Abstract
Methods for identification of primary HIV infections seem increasingly important to understand pathogenesis, and to prevent transmission, which is particularly efficient during acute infection. Most current algorithms for HIV testing are based on detection of HIV antibodies and are unable to identify early infections before seroconversion. The efficiency of prospective cohorts, which is a standard approach for identifying primary HIV-1 infection, depends on a variety of epidemiological and cultural factors including HIV incidence and stigma and, not surprisingly, varies significantly in different geographical areas. We report a voluntary counseling and testing (VCT)-based approach to identifying primary HIV-1C infection that was developed as part of a primary HIV-1 subtype C infection study in Botswana. The referral strategy was based on: (1) collaboration with VCT centers at city clinics operated by the Ministry of Health; (2) partnering with the busiest non-government VCT center; (3) educating healthcare workers and the community about primary HIV infection; and (4) pairing with diverse VCT providers, including NGOs and private-sector organizations. Acute HIV-1 infections were defined by a negative HIV-1 serology combined with a positive HIV-1 RT-PCR test. Recent HIV-1 infections were identified by detuned EIA testing according to the classic STARTH algorithm. The VCT-based referral strategy resulted in the successful identification of 57 cases of acute and early HIV infection. A referral strategy of expanded VCT with viral RNA (Ribonucleic acid) testing to a national program in Botswana may be a promising approach for identification of primary HIV infections on a countrywide level. The program should offer VCT with viral RNA testing to the general public, facilitate proper counseling and risk reduction, and allow initiation of early HAART, and may reduce new viral transmissions.
Keywords: HIV-1 subtype C, Acute infection, Recent infection, Primary infection, Southern Africa, Botswana
Introduction
The level of viral RNA load is associated with the efficiency of HIV transmission (Quinn et al., 2000) and the rate of HIV transmission is the highest during early stage of infection (Brenner et al., 2007; Pilcher, Eaton, Kalichman, Bisol, & de Souza Rda, 2006; Pilcher, Eron, Galvin, Gay, & Cohen, 2004; Wawer et al., 2005). Early identification of recently transmitted HIV infection has great potential for reducing HIV transmission and may represent a new, under-explored niche in the global fight against HIV.
It is still a challenge to identify early HIV infection. The absence of specific clinical signs and symptoms in acute HIV infection, asymptomatic forms, lack of awareness among healthcare providers regarding potential acute HIV infection and the shortage of nucleic acid tests for routine use in diagnostics for HIV infection still contribute to the hindrance of early HIV infection identification world-wide.
A combination of a ‘sensitive’ and ‘less-sensitive’ ELISA, or the detuned method (Janssen et al., 1998), has been widely used for distinguishing between recent and old HIV infections in many recent studies worldwide (Diaz, De Cock, Brown, Ghys, & Boerma, 2005; Janssen et al., 1998; Kellogg, Clements-Nolle, Dilley, Katz, & McFarland, 2001; Kellogg et al., 2001; Machado et al., 2002; McDougal et al., 2005; McFarland et al., 1999; Parekh et al., 2001; Parekh, Pau, Kennedy, Dobbs, & McDougal, 2001; Parekh et al., 2002; Rutherford, Schwarcz, & McFarland, 2000; Schwarcz et al., 2001; Young et al., 2003).
In this report we summarize a referral strategy of expanded Voluntary Counseling and Testing (VCT) with viral RNA testing in Botswana for identification of primary HIV infections.
Methods
Study structure
The referral strategy was used to identify primary HIV-1 infections in Botswana. The recruitment of patients was a part of the primary HIV-1 infection study in Botswana, the Tshedimoso study approved by the Institutional Review Boards in both Botswana and the US. The referral strategy was based on partnering with VCT centers in Gaborone, the capital of Botswana, and involved collaboration with VCT centers at city clinics operated by the Ministry of Health, partnership with the busiest non-government VCT center, educating healthcare providers and the community on primary HIV infection and pairing with diverse VCT providers, including NGOs and private-sector organizations. Information on referral sources was collected through patient self-reports at screening visits.
Referrals
The existing VCT centers based at the Gaborone City Council primary care clinics were targeted for referrals. Working relationships were established with the staff at clinics with high flows of patients. The study nurses were deployed to the clinics and initiated education of healthcare providers by providing presentations to physicians and nurses at morning meetings and to the patients in waiting areas of the clinic, which was important to initiate contact with potential candidates and provide them with the most basic information about primary HIV infection and the study goals. Healthcare providers were asked to refer their patients who met the referral criteria to the study staff for additional HIV testing to identify primary infection, if any.
The referred candidate received pre- and post-test counseling by the study staff nurse that included a detailed explanation of HIV infection, with particular emphasis on the acute and early phases, study goals and eligibility and exclusion criteria. If interested and eligible, the referred candidate was asked to sign a screening consent form and to donate blood for testing.
Screening algorithm
Referred candidates were tested by rapid and/or regular double ELISA for HIV-1 antibodies followed by RT-PCR or the detuned ELISA based on the results of the initial screening ELISA test. Primary HIV-1 infection was defined as the initial period of infection that includes acute HIV infection (pre-seroconversion) and recent HIV infection (seroconversion) (Lacabaratz-Porret et al., 2003).
Statistical analysis
Comparisons between groups were performed by t-test or Mann-Whitney rank sum test, and associations were assessed by linear regression test using SigmaStat v.3.1 software.
Results
Enrollment of patients with primary HIV-1 infection
The breakdown of screened and enrolled participants with primary HIV-1 infection over two years of the study is shown in Table 1. The total number of patients enrolled was 57, including 7 cases of acute HIV infection and 50 cases of recent HIV infection. The number of patients tested by rapid/regular EIA is higher than the sum of performed RT-PCR and detuned EIA because a fraction of patients, after receiving the results of the rapid EIA, were not interested in further testing and/or joining the study.
Table 1.
Screened and enrolled referrals
| Year 2005* | Year 2006 | Total | |
|---|---|---|---|
| Screening: | |||
| Rapid/regular EIA, # of tested candidates | 1,142 | 2,868 | 4,010 |
| RT-PCR, # of tested candidates | 814 | 1,172 | 1,986 |
| Detuned EIA, # of tested candidates | 322 | 230 | 552 |
| Enrolled participants: | |||
| Acute Infections, # of enrolled cases | 2 | 5 | 7 |
| Recent Infections, # of enrolled cases | 30 | 20 | 50 |
| Total Primary Infections, # of enrolled cases | 32 | 25 | 57 |
| Rate, enrolled/referred: | |||
| Acute Infections | 0.002 | 0.002 | 0.002 |
| Recent Infections | 0.026 | 0.007 | 0.012 |
| Total Primary Infections | 0.028 | 0.009 | 0.014 |
| Rate, enrolled/tested by RT-PCR or detuned EIA: | |||
| Acute Infections | 0.002 | 0.004 | 0.004 |
| Recent Infections | 0.093 | 0.087 | 0.091 |
| Total Primary Infections | 0.028 | 0.018 | 0.022 |
Referrals from November 2004 to December 2005
Table 1 also demonstrates the rate of identification of acute and recent HIV-1 infections in Botswana. The rate was calculated either as a ratio of enrolled to referred participants or as a ratio of enrolled to tested participants by RT-PCR or detuned EIA. The overall rate of enrollment of primary HIV-1 infections was 1.4% and 2.2% in relation to referred and tested candidates, respectively. The rate for acute infections was 0.2% and 0.4% and the rate for recent infections was 1.2% and 9.1% of referred and tested participants, respectively.
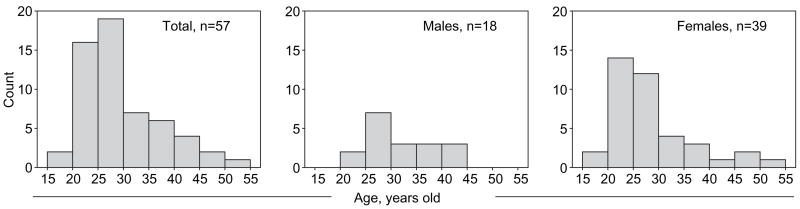
The overall gender ratio was 2.2:1, including 39 women and 18 men, which reflects the gender ratio of outpatients in clinics in Botswana. Figure 1 shows the age distribution of enrolled study participants, which was not normal but skewed toward younger ages. Table 2 provides descriptive statistics of age in the total group of primary HIV infections and the subsets of acute and recent HIV infections. The median values between male and female participants were at borderline significance (p=0.05 and p=0.04; Mann- Whitney rank sum test for the entire group and for recent infections, respectively). Other comparisons between groups did not reveal any statistically significant difference in age distribution (data not shown).
Figure 1.
Age distribution among study participants.
Table 2.
Age statistics for enrolled cases of acute and recent HIV-1 infections
| Group | Size, n | Mean | Std Dev | Median |
|---|---|---|---|---|
| Primary infections, total | 57 | 29.1 | 7.7 | 26.0 |
| Acute infection cases | 7 | 32.3 | 10.6 | 32.0 |
| Recent infection cases | 50 | 28.6 | 7.2 | 26.0 |
| Males in total group | 18 | 31.3 | 6.8 | 28.5 |
| Females in total group | 39 | 28.1 | 8.0 | 26.0 |
| Male participants with acute infections | 2 | 30.5 | 5.0 | 30.5 |
| Female participants with acute infections | 5 | 33.0 | 12.6 | 32.0 |
| Male participants with recent infections | 16 | 31.4 | 7.1 | 28.5 |
| Female participants with recent infections | 34 | 27.3 | 7.0 | 25.5 |
Referral criteria for identification of primary HIV infections in Botswana
The referral criteria for identification of acute and recent HIV-1 infections in Botswana were refined based on regression analysis at the end of the first year (Table 3). Young age and diagnosed STI were excluded, while multiple sexual partners and age difference with sexual partner were added as referral criteria. To target potential candidates within the pre-seroconversion period we emphasized recent unprotected sex.
Table 3.
Referral criteria for identification of primary HIV infections in Botswana
| Original Referral Criteria | Refined Referral Criteria |
|---|---|
|
|
Referral sources
Data on referral sources was collected from the subset of 442 consecutive candidates during screening for four months (Table 4). The majority of reported referrals came from healthcare providers at the clinics attached to the study VCT centers. While only about 17% of participants cited being worried about a recent HIV exposure as their reason for coming to the study VCT clinics, they represented 38% (5/13) of those participants who were eventually enrolled in the study. A majority of the patients referred by healthcare providers actually came to the clinic because they were worried about recent HIV exposure and then were referred to the study. Therefore, being worried about potential recent HIV exposure might be considered a dominant reason in identification of acute and recent HIV infections in Botswana.
Table 4.
Self-reported referral sources
| Referral source | Candidates who reported referral source, number (%) | Acute infections identified
|
Recent infections identified
|
Enrolled
|
||||
|---|---|---|---|---|---|---|---|---|
| # | rate | # | rate | # | rate | |||
| Referred by health care worker at clinic attached to VCT site | 281 | (63.6%) | 2 | 0.007 | 5 | 0.018 | 7 | 0.025 |
| Worried about recent exposure | 74 | (16.7%) | 1 | 0.014 | 6 | 0.081 | 5 | 0.068 |
| Referred by relative or family member | 24 | (5.4%) | 0 | 0 | 1 | 0.042 | 0 | 0 |
| Saw a poster | 19 | (4.3%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Heard presentation outside of clinic | 26 | (5.9%) | 1 | 0.038 | 0 | 0 | 0 | 0 |
| Other | 18 | (4.1%) | 1 | 0.056 | 0 | 0 | 1 | 0.056 |
| Total | 442 | (100%) | 5 | 0.011 | 12 | 0.027 | 13 | 0.029 |
Discussion
Our study demonstrated that identification of primary HIV infections is possible in Botswana. We suggest that development of a national program aimed at identifying primary HIV infections may be a critical step toward curbing the epidemic in the country. This approach, combined with early use of HAART, risk reduction counseling, partner notification and contact tracing, might deserve a new look and may be an important tool in fighting HIV/AIDS.
The results of this study highlight the challenges of identifying early HIV infections despite more than 25 years of experience with the HIV/AIDS epidemic. Although referrals have been recommended by the CDC (CDC, 1998, 2001; Gallant, 2004), only a small fraction of patients with acute HIV infection are actually referred for HIV testing by healthcare providers (Grant, 1998). In Botswana, a VCT-based referral strategy, coupled with advanced lab testing, appeared to be a promising future approach for identification of primary HIV infections. Collaboration with both government and non-government VCT centers combined with proper education of healthcare providers and community education and extended by pairing with diverse VCT providers including NGOs and private sector organizations, resulted in successful identification of 57 cases of acute and early HIV infection. It is important to note the relatively limited resources used in the study.
The referral system employed had a number of limitations. First, there is a lack of clearly defined signs and symptoms for the early phase of HIV infection. Second, budgetary constraints imposed limitations on study staffing, clinical space, transportation and advertising. Third was the limited coverage area. Fourth, clinic staff are normally overwhelmed by the flow of patients, which inevitably reduces referrals. Fifth, most screening was limited to morning hours. Finally, educational advertising was done only occasionally and not systematically.
Our main recommendation is to expand VCT with viral RNA testing up to nationwide coverage as a national program in Botswana. The current referral system can be transformed into an efficient network of VCT units linked to laboratories. The program could offer VCT with viral RNA testing to the general public and promote individual and community education on primary HIV infection and the importance of early HIV testing.
Acknowledgments
We are grateful to the patients who participated in the Tshedimoso study in Botswana. We thank Gaseboloke Mothowaeng, Florence Modise, S’khatele Molefhabangwe and Sarah Masole for their dedication and outstanding work in the clinic and outreach. We express thanks to Lemme Kebaabetswe, Busisiwe Mlotshwa and David Nkwe for excellent laboratory support. We greatly appreciate the enthusiasm and strong commitment of Carl Davis, Kenneth Onyait and Erik van Widenfelt in achieving the overall study goals. We thank the Botswana Ministry of Health, Gaborone City Council clinics and the Gaborone VCT Tebelopele for their ongoing support and collaboration. Finally, we thank Lendsey Melton for excellent editorial assistance. The primary HIV-1 subtype C infection study in Botswana, the Tshedimoso study, is supported and funded by NIH grant R01 AI057027. This work was also supported in part by the NIH grant D43 TW000004 through the AAMC FIC/Ellison Overseas Fellowships in Global Health and Clinical Research (EM and MK).
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, Matte C, et al. High rates of forward transmission events after acute/early HIV-1 infection. Journal of Infectious Diseases. 2007;195:951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- CDC. HIV partner counselingand referral services. US Department of Health and Human Services, Atlanta, GA: Public Health Service; 1998. [Google Scholar]
- CDC. Revised guidelines for HIV counseling, testing, and referral. Morbidity and Mortality Weekly Reports Recommendations and Report. 2001;50:1–57. [PubMed] [Google Scholar]
- Diaz T, De Cock K, Brown T, Ghys PD, Boerma JT. New strategies for HIV surveillance in resource-constrained settings: An overview. AIDS. 2005;19(Suppl 2):S1–S8. doi: 10.1097/01.aids.0000172871.80723.3e. [DOI] [PubMed] [Google Scholar]
- Gallant JE. HIV counseling, testing and referral. American Family Physician. 2004;70:295–302. [PubMed] [Google Scholar]
- Grant RM. Advances in the diagnosis and evaluation of acute HIV-1 infection. 1998 Conference on the Laboratory Science of HIV. Centers for Disease Control; Atlanta, GA. 16–18 September 1998.1998. [Google Scholar]
- Janssen RS, Satten GA, Stramer SL, Rawal BD, O’Brien TR, Weiblen BJ, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. Journal of the American Medical Association. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- Kellogg TA, Clements-Nolle K, Dilley J, Katz MH, McFarland W. Incidence of human immunodeficiency virus among male-to-female transgendered persons in San Francisco. Journal of Acquired Immune Deficiency Syndromes. 2001;28:380–384. doi: 10.1097/00126334-200112010-00012. [DOI] [PubMed] [Google Scholar]
- Kellogg TA, McFarland W, Perlman JL, Weinstock H, Bock S, Katz MH, et al. HIV incidence among repeat HIV testers at a county hospital, San Francisco, California, US. Journal of Acquired Immune Deficiency Syndromes. 2001;28:59–64. doi: 10.1097/00042560-200109010-00009. [DOI] [PubMed] [Google Scholar]
- Lacabaratz-Porret C, Urrutia A, Doisne JM, Goujard C, Deveau C, Dalod M, et al. Impact of antiretroviral therapy and changes in virus load on human immunodeficiency virus (HIV)-specific T cell responses in primary HIV infection. Journal of Infectious Diseases. 2003;187:748–757. doi: 10.1086/368333. [DOI] [PubMed] [Google Scholar]
- Machado DM, Delwart EL, Diaz RS, de Oliveira CF, Alves K, Rawal BD, et al. Use of the sensitive/less-sensitive (detuned) EIA strategy for targeting genetic analysis of HIV-1 to recently infected blood donors. AIDS. 2002;16:113–119. doi: 10.1097/00002030-200201040-00014. [DOI] [PubMed] [Google Scholar]
- McDougal JS, Pilcher CD, Parekh BS, Gershy-Damet G, Branson BM, Marsh K, et al. Surveillance for HIV-1 incidence using tests for recent infection in resource-constrained countries. AIDS. 2005;19(Suppl 2):S25–S30. doi: 10.1097/01.aids.0000172874.90133.7a. [DOI] [PubMed] [Google Scholar]
- McFarland W, Busch MP, Kellogg TA, Rawal BD, Satten GA, Katz MH, et al. Detection of early HIV infection and estimation of incidence using a sensitive/less-sensitive enzyme immunoassay testing strategy at anonymous counseling and testing sites in San Francisco. Journal of Acquired Immune Deficiency Syndromes. 1999;22:484–289. doi: 10.1097/00126334-199912150-00009. [DOI] [PubMed] [Google Scholar]
- Parekh BS, Hu DJ, Vanichseni S, Satten GA, Candal D, Young NL, et al. Evaluation of a sensitive/less-sensitive testing algorithm using the 3A11-LS assay for detecting recent HIV seroconversion among individuals with HIV-1 subtype B or E infection in Thailand. AIDS Research and Human Retroviruses. 2001;17:453–458. doi: 10.1089/088922201750102562. [DOI] [PubMed] [Google Scholar]
- Parekh BS, Kennedy MS, Dobbs T, Pau CP, Byers R, Green T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: A simple assay for detecting recent HIV infection and estimating incidence. AIDS Research & Human Retroviruses. 2002;18:295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- Parekh BS, Pau CP, Kennedy MS, Dobbs TL, McDougal JS. Assessment of antibody assays for identifying and distinguishing recent from long-term HIV Type 1 infection. AIDS Research & Human Retroviruses. 2001;17:137–146. doi: 10.1089/08892220150217229. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Eaton L, Kalichman S, Bisol C, de Souza Rda S. Approaching ‘HIV elimination’: Interventions for acute HIV infection. Current HIV/AIDS Report. 2006;3:160–168. doi: 10.1007/s11904-006-0011-4. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Eron JJ, Jr, Galvin S, Gay C, Cohen MS. Acute HIV revisited: New opportunities for treatment and prevention. Journal of Clinical Investigation. 2004;113:937–945. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. New England Journal of Medicine. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Rutherford GW, Schwarcz SK, McFarland W. Surveillance for incident HIV infection: New technology and new opportunities. Journal of Acquired Immune Deficiency Syndromes. 2000;25(Suppl 2):S115–S119. doi: 10.1097/00042560-200012152-00005. [DOI] [PubMed] [Google Scholar]
- Schwarcz S, Kellogg T, McFarland W, Louie B, Kohn R, Busch M, et al. Differences in the temporal trends of HIV seroincidence and seroprevalence among sexually transmitted disease clinic patients, 1989–1998: Application of the serologic testing algorithm for recent HIV seroconversion. American Journal of Epidemiology. 2001;153:925–934. doi: 10.1093/aje/153.10.925. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. Journal of Infectious Diseases. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- Young CL, Hu DJ, Byers R, Vanichseni S, Young NL, Nelson R, et al. Evaluation of a sensitive/less sensitive testing algorithm using the bioMerieux Vironostika-LS assay for detecting recent HIV-1 subtype B′ or E infection in Thailand. AIDS Research & Human Retroviruses. 2003;19:481–486. doi: 10.1089/088922203766774522. [DOI] [PubMed] [Google Scholar]