Abstract
We investigated hand function in mildly involved Multiple Sclerosis (MS) patients (N = 16; EDSS 1−5, 9-hole peg test 14−32 s) during static and dynamic manipulation tasks using an instrumented device. When compared with healthy controls (N = 16), the patients revealed impaired task performance regarding their ability to exert prescribed patterns of load force (L; force acting tangentially at the digits-object surface). Regarding the coordination of grip force (G; normal component) and L, the data only revealed elevated G/L ratio, although both the G and L coupling (maximum correlation coefficients and the time lags between them), and G modulation (gain and offset of G with respect to L) remained comparable in the two groups. Finally, most of the data suggested no MS-specific effects of switching from uni- to bimanual tasks, from available visual feedback to deprived feedback conditions. We conclude that the deterioration in the ability for precise control of external forces and overgripping could precede the decoupling of G and L and decreased G modulation in early phases of the disease. The results also suggest that the applied methodology could be sensitive enough to detect mild levels of impairment of hand function in MS and, possibly, other neurological diseases.
Keywords: hand function, grip, load, force coordination, coupling, task performance
The main hand function is to grasp and manipulate objects and external supports. This function is essential for everyday life, and, consequently, an impaired hand function (e.g., associated with neurological diseases or injuries) could be an important obstacle to living an independent life. When an externally fixed (e.g., a handle providing external support) or a free-moving object (a cup of coffee, or a tool) is held with the opposing tips of the thumb and fingers, a load force (L) is applied tangentially to the surfaces in contact to move the object or exert a reaction force. The slippage of the object is countered by friction force originating from grip force (G) acting normally to the surface. A consistent finding throughout the literature has been that the changes in G and L appear to be highly coordinated. This coordination is based not only on an ability of the neural controlling mechanisms to assess the skin-object friction coefficient and, based on it, apply a relatively low safety margin [i.e., G slightly higher than necessary to prevent slippage; (Johansson & Westling, 1984)], but also to modulate G in parallel with L without any considerable time delays between them [i.e., using anticipatory mechanisms (Flanagan & Wing, 1995, 1997)]. The result appears to be a stable and relatively low grip-to-load (G/L) ratio (Johansson, 1998), high coupling of G and L (as assessed by both high correlation coefficient and virtually no time lags between them [Blakemore, Goodbody, & Wolpert, 1998; Flanagan & Tresilian, 1994]), and a high level of modulation of G with respect to changes in L [i.e., high gain and low offset assessed through the slope and intercept, respectively, of the regression lines obtained from G-L diagrams (Flanagan, Tresilian, & Wing, 1993)].
A deterioration of the G and L coordination has been consistently seen in populations characterized by a prominent impairment of hand function, such as in neurological patients. This deterioration has been identified by an elevated G/L ratio, decreased coupling of G and L, or reduced modulation of G. For example, children with congenital hemiplegia exhibit poor control of grip because of both an impaired anticipatory scaling and abnormal timing of G and L coupling (Duque et al., 2003). Parkinson's patients also demonstrate excessive G and a prolonged adjustment of G to changes in L (Ingvarsson, Gordon, & Forssberg, 1997). Patients with cerebellar atrophy apply both an exaggerated G and exhibit a disrupted coupling of G and L, although their anticipatory control mechanisms are mainly preserved (Rost, Nowak, Timmann, & Hermsdorfer, 2005). An inaccurate temporal coupling of G and L has been also seen in motor neuron disease (Nowak, Hermsdorfer, & Topka, 2003). Stroke patients with subcortical lesions demonstrate both an elevated G and impaired anticipatory control that affect G and L coupling (Nowak & Hermsdorfer, 2003), although stroke patients with cerebral lesions appear to have preserved the feed-forward mechanism for anticipatory G control, although G remains elevated and partly decoupled from L (Hermsdorfer, Hagl, Nowak, & Marquardt, 2003). Nevertheless, there is a general lack of data regarding specific aspects of impairment of hand function in various neurological populations. For example, although most of the authors have focused on the previously discussed coordination of G and L, the ability to exert an accurate pattern of L needed for accurate manipulation of an external object or using external support has been mainly neglected. Changes in force coordination associated with the progress of neural diseases are also largely unknown.
Multiple sclerosis (MS) is the most common autoimmune, inflammatory, demyelinating disease of the central nervous system (CNS). MS is characterized by a combination of damage to both gray matter and white matter, with an ensuing loss of tissue leading to cortical atrophy. The neurological symptoms include sensory disturbances, motor impairment, intention tremor, ataxia, memory loss, postural imbalance, and impaired motor coordination (Matthews, 1991). The clinical symptoms of MS are highly variable depending on the site and extent of CNS involvement. However, one of the common and important clinical features is impaired hand function caused by deterioration of hand dexterity (Feys, Duportail, Kos, Van Asch, & Ketelaer, 2002).
In our recent study we tested mildly involved MS patients (EDSS 1.5−4) in a variety of bimanual static tasks, looking for disease-specific aspects of impairment of hand function (Marwaha, Hall, Knight, & Jaric, 2006). When compared with healthy controls, the patients revealed an elevated G/L ratio and lower task performance regarding the accuracy of the instructed L profile, although both the coupling of G and L and modulation of G were preserved. However, several potentially important aspects of the hand function remained unexplored. For example, the corpus callosum plays a major role in interhemispheric transfer and integration of sensorimotor information in bimanual movements (Seymour, Reuter-Lorenz, & Gazzaniga, 1994), and it appears to be affected frequently even in mildly involved MS patients (Mendez, 1995; Tsolaki et al., 1994). Therefore, it remains possible that the MS patients could reveal a noticeable deterioration in bimanual manipulation tasks. It has also been shown that in MS patients both the intention tremor (i.e., voluntary withering in an attempt to coordinate delicate movements) and aiming errors can increase with the presence of visual feedback (Feys, Helsen, Lavrysen, Nuttin, & Ketelaer, 2003; Feys et al., 2003; Feys et al., 2005; Mitoma, 1996; Schenk, Walther, & Mai, 2000). Since the opposite result could be expected from a healthy population, the availability of visual feedback when producing a prescribed L profile could have different effects on MS patients than on healthy individuals. Finally, we have recently shown that the coordination of G and L in static manipulation tasks (Jaric, Collins, Marwaha, & Russell, 2006) could be less affected by task complexity than the same coordination in tasks performed with free-moving objects (c.f., Zatsiorsky, Gao, & Latash, 2005). Therefore, testing of both static and dynamic manipulation tasks could be needed for a more complete assessment of hand function in various populations.
The main aim of our study was to assess specific aspects of impairment of hand function in mildly involved MS patients in both static and dynamic manipulation tasks. Specifically, we assessed hand function through the indices of both task performance (i.e., the ability to exert the prescribed L profile) and force coordination reflected through low G/L ratio, a high coupling of G and L, and a high modulation of G (see previous text for details). We hypothesized that even the mildly involved patients will demonstrate impaired task performance and decreased force coordination when compared with healthy controls. When compared with our previous study, performed on a similar group of MS patients (Marwaha, Hall, Knight, & Jaric, 2006), the present one has been extended from purely static to both static and dynamic tasks. Specifically, we expected that the hypothesized differences would be more prominent in the tasks performed bimanually rather than unimanually, as well as in the tasks in which visual feedback is available. If the results support the hypotheses, the findings would not only reveal the specific aspects of impairment of hand function in mildly impaired MS patients, but also speak in favor of the applied approach as a methodology sensitive enough to detect a mild level of impairment of hand function in MS. The latter could be of particular importance due to a general lack of objective quantitative methods for assessment of hand function in MS, as well as in other neurological diseases (Jaric, Knight, Collins, & Marwaha, 2005).
Materials and Methods
Subjects
Sixteen MS patients (12 females and 4 males) aged between 30 and 58 years (mean ± SD 45.1 ± 9.4 years) and an equal number of age (range 31 to 55, 43.4 ± 7.5 years) and gender matched healthy controls participated in the study. The proportion of males and females was selected because of the higher prevalence (60 to 75%) of MS in females (Whitacre, 2001). MS patients were recruited through the Delaware chapter of the National Multiple Sclerosis Society, as well as from the MS Clinic at the Physical Therapy Department of the University of Delaware, while healthy controls were recruited by public advertisement. All MS patients were right handed, although two controls were left handed, as assessed by the Edinburgh Scale (Oldfield, 1971). The experimental procedure was conducted in accordance with the Helsinki Declaration, and the subjects provided their informed consent according to the procedures approved by the Human Subjects Review Board of the University of Delaware.
An experienced neurologist screened the MS patients. All patients met the following inclusion criteria in order to control for the heterogeneity of expression and symptomatology of the disease: They had relapsing-remitting type of MS, the patients were also without signs of cognitive impairment and all were capable of independent living, they had normal or corrected to normal vision, the Expanded Disability Status Scale [EDSS (Kurtzke, 1983)] score was 5 or less, and the 9-hole peg test (Grice et al., 2003) time was less than 40 s. Patients were excluded if their history suggested drug or alcohol abuse, psychiatric, or any other concurrent medical problems, or if they were unable to follow simple instructions. The EDSS and 9-hole peg test were performed on the MS patients, while only the 9-hole peg test was performed on healthy controls.
Experimental Device and Procedure
The experimental device used in the study (see Figure 1A) consisted of two externally fixed vertical handles covered with rubber with a 3 cm aperture and positioned 13 cm apart. Two force transducers (miniature single-axis strain gauge load cells WMC-50, Interface Inc.) allowed for simultaneous recording of grip force (G) of each hand applied against the handles. An additional pair of multi-access force transducers that was positioned below each handle (Mini40, ATI, Apex, NC) was used to record load forces (L) exerted in a vertical direction. The device was positioned in front of the standing participant, and its height was individually adjusted for each of them. In the static manipulation tasks, the device was externally fixed. In the dynamic manipulation (i.e., lifting task), the device was detached, and additional weights in steps of 100 g of mass served to adjust the total weight to the selected level of L (see further text for details). Although due to the cross-sectional approach applied in the study the minimum individual G/L ratios were not necessary to assess, a pilot study revealed the ratio (average across four subjects) of about 0.40.
Figure 1.
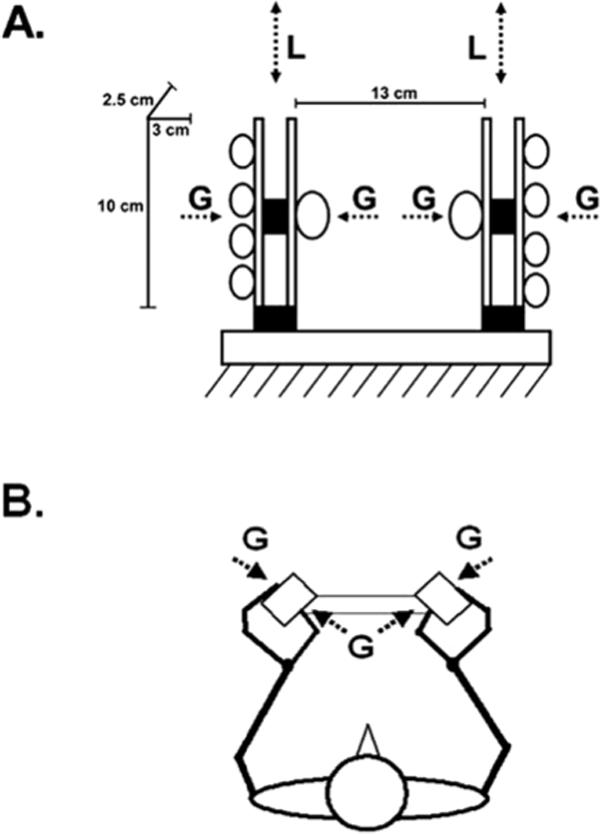
(A) Schematic representation of the experimental device. The circles illustrate the position of the tips of all five digits applying precision grasp against two handles. On the left hand side the dimensions of the opposing grasping surfaces and the distance between them is depicted. The lower shaded rectangles illustrate the force sensors recording the instructed load force (L) exerted in vertical direction, while the upper ones recorded the grip force (G). (B) The stick diagram illustrates the horizontal projection of the body position and hand position, as well as the corresponding G.
The experimental procedure was conducted within a single session. The sessions were performed in the morning to minimize the effects of fatigue that are commonly reported by MS patients later in the day. The subjects were asked to keep their upper arms in a neutral position (held close to the body), elbows at 90° of flexion, and the forearms in the midprone position. The handles were rotated 45° (see Figure 1B) to allow comfortable wrist and finger joint positions that correspond to a number of everyday tasks. The handles were grasped by using the precision grip exerted from the opposite sides by the tips of the thumb and all four fingers.
As the first step of the experimental procedure the maximum pinch grip force (G) of each hand was recorded. About 10% of the maximum pinch G of the weaker hand served as the instructed peak L in the main experiment. According to our previous findings, this level was well below the one that could cause fatigue over the course of the experiment (Jaric, Knight, Collins, & Marwaha, 2005). As a result, the maximum level of the prescribed L was participant specific and ranged from 4 to 10 N. Thereafter, the tasks were explained and demonstrated, and the participants practiced the tasks over approximately 15 min. Note that G was not mentioned through the entire experiment. Finally, the subjects were tested on three different manipulation tasks including one performed under dynamic (lifting task) and two under static conditions (ramp-and-hold and oscillatory task) in which they were instructed to produce different L patterns. The three experimental tasks were performed in random sequence. Prior to the main experiment the participants cleaned their hands with an alcohol swab and dried them with paper tissues.
To extend our previous study from purely static to dynamic manipulation tasks (see the introduction for details), we performed the lifting task that closely corresponded to the natural grasping and lifting of an object. In particular, the subjects were instructed to prepare their hands for grasping, placing the tips of the digits close to the grasping surfaces without touching them, grasp the handles, lift them approximately an inch (2.5 cm), and hold them as steady as possible, and put them back on the table. All three steps were cued by auditory beeps, and the intervals between the consecutive steps were 3 s. The task was performed either unimanually or bimanually. A total of nine trials were performed under three hand conditions (left hand only, right hand only, and both hands), and the sequence of the trials was fully randomized.
Static manipulations included the ramp-and-hold task and the oscillation task. Both tasks were performed bimanually against externally fixed handles. In the ramp-and-hold task, subjects were asked to match a prescribed load force (L) profile by pulling both handles up. The profile included the following three segments lasting four seconds each: 0 L, increasing ramp L, and constant L. Four computer-generated beeps indicated the beginning and end of each segment. In the oscillatory task, the participants were instructed to exert an oscillatory pattern of L by pulling upward against the handles (pull up and relax) paced by a metronome set at 1.33 Hz. According to our previous findings, this frequency was within the comfortable range (Jaric, Collins, Marwaha, & Russell, 2006). The duration of each trial was 12 s (i.e., approximately 16 periods).
Both static manipulation tasks were performed with and without visual feedback. Under the visual feedback condition, a computer monitor placed in front of the participants showed either the prescribed profile (in the ramp-and-hold task) or two horizontal lines depicting the upper and lower level for the oscillatory force profile, as well as the current average L exerted by the subject's two hands. The trials were performed in pairs so that the trial with visual feedback was immediately followed by a trial without visual feedback in which the subject was asked to repeat the same L profile exerted within the preceding trial. Thereafter, the exerted L profile of the second (i.e., without visual feedback) trial was shown to the subject to provide the information about his or her performance. A total number of six trials of both the ramp and oscillation tasks were performed, and the data from the last two were collected for further analysis.
Data Processing
G and L signals from all four transducers were A/D converted and recorded at a rate of 200 per s. Two custom-made LabView (National Instruments, Austin, TX, USA) programs were used for data acquisition and processing. L and G signals for each hand were low-pass filtered at 10 Hz with a fourth-order Butterworth filter. In the lifting task, the lifting phase (from 0.5 s before to 0.5 s after the instant of 50% of the maximum L) and the holding phase (the following 1.5 s) were analyzed. In the ramp-and-hold task, the 4 s of the ramp and the middle 2 s of the holding phase were separately analyzed. Finally, to avoid analyzing both the initial adjustment and a possible effect of the end of the trial, the first 6 s and the last 1 s were skipped from the oscillation task, and the remaining 5 s between them were analyzed.
Since the prescribed maximum L was selected individually for each subject, the task performance of both the lifting task and ramp-and-hold task was assessed from the holding phase by the coefficient of variation (CV) of L. This measure presumably assessed the subjects’ ability to keep an object in a steady position (lifting task) or exert the prescribed constant load force (ramp-and-hold task). The task performance of the oscillation task was assessed by variable errors calculated as the standard deviations of the L peaks.
Force coordination was assessed through a number of variables already found in the literature. To evaluate the magnitude of applied G relative to the magnitude of L exerted during a particular task, the averaged G force and L force were assessed either from the holding phases (the lifting and ramp-and-hold task) or from all 5 s of the analyzed segment of the oscillation task. Thereafter, the G/L ratio was calculated. In line with previous studies, lower G/L was interpreted as an index of better coordination of G and L (Johansson & Westling, 1988; Rost, Nowak, Timmann, & Hermsdorfer, 2005). Since no difference was found between the dominant and nondominant hand when performing the experimental tasks, the indices of coordination for two hands (see further text for their description) were averaged prior to the statistical analysis.
The coupling of G and L in the lifting task was assessed by cross-correlation of the first derivatives of G (dG/dt) and L (dL/dt) recorded during the lifting phase (see Duque et al., 2003, for a similar approach). In the ramp-and-hold task (the ramp phase) and oscillation task, the coupling of G and L was assessed by calculating the maximum cross-correlation coefficients between the time series of G and L (Flanagan & Tresilian, 1994; Marwaha, Hall, Knight, & Jaric, 2006). However, similar to the findings of previous studies (Jaric, Collins, Marwaha, & Russell, 2006; Jaric, Russell, Collins, & Marwaha, 2005), the time lags appeared to be exceptionally small and inconsistent for all three tasks, demonstrating no significant effects even at the level of p < .1. Therefore, the maximum correlation coefficients alone were taken for further analysis (see Marwaha, Hall, Knight, & Jaric, 2006, for a similar approach). In general, the higher correlation coefficients were interpreted as higher coordination of G and L.
In line with a number of previous studies, the modulation of G with respect to changes in L has been interpreted as an index of force coordination (Flanagan, Tresilian, & Wing, 1993; Jaric, Collins, Marwaha, & Russell, 2006; Zatsiorsky, Gao, & Latash, 2005). The modulation was assessed by the regression lines calculated from G-L diagrams (see Figures 3B and 4B for illustration), and the associated slope and intercept have been interpreted as gain and offset of G (Flanagan, Tresilian, & Wing, 1993). As a result, highly modulated G was expected to reveal a high gain and low offset.
Figure 3.
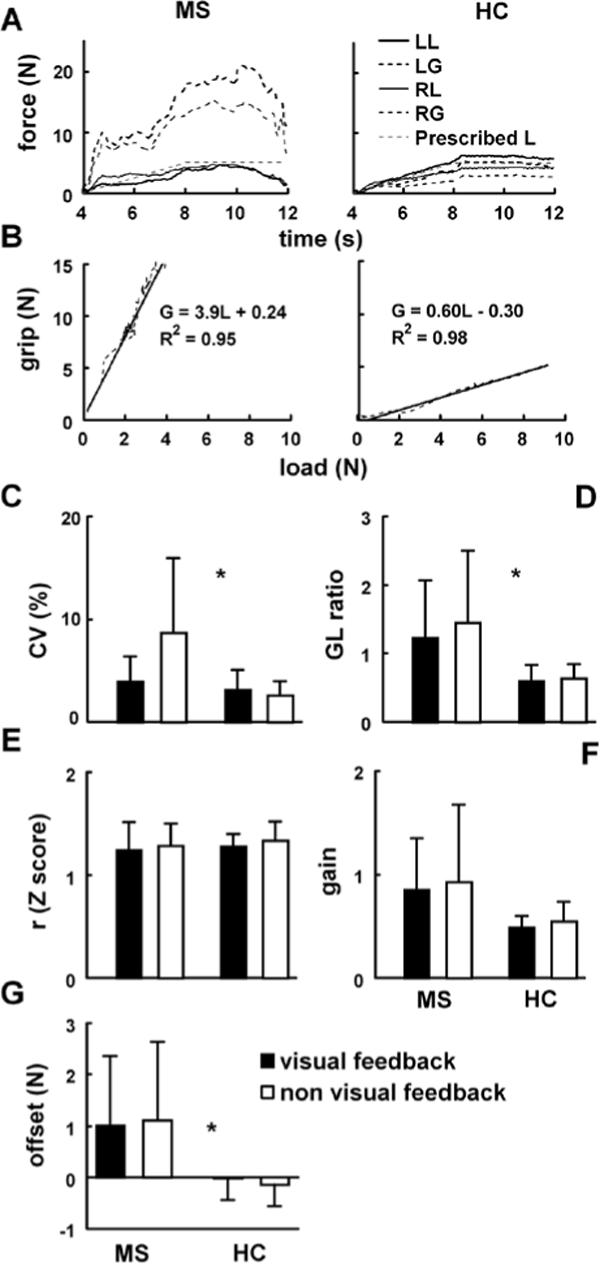
(A) Right (RG) and left grip (LG), and right (RL) and left load (LL) forces recorded in the ramp-and-hold task in a representative MS patient and a healthy control. (B) Grip-load diagrams represent the data depicted in Figure 3A together with the regression lines and the corresponding coefficients of determination (R2). The remaining graphs illustrate averaged across the subjects coefficients of variation (CV;) C), G/L ratios (D), Z-transformed correlation coefficients (E), and gain (F) and offset (G) that correspond to the slopes and intercepts of the regression lines. Error bars represent standard deviations.
Figure 4.
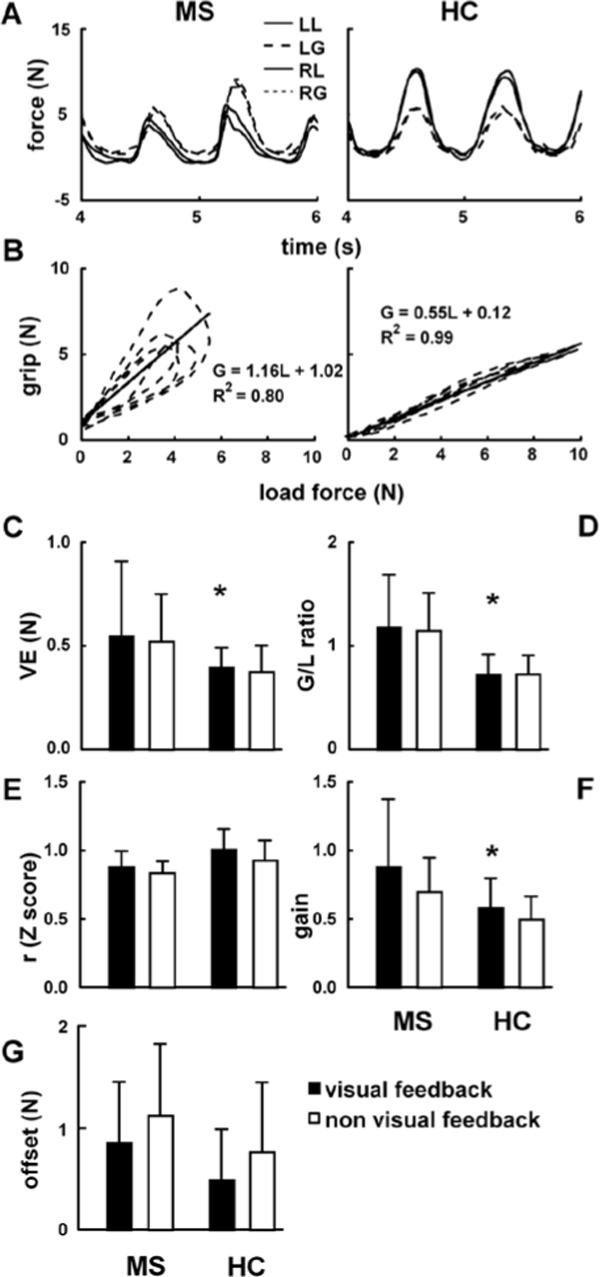
(A) Right (RG) and left grip (LG), and right (RL) and left load (LL) forces recorded in the static oscillation task in a representative MS patient and healthy control. (B) Grip-load diagrams represent the data depicted in Figure 3A together with the regression lines and the corresponding coefficients of determination (R2). The remaining graphs illustrate the averaged across the subjects’ coefficients of variation (CV (C), I/I ratios (D), Z-transformed correlation coefficients (E), and gain (F) and offset (G) that correspond to the slopes and intercepts of the regression lines. Error bars represent standard deviations.
Statistical Analysis
In the lifting task, a mixed two-way ANOVA (Group × Hand and Group × Force) were used where the group (MS subjects vs. control subjects) was between-subject, while the hand (uni- vs. bimanual) and force (G-L vs. G-G vs. L-L) were within-subject factors. In the ramp-and-hold and oscillation task a mixed 2-way ANOVA (group × feedback) was used where the group was between-subject and feedback (with vs. without visual feedback) was within-subject factor. The cross-correlation coefficients were Z-transformed prior to the statistical analysis. The Bonferroni post hoc test was carried out when necessary in all statistical tests, and the level of significance was set at p < .05. Statistical analysis was performed in SPSS 10.0 for windows (SPSS Inc, Chicago, USA).
Results
The EDSS score, averaged across the MS patients, was (mean ± SD) 3.2 ± 1.1 (range 1−5). The averaged time expended by MS patients to perform the 9-hole peg test using the dominant hand was 19.8 ± 4.5 and using the nondominant hand was 20.8 ± 5.4 s, while for the healthy controls the time was 15.6 ± 1.5 and 17.1 ± 1.9 seconds, respectively (see Table 1). The mixed two-way ANOVA (Group × Hand) revealed a longer time for test execution in MS patients than in healthy controls [F(1,30) = 9.9, p < .05] and the subjects performed the 9-hole peg test quicker using their dominant hand [F(1,30) = 10.2, p < .05].
Table 1.
Patients’ Clinical Data
| Patient | Sex | Age | EDSS | Dom 9-hole peg (s) | NDom 9-hole peg (s) |
|---|---|---|---|---|---|
| P1 | M | 37 | 2.0 | 14 | 14 |
| P2 | F | 38 | 3.5 | 19 | 19 |
| P3 | F | 46 | 4.0 | 21 | 20 |
| P4 | M | 57 | 4.5 | 24 | 24 |
| P5 | M | 57 | 3.5 | 19 | 18 |
| P6 | M | 53 | 4.5 | 24 | 32 |
| P7 | F | 39 | 2.5 | 25 | 32 |
| P8 | F | 55 | 4.0 | 31 | 27 |
| P9 | F | 58 | 4.0 | 22 | 22 |
| P10 | F | 33 | 1.0 | 16 | 16 |
| P11 | F | 30 | 5.0 | 16 | 18 |
| P12 | F | 34 | 2.0 | 18 | 16 |
| P13 | F | 43 | 3.5 | 16 | 17 |
| P14 | F | 49 | 3.0 | 18 | 21 |
| P15 | F | 40 | 2.0 | 14 | 19 |
| P16 |
F |
51 |
3.5 |
20 |
18 |
| Mean ± SD | 45 ± 9 | 3.2 ± 1.1 | 19.8 ± 4.5 | 20.8 ± 5.4 | |
| Range | 30−58 | 1−5 | 14−31 | 14−32 |
Abbreviations: Dom, dominant hand; NDom, nondominant hand; EDSS, expanded disability status scale.
Figure 2A illustrates the profiles of the time series of G and L recorded from a representative MS patient (P16) and a healthy subject (C6) performing the lifting task. Note that this patient demonstrated a remarkably higher grip (G) relative to load forces (L) than the selected healthy subject. Figure 2B depicts the smoothness of L observed from the holding phase and assessed by CV. The results showed the main effect of group [F(1,30) = 4.5, p < .05], revealing a higher CV (i.e., lower smoothness) in MS patients, as well as the main effect of hand [CV higher under uni- than under bimanual conditions; F(1,30) = 20.6, p < .01] and no interaction. The G/L ratio also revealed the main effects of group [a higher ratio in MS patients; F(1,30) = 26.3, p < 0.01], but no effect of hand [F(1,30) = 0.16, p > .05], and no interaction (see Figure 2C). Figure 2D illustrates Z-transformed correlation coefficients as an index of force coupling obtained from the interlimb (G-G and L- L) and within limb forces (G-L, averaged for two hands) during the lifting phase. The results revealed the effect of force [F(2,60) = 4.8, p < .05], but no effect of group [F(1,30) = 1.5, p > .05] and no interaction between group and hand. The post hoc analysis suggested that the coupling of interlimb L-L was higher than the within limb force coupling (i.e., G-L).
Figure 2.
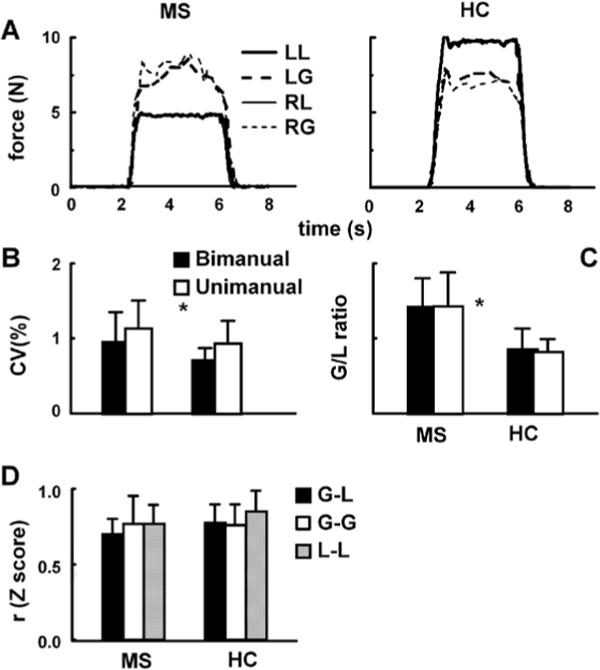
(A) Right (RG) and left grip (LG), and right (RL) and left load force (LL) recorded in the lifting task in a representative MS patient and healthy control. The remaining graphs illustrate coefficients of variation (CV) (B), G/L ratios (C), and Z-transformed correlation coefficients (D) averaged across the subjects. Error bars represent standard deviations.
Figure 3A shows G and L time series of the ramp-and-hold task performed by the same subjects as shown in Figure. 2A. Both the overall level of G and the extent of G modulation (see Figure 3B) appear to be higher in the patient. As illustrated by Figure 3C, CV was higher in MS patients than in healthy subjects [F(1,30) = 9.4, p < .01], while the group-feedback interaction [F(1,30) = 10.6, p < .01] suggested that the patients decreased the smoothness of L (i.e., increased their CV) when deprived of visual feedback, but the healthy subjects did not. The G/L ratio demonstrated the main effect of group [G/L higher in MS patients; F(1,30) = 13.7, p < .05], but no main effect of feedback [F(1,30) = 3.1, p > .05] and no interaction (Figure 3D). Regarding the force coupling as assessed by the maxima of the cross-correlation coefficients between G and L, there was neither an effect of group [F(1,30) = 0.5, p > .05], nor of feedback [F(1,30) = 1.8, p > .05] and no interaction (see the Z-transformed data in Figure 3E). Finally, we assessed the modulation of G from the regression lines of the G-L diagrams (see Figure 3B for an illustration of the diagrams). MS patients showed higher offset [F(1,30) = 11.4, p < .05] and higher gain [F(1,30) = 5.7, p < .05] than healthy controls. There were no effects of feedback on either gain [F(1,30) = 2.0, p > .05] or offset [F(1,30) = 0.1, p > .05] and no interactions.
Figure 4A illustrates the temporal patterns of G and L obtained from the oscillation task performed by the same subjects as depicted in Figures 2 and 3, while Figure 4B shows the corresponding G-L diagrams and the regression lines. Note a high level of G relative to L, as well as both a relatively high gain and low correlation coefficient demonstrated by the MS patient, as compared with the healthy control. Also note that this patient also demonstrated an ellipsoidal pattern of G-L diagrams that reveals some temporal delay between G and L. Higher variable errors suggested lower task performance in MS patients than in control subjects [F(1,30) = 4.2, p< .05], while both the effect of feedback (F = 0.4, p > .05; see Figure 4C) and the interaction were not significant. Figure 4D illustrates the G/L ratio averaged across the subjects. Similar to the results of other tested tasks, there was an effect of group [higher G/L in MS patients; F(1,30) = 14.5, p < .05], but no effect of feedback [F(1,30) = 0.2, p > .05] and no interaction. The Z-transformed maxima of the cross-correlation coefficients demonstrated no effect of group [F(1,30) = 3.2, p > .05], while the trials with visual feedback demonstrated higher values than the trials without the feedback [F(1,30) = 9.4, p < .05; see Figure 4E]. As an index of G modulation, the gain was higher in MS patients than control subjects [F(1,30) = 5.8, p < .05], as well as higher with than without visual feedback [F(1,30) = 16.4, p < 0.01; Figure 4F]. The offset revealed no effect of group (F(1,30) = 2.9, p >.05), while the effect of feedback [F(1,30) = 14.7, p < 0.01; see Figure 4G] indicated an increased offset when there was no visual feedback.
Discussion
The main aim of this study was to assess the specific aspects of impairment of hand function in mildly involved MS patients. In addition to comparing various indices of both the functional performance and G and L coordination observed from the patients and healthy controls, we also evaluated the differences between uni- and bimanual tasks, as well as the differences between the tasks performed with and without visual feedback.
Prior to discussing the main findings of the study, several important methodological aspects deserve to be pointed out. First, we tested simple manipulation tasks that closely resemble everyday activities, such as lifting and holding an object or exerting a force against a fixed object or external support. Second, most of the derived variables have already demonstrated either a moderate or high reliability (Jaric, Knight, Collins, & Marwaha, 2005). Therefore, one could conclude that the study provided both valid and reliable data regarding hand function in the tested subjects. However, the most important methodological aspect could be the sample of the MS patients tested. Specifically, we evaluated mildly involved patients who were capable of leading not only an independent, but also an active, professional life. Most of them claimed that they did not have any problem with hand function except a somewhat pronounced fatigue. Therefore, it is not surprising that despite some individual EDSS scores being as high as 5, their mean 9-hole peg test score was merely 25% higher than in the healthy controls. Conversely, moderately affected MS patients tested in a recent study revealed EDSS scores within the range 6−8, while their time to complete 9-hole peg test score was several times longer than in the healthy controls (Feys, Helsen, Lavrysen, Nuttin, & Ketelaer, 2003).
Regarding task performance, a deterioration of task performance in MS patients was noticed in all three tasks. This finding is in line with our previous results (Jaric, Knight, Collins, & Marwaha, 2005; Marwaha, Hall, Knight, & Jaric, 2006) and generally suggests that the ability to exert an accurate pattern of external forces could be one of the first signs of functional impairment of the hands associated with the progress of the disease.
Force coordination was assessed through G/L ratio, force coupling, and G modulation. When compared with healthy controls, MS patients showed a higher G/L ratio in all three tasks This result is in line with findings obtained from other neural pathologies (see the introduction for details) and suggests that, in addition to inaccurate L pattern, an elevated G/L ratio could also be an early sign of hand function impairment in MS. Although we did not present the data that could provide more direct evidence (e.g., imaging revealing brain activity during task performance [Duque et al., 2003]), we could speculate on potential neural factors that could lead to the observed phenomena. For example, the ability to provide an accurate pattern of the prescribed external force (i.e., G/L ratio seem to be based considerably on sensorimotor integration. Specifically, information from proprioceptors and or or vision is required for an accurate and steady L (Hermsdorfer, Hagl, & Nowak, 2004), while G/L ratio is not only elevated in neurological patients, but also in healthy individuals when they are deprived of sensory information from skin receptors (Johansson, 1998; Johansson & Westling, 1984).
Regarding the remaining indices of force coordination, the findings mainly suggest no differences between the MS patients and healthy controls. In particular, performance of all three tasks produced a similar level of and coupling in, the two groups in both the almost negligible time lags and high correlations between the forces. These findings suggest that the elaborate anticipatory control of and L is preserved in early stages of the disease (see Marwaha, Hall, Knight, & Jaric, 2006, for a similar finding). With respect to the modulation of G, one could interpret a higher G gain observed in both the ramp-and-hold and oscillatory task as a surprising indication of higher G and L coordination in MS patients. However, as illustrated by G-L diagrams (see Figures 3B and 4B), a higher G/L ratio requires either a higher gain, or higher offset, or both. Taking also into account that the MS patients demonstrated a higher offset in the ramp-and-hold task, it remains possible that the discussed difference in gain between the two groups was a consequence of a higher G/L ratio demonstrated by MS patients.
Besides the assessment of differences in G and L coordination between the two groups, we also evaluated two effects that could lead to different outcomes in MS patients as compared with healthy controls. First, due to frequent demyelinization occurring in the corpus callosum, even in majority of the moderately affected MS subjects (Mendez, 1995; Seymour, Reuter-Lorenz, & Gazzaniga, 1994), we hypothesized that the patients’ hand function would be particularly affected in bimanual, as compared with unimanual tasks. We did not find any group-specific effect of switching from uni- to bimanual tasks, although somewhat better performance was observed in bimanual than in unimanual lifting tasks in both groups. However, if the inter-hemispheric transfer and the related force coordination were affected, one could expect a deteriorated coupling of the interlimb forces (G-G and L-L). In fact, both groups demonstrated L-L coupling (i.e., maximum correlation coefficients between the L of two hands) to be higher than either another interlimb (G-G) or within-limb (G-L) coupling, which was in line with our previous finding obtained from healthy individuals (Jaric, Collins, Marwaha, & Russell, 2006). We also hypothesized that when compared with the same data observed from the healthy subjects, the hand function of MS patients would be particularly affected in the task performed with visual feedback. However, except an increase in L smoothness associated with the no feedback conditions of the ramp-and-hold task (which contradicts our hypothesis), no Group × Feedback interactions were observed from either the ramp-and-hold or oscillation tasks. Therefore, we concluded that the results did not support the hypothesized effects.
The present study revealed a lack of differences between the patients and controls regarding some indices of force coordination, as well as regarding the hypothesized effects bimanual (vs. unimanual) manipulation and the effect of vision (see previous paragraph). We believe that a part of explanation could be found in adaptation within the CNS. For example, recent brain imaging studies conducted on mildly involved MS patients have suggested normal neurological functioning due to cortical reorganization (Lee et al., 2000; Pantano et al., 2005; Reddy, Narayanan, Arnoutelis et al., 2000; Reddy, Narayanan, Matthews et al., 2000). In addition, no performance deficits in reaction time motor tasks was observed between mildly affected MS patients (with a mean EDSS score of 1.4) indicating an adaptive mechanism in the cortex (Cerasa et al., 2006). An increased activity in the contralateral cerebellar hemisphere in mildly impaired MS patients [EDSS score of about 1.5 (Saini et al., 2004)] was similar to the increased contralateral activity found in association with motor learning in healthy subjects (Doyon et al., 2002). Of particular importance could be the finding that the patients with lesions of the corpus callosum may show deficits in the acquisition of novel bimanual activities but not necessarily in the execution of previously learned bimanual activities (Andres et al., 1999; Geffen, Jones, & Geffen, 1994; Sperry, 1968). Since lifting objects both uni- and bimanually could have been learned well before the occurrence of MS, this finding could partly explain the lack of differences observed between uni- and bimanual lifting. However, since the potential mechanisms of adaptation and recovery become gradually impaired with the progression of the disease (Saini et al., 2004), one could expect a more pronounced impairment of hand function in more involved MS patients, as well as the hypothesized effect of visual feedback.
To conclude, the present study represents an extension of our previous study (Marwaha, Hall, Knight, & Jaric, 2006) to both static and dynamic tasks, as well as to the effects of visual feedback and of uni- versus bimanual manipulation. The results revealed some specific aspects of the impairment of hand function in mildly involved MS patients. In particular, we found that the deterioration in the ability for precise control of the observed findings is partly based on a high involvement of sensorimotor integration. Extension of the applied methodological approach to more involved MS patients could also deserve attention. However, of particular importance could be that the applied methodological approach was sensitive enough to detect impairment of hand function in mildly involved MS patients. As a result, a similar approach could also be able to detect the moderate effects of applied pharmacologic and therapeutic procedures, as well as the changes in hand function associated with relapsing and remitting phases of the disease. The findings of this study, as well as both the importance of hand function per se and a general lack of objective quantitative tests of hand function, strongly speak in favor of extending this line of research to both more involved MS patients and other manipulation tasks and conditions. Finally, taking into account the similarity of findings regarding the deterioration of hand function in various groups of patients (see the introduction for details), the applied methodology could also detect the impairment of hand function in a variety of neurological diseases.
Acknowledgments
We thank Mr. K. Seaman P.T. for the help in selection and Dr. S. Radosavljevic Jaric for clinical evaluation of MS patients. We also thank two anonymous reviewers for their valuable comments and suggestions. The study was supported in part by grant HD-48481 from the National Institute of Health to S. Jaric. P. Freitas, Jr, has been partly supported by Fulbright Program (15053184) and the Brazilian Government through the Coordination for the Training and Improvement of Higher Education Personnel (CAPES # 2051-04/4).
References
- Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain. 1999;122(Pt 5):855–870. doi: 10.1093/brain/122.5.855. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: The role of sensorimotor context estimation. Journal of Neuroscience. 1998;18(18):7511–7518. doi: 10.1523/JNEUROSCI.18-18-07511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasa A, Fera F, Gioia MC, Liguori M, Passamonti L, Nicoletti G, et al. Adaptive cortical changes and the functional correlates of visuo-motor integration in relapsing-remitting multiple sclerosis. Brain Research Bulletin. 2006;69(6):597–605. doi: 10.1016/j.brainresbull.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(2):1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Thonnard JL, Vandermeeren Y, Sebire G, Cosnard G, Olivier E. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain. 2003;126:732–747. doi: 10.1093/brain/awg069. [DOI] [PubMed] [Google Scholar]
- Feys P, Duportail M, Kos D, Van Asch P, Ketelaer P. Validity of the TEMPA for the measurement of upper limb function in multiple sclerosis. Clinical Rehabilitation. 2002;16(2):166–173. doi: 10.1191/0269215502cr471oa. [DOI] [PubMed] [Google Scholar]
- Feys P, Helsen WF, Lavrysen A, Nuttin B, Ketelaer P. Intention tremor during manual aiming: A study of eye and hand movements. Multiple Sclerosis. 2003;9(1):44–54. doi: 10.1191/1352458503ms863oa. [DOI] [PubMed] [Google Scholar]
- Feys P, Helsen WF, Liu X, Lavrysen A, Loontjens V, Nuttin B, et al. Effect of visual information on step-tracking movements in patients with intention tremor due to multiple sclerosis. Multiple Sclerosis. 2003;9(5):492–502. doi: 10.1191/1352458503ms949oa. [DOI] [PubMed] [Google Scholar]
- Feys P, Helsen WF, Liu X, Nuttin B, Lavrysen A, Swinnen SP, et al. Interaction between eye and hand movements in multiple sclerosis patients with intention tremor. Movement Disorders. 2005;20(6):705–713. doi: 10.1002/mds.20382. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Tresilian J, Wing AM. Coupling of grip force and load force during arm movements with grasped objects. Neuroscience Letters. 1993;152(1−2):53–56. doi: 10.1016/0304-3940(93)90481-y. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Tresilian JR. Grip-load force coupling: a general control strategy for transporting objects. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(5):944–957. doi: 10.1037//0096-1523.20.5.944. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The stability of precision grip forces during cyclic arm movements with a hand-held load. Experimental Brain Research. 1995;105(3):455–464. doi: 10.1007/BF00233045. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Wing AM. The role of internal models in motion planning and control: Evidence from grip force adjustments during movements of hand-held loads. Journal of Neurosicence. 1997;17(4):1519–1528. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen GM, Jones DL, Geffen LB. Interhemispheric control of manual motor activity. Behavioral Brain Research. 1994;64(1−2):131–140. doi: 10.1016/0166-4328(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Grice KO, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available nine hole peg test for finger dexterity. American Journal of Occupational Therapy. 2003;57(5):570–573. doi: 10.5014/ajot.57.5.570. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA. Deficits of anticipatory grip force control after damage to peripheral and central sensorimotor systems. Human Movement Science. 2004;23(5):643–662. doi: 10.1016/j.humov.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA, Marquardt C. Grip force control during object manipulation in cerebral stroke. Clinical Neurophysiology. 2003;114(5):915–929. doi: 10.1016/s1388-2457(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Ingvarsson PE, Gordon AM, Forssberg H. Coordination of manipulative forces in Parkinson's disease. Experimental Neurology. 1997;145(2 Pt 1):489–501. doi: 10.1006/exnr.1997.6480. [DOI] [PubMed] [Google Scholar]
- Jaric S, Collins JJ, Marwaha R, Russell E. Interlimb and within limb force coordination in static bimanual manipulation task. Experimental Brain Research. 2006;168(1−2):88–97. doi: 10.1007/s00221-005-0070-6. [DOI] [PubMed] [Google Scholar]
- Jaric S, Knight CA, Collins JJ, Marwaha R. Evaluation of a method for bimanual testing coordination of hand grip and load forces under isometric conditions. Journal of Electromyography and Kinesiology. 2005;15(6):556–563. doi: 10.1016/j.jelekin.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Jaric S, Russell EM, Collins JJ, Marwaha R. Coordination of hand grip and load forces in uni- and bidirectional static force production tasks. Neuroscience Letters. 2005;381(1−2):51–56. doi: 10.1016/j.neulet.2005.01.086. [DOI] [PubMed] [Google Scholar]
- Johansson RS. Sensory input and control of grip. Novartis Foundation Symposium. 1998;218:45–59. doi: 10.1002/9780470515563.ch4. discussion 59−63. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Experimental Brain Research. 1984;56(3):550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Experimental Brain Research. 1988;71(1):59–71. doi: 10.1007/BF00247522. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple-sclerosis:An expanded disability status scale (Edss). Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lee M, Reddy H, Johansen-Berg H, Pendlebury S, Jenkinson M, Smith S, et al. The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Annals of Neurology. 2000;47(5):606–613. [PubMed] [Google Scholar]
- Marwaha R, Hall SJ, Knight CA, Jaric S. Load and grip force coordination in static bimanual manipulation tasks in multiple sclerosis. Motor Control. 2006;10(2):160–177. doi: 10.1123/mcj.10.2.160. [DOI] [PubMed] [Google Scholar]
- Matthews WB. Multiple sclerosis and other demyelinating diseases: Clinical features. In: S.M., O.J., editors. Clinical Neurology. Churchill Livingstone; New York: 1991. pp. 1106–1116. [Google Scholar]
- Mendez MF. The neuropsychiatry of multiple sclerosis. International Journal of Psychiatry and Medicine. 1995;25(2):123–135. doi: 10.2190/NK8F-MTUW-QHH1-0531. [DOI] [PubMed] [Google Scholar]
- Mitoma H. Intention tremor exaggerated by visually guided movement. European Neurology. 1996;36(3):177–178. doi: 10.1159/000117241. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Deficits of predictive grip force control during object manipulation in acute stroke. Journal of Neurology. 2003;250(7):850–860. doi: 10.1007/s00415-003-1095-z. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J, Topka H. When motor execution is selectively impaired: Control of manipulative finger forces in amyotrophic lateral sclerosis. Motor Control. 2003;7(3):304–320. doi: 10.1123/mcj.7.3.304. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Lenzi D, Caramia F, Iannetti GD, Piattella MC, et al. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain. 2005;128:2146–2153. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Arnoutelis R, Jenkinson M, Antel J, Matthews PM, et al. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain. 2000;123:2314–2320. doi: 10.1093/brain/123.11.2314. [DOI] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Matthews PM, Hoge RD, Pike GB, Duquette P, et al. Relating axonal injury to functional recovery in MS. Neurology. 2000;54(1):236–239. doi: 10.1212/wnl.54.1.236. [DOI] [PubMed] [Google Scholar]
- Rost K, Nowak DA, Timmann D, Hermsdorfer J. Preserved and impaired aspects of predictive grip force control in cerebellar patients. Clinical Neurophysiologyl. 2005;116(6):1405–1414. doi: 10.1016/j.clinph.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Saini S, DeStefano N, Smith S, Guidi L, Amato MP, Federico A, et al. Altered cerebellar functional connectivity mediates potential adaptive plasticity in patients with multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75(6):840–846. doi: 10.1136/jnnp.2003.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk T, Walther EU, Mai N. Closed- and open-loop handwriting performance in patients with multiple sclerosis. European Journal of Neurology. 2000;7(3):269–279. doi: 10.1046/j.1468-1331.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- Seymour SE, Reuter-Lorenz PA, Gazzaniga MS. The disconnection syndrome: Basic findings reaffirmed. Brain. 1994;117(Pt 1):105–115. doi: 10.1093/brain/117.1.105. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Hemisphere deconnection and unity in conscious awareness. The American Psychologist. 1968;23(10):723–733. doi: 10.1037/h0026839. [DOI] [PubMed] [Google Scholar]
- Tsolaki M, Drevelegas A, Karachristianou S, Kapinas K, Divanoglou D, Routsonis K. Correlation of dementia, neuropsychological and MRI findings in multiple sclerosis. Dementia. 1994;5(1):48–52. doi: 10.1159/000106694. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nature Immunology. 2001;2(9):777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Gao F, Latash ML. Motor control goes beyond physics: Differential effects of gravity and inertia on finger forces during manipulation of hand-held objects. Experimental Brain Research. 2005;162(3):300–308. doi: 10.1007/s00221-004-2152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


