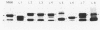Abstract
Lipooligosaccharides (LOS) of the eight immunotypes found in serogroup B Neisseria meningitidis were purified from their prototype strains grown in tryptic soy broth. Rabbit antisera to these LOS were prepared. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by silver staining revealed that most of the LOS antigens contained two major components; the larger components had apparent molecular weights (Mrs) in the range of 4,800 +/- 300, and the smaller components had an apparent Mr of 4,300. Immunoblot analysis showed that the larger major component of an LOS, in general, was much more immunogenic because the rabbits produced antibodies exclusively or primarily to this component even though the LOS immunogen contained both large and small major components. Antibodies to the smaller 4,300-Mr components were infrequently observed but, when present, were cross-reactive with the same-size components of all heterologous LOS. Hence, the immunotype epitopes reside in the larger major components of all immunotypes except type 5, in which a smaller major component having an apparent Mr of 4,400 carries the epitope. Rabbit antisera to types 1, 5, and 6 were immunotype specific. Antisera to other types had cross-reactivities with some heterologous LOS, and the larger components, but not the 4,300-Mr components, of the LOS were primarily responsible for the cross-reactivities. This finding suggests that the larger components of cross-reactive LOS have a similar structure in addition to their type-specific sugar moieties. The LOS of N. meningitidis M986, a strain used for the production of a serotype 2a vaccine, was found to contain the immunotype 7 epitope.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRANHAM S. E. Serological relationships among meningococci. Bacteriol Rev. 1953 Sep;17(3):175–188. doi: 10.1128/br.17.3.175-188.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. I. Serological typing by a microbactericidal method. Infect Immun. 1972 Jan;5(1):98–102. doi: 10.1128/iai.5.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Leive L., Mäkelä P. H., Rietschel E. T., Strittmatter W., Morrison D. C. Lipopolysaccharide nomenclature--past, present, and future. J Bacteriol. 1986 Jun;166(3):699–705. doi: 10.1128/jb.166.3.699-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. I., Jr, Wunderlich A. C., Lyons J., Davis C. E., Guiney D. G., Braude A. I. Role of magnesium in the enzyme-linked immunosorbent assay for lipopolysaccharides of rough Escherichia coli strain J5 and Neisseria gonorrhoeae. J Infect Dis. 1980 Oct;142(4):532–537. doi: 10.1093/infdis/142.4.532. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Bhattacharjee A. K., Kenne L., Kenny C. P., Calver G. The R-type lipopolysaccharides of Neisseria meningitidis. Can J Biochem. 1980 Feb;58(2):128–136. doi: 10.1139/o80-018. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., van Boxtel R., de Jong M. Atrophic rhinitis in swine: correlation of Pasteurella multocida pathogenicity with membrane protein and lipopolysaccharide patterns. Infect Immun. 1984 Oct;46(1):48–54. doi: 10.1128/iai.46.1.48-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977 May;16(2):471–475. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]