Abstract
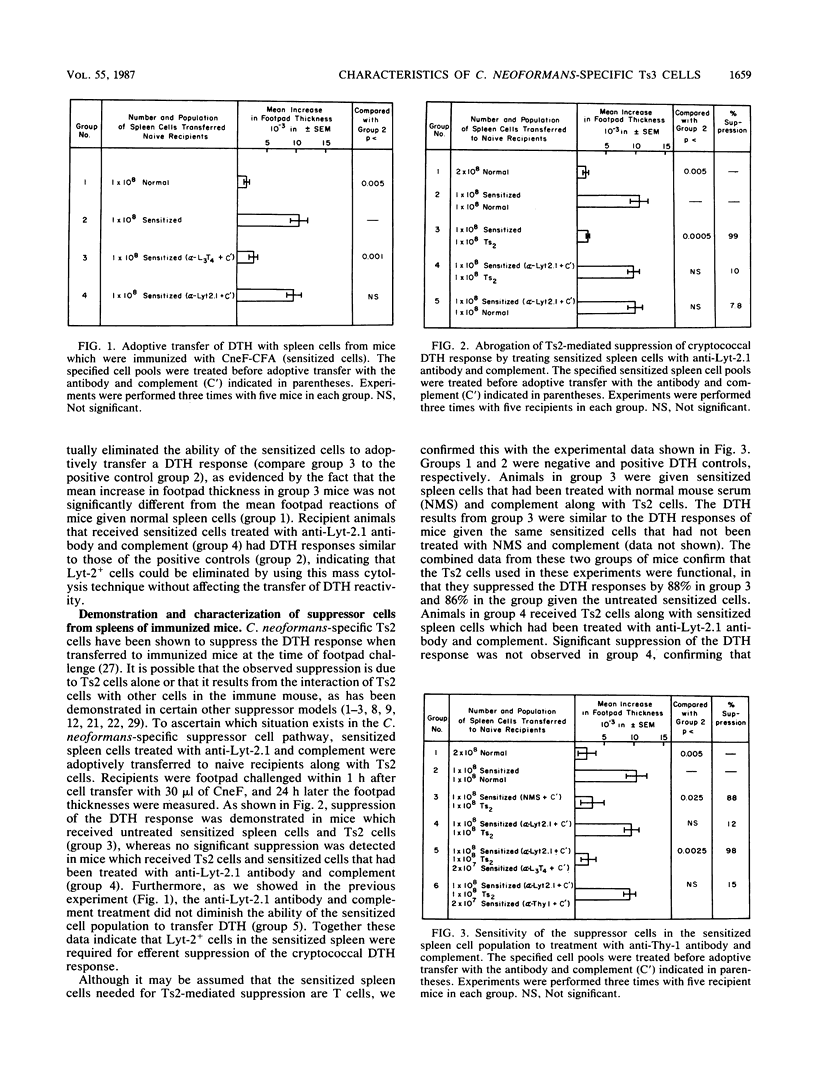
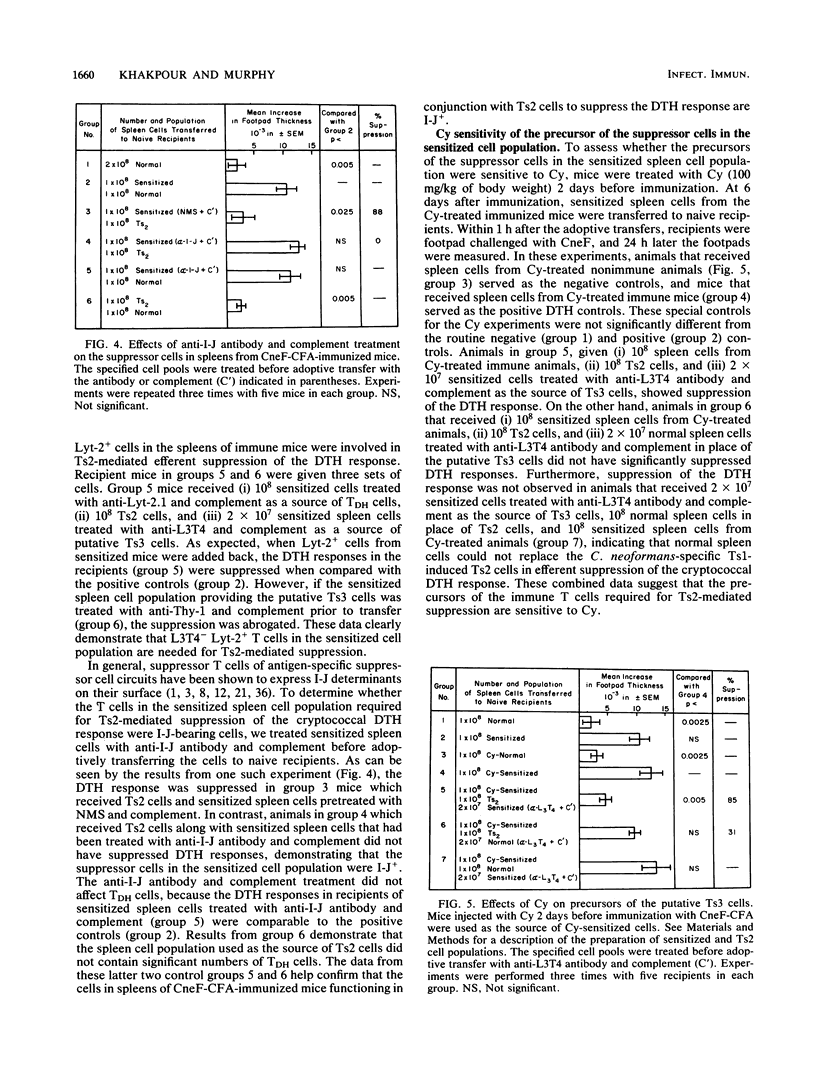
Previous studies from our laboratory have shown that a high dose of cryptococcal culture filtrate antigen (CneF) administered intravenously induces a complex suppressor cell cascade which down-regulates the cell-mediated immune response to Cryptococcus neoformans antigens. The primary objective of this investigation was to determine whether a suppressor cell induced by immunization is required for efferent suppression of the cryptococcal delayed-type hypersensitivity (DTH) response. Our approach to this problem was to immunize CBA/J mice with CneF emulsified in complete Freund adjuvant and then 6 days later to collect spleen cells from the immunized mice and adoptively transfer these cells along with C. neoformans-specific second-order suppressor T cells (Ts2) to naive syngeneic recipients at the time of footpad challenge of the recipients with CneF. To establish which populations of cells in the spleens of immunized mice play a suppressive role, mass cytolysis with specific antibodies and complement was performed before the spleen cells were transferred to naive animals. Since the phenotype of the cells responsible for the transfer of the cryptococcal DTH response had not been completely determined, we first demonstrated that the cells responsible for DTH were L3T4+ Lyt-2- cells. Subsequently, we established that a Thy-1+ L3T4- Lyt-2+ I-J+ cell population induced by immunization was required along with C. neoformans-specific Ts2 cells for efferent suppression of the cryptococcal DTH response. In addition, we demonstrated that the suppressor cells in the immune cell population were derived from cyclophosphamide-sensitive precursors. These data indicate that a third suppressor cell population is required for efferent suppression of the cryptococcal DTH response. As in the azobenzenearsonate and 4-hydroxy-3-nitrophenyl acetyl hapten suppressor models, the Ts2 cells in the circuit mediate their effects through this third suppressor component. Since the mode of induction and the phenotype of the third C. neoformans-specific suppressor cells are similar to those reported for Ts3 cells in other antigen-specific suppression models, we referred to this third suppressor cell in the C. neoformans-specific suppressor cell cascade as a Ts3 cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Colizzi V., Zembala M. An overview of T-suppressor cell circuits. Annu Rev Immunol. 1986;4:37–68. doi: 10.1146/annurev.iy.04.040186.000345. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Dorf M. E., Colizzi V., Zembala M., James B. M. Equivalence of conventional anti-picryl T suppressor factor in the contact sensitivity system and monoclonal anti-NP TsF3: their final non-specific effect via the T acceptor cell. Immunology. 1984 Nov;53(3):491–497. [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B., Greene M. I., Sy M. S., Dorf M. E. Suppressor T cell circuits. Ann N Y Acad Sci. 1982;392:300–308. doi: 10.1111/j.1749-6632.1982.tb36115.x. [DOI] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiacomo A., North R. J. T cell suppressors of antitumor immunity. The production of Ly-1-,2+ suppressors of delayed sensitivity precedes the production of suppressors of protective immunity. J Exp Med. 1986 Oct 1;164(4):1179–1192. doi: 10.1084/jem.164.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973 Jun;127(6):694–697. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Okuda K., Minami M. Dissection of a suppressor cell cascade. Curr Top Microbiol Immunol. 1982;100:61–67. doi: 10.1007/978-3-642-68586-6_7. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974 Oct;14(1):12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- Green D. R., Flood P. M., Gershon R. K. Immunoregulatory T-cell pathways. Annu Rev Immunol. 1983;1:439–463. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Sugimoto M., Benacerraf B. Mechanisms of regulation of cell-mediated immune responses. I. Effect of the route of immunization with TNP-coupled syngeneic cells on the induction and suppression of contact sensitivity to picryl chloride. J Immunol. 1978 May;120(5):1604–1611. [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. Function and antigen recognition pattern of L3T4+ T-cell clones from Mycobacterium tuberculosis-immune mice. Infect Immun. 1986 Nov;54(2):291–296. doi: 10.1128/iai.54.2.291-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Butler L. D., Claman H. N. Suppressor T cell circuits in contact sensitivity. I. Two mechanistically distinct waves of suppressor T cells occur in mice tolerized with syngeneic DNP-modified lymphoid cells. J Immunol. 1982 Aug;129(2):461–468. [PubMed] [Google Scholar]
- Minami M., Furusawa S., Dorf M. E. I-J restrictions on the activation and interaction of parental and F1-derived TS3 suppressor cells. J Exp Med. 1982 Aug 1;156(2):465–479. doi: 10.1084/jem.156.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M., Furusawa S., Dorf M. E. I-J restrictions on the activation and interaction of parental and F1-derived TS3 suppressor cells. J Exp Med. 1982 Aug 1;156(2):465–479. doi: 10.1084/jem.156.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley R. L., Murphy J. W., Cox R. A. Immunoadsorption of Cryptococcus-specific suppressor T-cell factors. Infect Immun. 1986 Mar;51(3):844–850. doi: 10.1128/iai.51.3.844-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Effects of first-order Cryptococcus-specific T-suppressor cells on induction of cells responsible for delayed-type hypersensitivity. Infect Immun. 1985 May;48(2):439–445. doi: 10.1128/iai.48.2.439-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol. 1985 Jan;134(1):577–584. [PubMed] [Google Scholar]
- Okuda K., Minami M., Furusawa M., Dorf M. E. Analysis of T cell hybridomas. II. Comparisons among three distinct types of monoclonal suppressor factors. J Exp Med. 1981 Dec 1;154(6):1838–1851. doi: 10.1084/jem.154.6.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Minami M., Sherr D. H., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. XI. Pseudogenetic restrictions of hybridoma suppressor factors. J Exp Med. 1981 Aug 1;154(2):468–479. doi: 10.1084/jem.154.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpff S. C., Bennett J. E. Abnormalities in cell-mediated immunity in patients with Cryptococcus neoformans infection. J Allergy Clin Immunol. 1975 Jun;55(6):430–441. doi: 10.1016/0091-6749(75)90082-2. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Lo D., Korngold R. Functions of purified L3T4+ and Lyt-2+ cells in vitro and in vivo. Immunol Rev. 1986 Jun;91:195–218. doi: 10.1111/j.1600-065x.1986.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Sunday M. E., Benacerraf B., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. VIII. Suppressor cell pathways in cutaneous sensitivity responses. J Exp Med. 1981 Apr 1;153(4):811–822. doi: 10.1084/jem.153.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday M. E., Weinberger J. Z., Benacerraf B., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. J Immunol. 1980 Oct;125(4):1601–1605. [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Moorhead J. W., Claman H. N. Active suppression of 1-fluoro-2,4-dinitrobenzene-immune T cells. Requirement of an auxiliary T cell induced by antigen. J Exp Med. 1979 May 1;149(5):1197–1207. doi: 10.1084/jem.149.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Uracz W., Abe R., Asano Y. I-J as an inducible T cell receptor for self. Immunol Rev. 1985 Apr;83:105–124. doi: 10.1111/j.1600-065x.1985.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Tsurufuji M., Benacerraf B., Sy M. S. An antigen-specific signal is required for the activation of second-order suppressor T cells in the regulation of delayed-type hypersensitivity to 2,4,6-trinitrobenzene sulfonic acid. J Exp Med. 1983 Sep 1;158(3):932–945. doi: 10.1084/jem.158.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger J. Z., Germain R. N., Ju S. T., Greene M. I., Benacerraf B., Dorf M. E. Hapten-specific T-cell responses to 4-hydroxy-3-nitrophenyl acetyl. II. Demonstration of idiotypic determinants on suppressor T cells. J Exp Med. 1979 Oct 1;150(4):761–776. doi: 10.1084/jem.150.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R. B., Nepom J. T., Sy M. S., Takaoki M., Gramm C. F., Fox I., Germain R. N., Nelles M. J., Greene M. I., Benacerraf B. Suppressor factor from a T cell hybrid inhibits delayed-type hypersensitivity responses to azobenzenearsonate. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6441–6445. doi: 10.1073/pnas.78.10.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]



