 2006;70:1643–1653. doi: 10.1124/mol.106.028274. [DOI] [PubMed] [Google Scholar]
- 75.Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- 76.Szymkiewicz I, Kowanetz K, Soubeyran P, Dinarina A, Lipkowitz S, Dikic I. CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases. J Biol Chem. 2002 doi: 10.1074/jbc.M205535200. [DOI] [PubMed] [Google Scholar]
- 77.Haglund K, Shimokawa N, Szymkiewicz I, Dikic I. Cbl-directed monoubiquitination of CIN85 is involved in regulation of ligand-induced degradation of EGF receptors. Proc Natl Acad Sci U S A. 2002;99:12191–12196. doi: 10.1073/pnas.192462299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A-431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hopkins CR, Miller K, Beardmore JM. Receptor-mediated endocytosis of transferrin and epidermal growth factor receptors: a comparison of constitutive and ligand-induced uptake. J Cell Sci. 1985;(Suppl 3):173–186. doi: 10.1242/jcs.1985.supplement_3.17. [DOI] [PubMed] [Google Scholar]
- 80.Miller K, Beardmore J, Kanety H, Schlessinger J, Hopkins CR. Localization of epidermal growth factor (EGF) receptor within the endosome of EGF-stimulated epidermoid carcinoma (A431) cells. J Cell Biol. 1986;102:500–509. doi: 10.1083/jcb.102.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dunn WA, Hubbard AC. Receptor-mediated endocytosis of epidermal growth factor by hepatocytes in the perfused rat liver: ligand and receptor dynamics. J Cell Biol. 1984;98:2148–2159. doi: 10.1083/jcb.98.6.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 83.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 84.Sorkin A, Teslenko L, Nikolsky N. The endocytosis of epidermal growth factor in A431 cells: a pH of microenvironment and the dynamics of receptor complexes dissociation. Exp Cell Res. 1988;175:192–205. doi: 10.1016/0014-4827(88)90266-2. [DOI] [PubMed] [Google Scholar]
- 85.Galperin E, Verkhusha VV, Sorkin A. Three-chromophore FRET micrsocopy to analyze multiprotein interactions in living cells. Nature Methods. 2004;1:209–217. doi: 10.1038/nmeth720. [DOI] [PubMed] [Google Scholar]
- 86.Di Gugliemo GM, Baass PC, Ou WJ, Posner B, Bergeron JJM. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorkin A, Carpenter G. Dimerization of internalized growth factor receptors. J Biol Chem. 1991;266:23453–23460. [PubMed] [Google Scholar]
- 88.McKanna JA, Haigler HT, Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci U S A. 1979;76:5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carpentier JL, White MF, Orci L, Kahn CR. Direct visualization of the phosphorylated epidermal growth factor receptor during its internalization in A-431 cells. J Cell Biol. 1987;105:2751–2762. doi: 10.1083/jcb.105.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murk JL, Humbel BM, Ziese U, Griffith JM, Posthuma G, Slot JW, Koster AJ, Verkleij AJ, Geuze HJ, Kleijmeer MJ. Endosomal compartmentalization in three dimensions: implications for membrane fusion. Proc Natl Acad Sci U S A. 2003;100:13332–13337. doi: 10.1073/pnas.2232379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sorkin A, Krolenko S, Kudrjavtceva N, Lazebnik J, Teslensko L, Soderquist AM, Nikolsky N. Recycling of epidermal growth factor-receptor complexes in A431 cells: identification of dual pathways. J Cell Biology. 1991;112:55–63. doi: 10.1083/jcb.112.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dickson RB, Hanover JA, Willingham MC, Pastan I. Prelysosomal divergence of transferrin and epidermal growth factor during receptor-mediated endocytosis. Biochemistry. 1983;22:5667–5674. doi: 10.1021/bi00293a033. [DOI] [PubMed] [Google Scholar]
- 93.Hopkins CR. Selective membrane protein trafficking: vectorial flow and filter. Trends Biochem Sci. 1992;17:27–32. doi: 10.1016/0968-0004(92)90423-7. [see comments] [DOI] [PubMed] [Google Scholar]
- 94.Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nickerson DP, Russell MR, Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO reports. 2007;8:644–650. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 97.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roxrud I, Raiborg C, Pedersen NM, Stang E, Stenmark H. An endosomally localized isoform of Eps15 interacts with Hrs to mediate degradation of epidermal growth factor receptor. J Cell Biol. 2008;180:1205–1218. doi: 10.1083/jcb.200708115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sachse M, Urbe S, Oorschot V, Strous GJ, Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol Biol Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malerod L, Stuffers S, Brech A, Stenmark H. Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007;8:1617–1629. doi: 10.1111/j.1600-0854.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 101.Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- 102.Hanson PI, Roth R, Lin Y, Heuser JE. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol. 2008;180:389–402. doi: 10.1083/jcb.200707031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shim S, Merrill SA, Hanson PI. Novel interactions of ESCRT-III with LIP5 and VPS4 and their implications for ESCRT-III disassembly. Mol Biol Cell. 2008;19:2661–2672. doi: 10.1091/mbc.E07-12-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- 105.Bishop N, Horman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol. 2002;157:91–102. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol. 2003;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bache KG, Stuffers S, Malerod L, Slagsvold T, Raiborg C, Lechardeur D, Walchli S, Lukacs GL, Brech A, Stenmark H. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol Biol Cell. 2006;17:2513–2523. doi: 10.1091/mbc.E05-10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, Luzio JP. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem. 2006;281:5094–5105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- 109.Raiborg C, Malerod L, Pedersen NM, Stenmark H. Differential functions of Hrs and ESCRT proteins in endocytic membrane trafficking. Exp Cell Res. 2008;314:801–813. doi: 10.1016/j.yexcr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 110.French AR, Tadaki NSKDK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J Biol Chem. 1995;270:4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- 111.Tsacoumangos A, Kil SJ, Ma L, Sonnichsen FD, Carlin C. A novel dileucine lysosomal-sorting-signal mediates intracellular EGF-receptor retention independently of protein ubiquitylation. J Cell Sci. 2005;118:3959–3971. doi: 10.1242/jcs.02527. [DOI] [PubMed] [Google Scholar]
- 112.Kurten RC, Cadena DL, Gill GN. Enhanced degradation of EGF receptors by a sorting nexin, SNX-1. Science. 1996;272:1008–1010. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- 113.White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. Embo J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carpenter G. Nuclear localization and possible functions of receptor tyrosine kinases. Curr Opin Cell Biol. 2003;15:143–148. doi: 10.1016/s0955-0674(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 115.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 116.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 117.Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC, Hung MC. Nuclear-cytoplasmic transport of EGFR involves receptor endocytosis, importin beta1 and CRM1. J Cell Biochem. 2006;98:1570–1583. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- 118.Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell. 2007;18:1064–1072. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rubin C, Gur G, Yarden Y. Negative regulation of receptor tyrosine kinases: unexpected links to c-Cbl and receptor ubiquitylation. Cell Res. 2005;15:66–71. doi: 10.1038/sj.cr.7290268. [DOI] [PubMed] [Google Scholar]
- 120.Wong ES, Fong CW, Lim J, Yusoff P, Low BC, Langdon WY, Guy GR. Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. Embo J. 2002;21:4796–4808. doi: 10.1093/emboj/cdf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rubin C, Litvak V, Medvedovsky H, Zwang Y, Lev S, Yarden Y. Sprouty fine-tunes EGF signaling through interlinked positive and negative feedback loops. Curr Biol. 2003;13:297–307. doi: 10.1016/s0960-9822(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 122.Hanafusa H, Torii S, Yasunaga T, Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 123.Feng Q, Baird D, Peng X, Wang J, Ly T, Guan JL, Cerione RA. Cool-1 functions as an essential regulatory node for EGF receptor- and Src-mediated cell growth. Nat Cell Biol. 2006;8:945–956. doi: 10.1038/ncb1453. [DOI] [PubMed] [Google Scholar]
- 124.Wainszelbaum MJ, Charron AJ, Kong C, Kirkpatrick DS, Srikanth P, Barbieri MA, Gygi SP, Stahl PD. The hominoid-specific oncogene TBC1D3 activates Ras and modulates epidermal growth factor receptor signaling and trafficking. J Biol Chem. 2008;283:13233–13242. doi: 10.1074/jbc.M800234200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schmidt MH, Hoeller D, Yu J, Furnari FB, Cavenee WK, Dikic I, Bogler O. Alix/AIP1 antagonizes epidermal growth factor receptor downregulation by the Cbl-SETA/CIN85 complex. Mol Cell Biol. 2004;24:8981–8993. doi: 10.1128/MCB.24.20.8981-8993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tanos B, Pendergast AM. Abl tyrosine kinase regulates endocytosis of the epidermal growth factor receptor. J Biol Chem. 2006;281:32714–32723. doi: 10.1074/jbc.M603126200. [DOI] [PubMed] [Google Scholar]
- 127.Kowanetz K, Crosetto N, Haglund K, Schmidt MH, Heldin CH, Dikic I. Suppressors of T-cell receptor signaling Sts-1 and Sts-2 bind to Cbl and inhibit endocytosis of receptor tyrosine kinases. J Biol Chem. 2004;279:32786–32795. doi: 10.1074/jbc.M403759200. [DOI] [PubMed] [Google Scholar]
- 128.Gur G, Rubin C, Katz M, Amit I, Citri A, Nilsson J, Amariglio N, Henriksson R, Rechavi G, Hedman H, Wides R, Yarden Y. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. Embo J. 2004;23:3270–3281. doi: 10.1038/sj.emboj.7600342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kario E, Marmor MD, Adamsky K, Citri A, Amit I, Amariglio N, Rechavi G, Yarden Y. Suppressors of cytokine signaling 4 and 5 regulate epidermal growth factor receptor signaling. J Biol Chem. 2005;280:7038–7048. doi: 10.1074/jbc.M408575200. [DOI] [PubMed] [Google Scholar]
- 130.Su X, Kong C, Stahl PD. GAPex-5 mediates ubiquitination, trafficking, and degradation of epidermal growth factor receptor. J Biol Chem. 2007;282:21278–21284. doi: 10.1074/jbc.M703725200. [DOI] [PubMed] [Google Scholar]
- 131.Miura GI, Roignant JY, Wassef M, Treisman JE. Myopic acts in the endocytic pathway to enhance signaling by the Drosophila EGF receptor. Development. 2008;135:1913–1922. doi: 10.1242/dev.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tashiro K, Konishi H, Sano E, Nabeshi H, Yamauchi E, Taniguchi H. Suppression of the ligand-mediated down-regulation of epidermal growth factor receptor by Ymer, a novel tyrosine-phosphorylated and ubiquitinated protein. J Biol Chem. 2006;281:24612–24622. doi: 10.1074/jbc.M604184200. [DOI] [PubMed] [Google Scholar]
- 133.Bakowska JC, Jupille H, Fatheddin P, Puertollano R, Blackstone C. Troyer syndrome protein spartin is mono-ubiquitinated and functions in EGF receptor trafficking. Mol Biol Cell. 2007;18:1683–1692. doi: 10.1091/mbc.E06-09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen F, Lin Q, Gu Y, Childress C, Yang W. Activated Cdc42-associated kinase 1 is a component of EGF receptor signaling complex and regulates EGF receptor degradation. Mol Biol Cell. 2007;18:732–742. doi: 10.1091/mbc.E06-02-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Urbe S, McCullough J, Row P, Prior IA, Welchman R, Clague MJ. Control of growth factor receptor dynamics by reversible ubiquitination. Biochem Soc Trans. 2006;34:754–756. doi: 10.1042/BST0340754. [DOI] [PubMed] [Google Scholar]
- 136.McCullough J, Clague MJ, Urbe S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol. 2004;166:487–492. doi: 10.1083/jcb.200401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell. 2005;16:5163–5174. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol. 2006;16:551–559. doi: 10.1016/j.tcb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 139.Row PE, Liu H, Hayes S, Welchman R, Charalabous P, Hofmann K, Clague MJ, Sanderson CM, Urbe S. The MIT domain of UBPY constitutes a CHMP binding and endosomal localization signal required for efficient epidermal growth factor receptor degradation. J Biol Chem. 2007;282:30929–30937. doi: 10.1074/jbc.M704009200. [DOI] [PubMed] [Google Scholar]
- 140.Alwan HA, van Leeuwen JE. UBPY-mediated EGFR deubiquitination promotes EGFR degradation. J Biol Chem. 2006 doi: 10.1074/jbc.M604711200. [DOI] [PubMed] [Google Scholar]
- 141.Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem. 2006;281:12618–12624. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- 142.Niendorf S, Oksche A, Kisser A, Lohler J, Prinz M, Schorle H, Feller S, Lewitzky M, Horak I, Knobeloch KP. Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol Cell Biol. 2007;27:5029–5039. doi: 10.1128/MCB.01566-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Z, Zhang L, Yeung TK, Chen X. Endocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulation. Mol Biol Cell. 1999;10:1621–1636. doi: 10.1091/mbc.10.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Longva KE, Pedersen NM, Haslekas C, Stang E, Madshus IH. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int J Cancer. 2005;116:359–367. doi: 10.1002/ijc.21015. [DOI] [PubMed] [Google Scholar]
- 145.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. Embo J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Worthylake R, Wiley HS. Structural aspects of the epidermal growth factor receptor required for transmodulation of erbB-2/neu. J Biol Chem. 1997;272:8594–8601. doi: 10.1074/jbc.272.13.8594. [DOI] [PubMed] [Google Scholar]
- 147.Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274:8865–8874. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
- 148.Kornilova ES, Taverna D, Hoeck W, Hynes NE. Surface expression of erbB-2 protein is post-transcriptionally regulated in mammary epithelial cells by epidermal growth factor and by the culture density. Oncogene. 1992;7:511–519. [PubMed] [Google Scholar]
- 149.Haslekas C, Breen K, Pedersen KW, Johannessen LE, Stang E, Madshus IH. The inhibitory effect of ErbB2 on epidermal growth factor-induced formation of clathrin-coated pits correlates with retention of epidermal growth factor receptor-ErbB2 oligomeric complexes at the plasma membrane. Mol Biol Cell. 2005;16:5832–5842. doi: 10.1091/mbc.E05-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lenferink AEG, Pinkas-Kramarski R, van de Poll MLM, van Vugt MJH, Klapper LN, Tzahar E, Waterman H, Sela M, van Zoelen JJ, Yarden Y. Differential endocytic routing of momo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Offterdinger M, Bastiaens PI. Prolonged EGFR signaling by ERBB2-mediated sequestration at the plasma membrane. Traffic. 2008;9:147–155. doi: 10.1111/j.1600-0854.2007.00665.x. [DOI] [PubMed] [Google Scholar]
- 152.Hommelgaard AM, Lerdrup M, van Deurs B. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol Biol Cell. 2004;15:1557–1567. doi: 10.1091/mbc.E03-08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adelaide J, Margolis B, Birnbaum D. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol. 2000;2:407–414. doi: 10.1038/35017038. [DOI] [PubMed] [Google Scholar]
- 154.Jaulin-Bastard F, Saito H, Le Bivic A, Ollendorff V, Marchetto S, Birnbaum D, Borg JP. The ERBB2/HER2 receptor differentially interacts with ERBIN and PICK1 PSD-95/DLG/ZO-1 domain proteins. J Biol Chem. 2001;276:15256–15263. doi: 10.1074/jbc.M010032200. [DOI] [PubMed] [Google Scholar]
- 155.Tikhomirov O, Carpenter G. Geldanamycin induces ErbB-2 degradation by proteolytic fragmentation. J Biol Chem. 2000;275:26625–26631. doi: 10.1074/jbc.M003114200. [DOI] [PubMed] [Google Scholar]
- 156.Lerdrup M, Bruun S, Grandal MV, Roepstorff K, Kristensen MM, Hommelgaard AM, van Deurs B. Endocytic down-regulation of ErbB2 is stimulated by cleavage of its C-terminus. Mol Biol Cell. 2007;18:3656–3666. doi: 10.1091/mbc.E07-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Levkowitz G, Klapper LN, Tzahar E, Freywald A, Sela M, Yarden Y. Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene. 1996;12:1117–1125. [PubMed] [Google Scholar]
- 158.Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. J Biol Chem. 2003;278:23343–23351. doi: 10.1074/jbc.M300477200. [DOI] [PubMed] [Google Scholar]
- 159.Gilboa L, Ben-Levy R, Yarden Y, Henis YI. Roles for a cytoplasmic tyrosine and tyrosine kinase activity in the interactions of Neu receptors with coated pits. J Biol Chem. 1995;270:7061–7067. doi: 10.1074/jbc.270.13.7061. [DOI] [PubMed] [Google Scholar]
- 160.Klapper LN, Waterman H, Sela M, Yarden Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 2000;60:3384–3388. [PubMed] [Google Scholar]
- 161.Chan R, Hardy WR, Dankort D, Laing MA, Muller WJ. Modulation of Erbb2 signaling during development: a threshold level of Erbb2 signaling is required for development. Development. 2004;131:5551–5560. doi: 10.1242/dev.01425. [DOI] [PubMed] [Google Scholar]
- 162.Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G, Wang SC, Hung MC. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25:11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Omerovic J, Santangelo L, Puggioni EM, Marrocco J, Dall’Armi C, Palumbo C, Belleudi F, Di Marcotullio L, Frati L, Torrisi MR, Cesareni G, Gulino A, Alimandi M. The E3 ligase Aip4/Itch ubiquitinates and targets ErbB-4 for degradation. Faseb J. 2007;21:2849–2862. doi: 10.1096/fj.06-7925com. [DOI] [PubMed] [Google Scholar]
- 164.Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem. 1998;273:13819–13827. doi: 10.1074/jbc.273.22.13819. [DOI] [PubMed] [Google Scholar]
- 165.Baulida J, Kraus MH, Alimandi C, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 166.Baulida J, Carpenter G. Heregulin degradation in the absence of rapid receptor-mediated internalization. Exp Cell Res. 1997;232:167–172. doi: 10.1006/excr.1997.3515. [DOI] [PubMed] [Google Scholar]
- 167.Liu Y, Tao YM, Woo RS, Xiong WC, Mei L. Stimulated ErbB4 internalization is necessary for neuregulin signaling in neurons. Biochem Biophys Res Commun. 2007;354:505–510. doi: 10.1016/j.bbrc.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Longart M, Chatani-Hinze M, Gonzalez CM, Vullhorst D, Buonanno A. Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons. Brain Res Bull. 2007;73:210–219. doi: 10.1016/j.brainresbull.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Waterman H, Alroy I, Strano S, Seger R, Yarden Y. The C-terminus of the kinase-defective neuregulin receptor ErbB-3 confers mitogenic superiority and dictates endocytic routing. Embo J. 1999;18:3348–3358. doi: 10.1093/emboj/18.12.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qiu XB, Goldberg AL. Nrdp1/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc Natl Acad Sci USA. 2002;99:14843–14848. doi: 10.1073/pnas.232580999. [DOI] [PMC free article] [PubMed] [Google Scholar]