Abstract
Our laboratory demonstrated previously that PGE2-induced modulation of hippocampal synaptic transmission is via a presynaptic PGE2 EP2 receptor. However, little is known about whether the EP2 receptor is involved in hippocampal long-term synaptic plasticity and cognitive function. Here we show that long-term potentiation (LTP) at the hippocampal perforant path synapses was impaired in mice deficient in the EP2 (KO), while membrane excitability and passive properties in granule neurons were normal. Importantly, escape latency in the water maze in EP2 KO was longer than that in age-matched EP2 wild-type littermates (WT). We also observed that LTP was potentiated in EP2 WT animals that received lipopolysaccharide (LPS, i.p.), but not in EP2 KO. Bath application of PGE2 or butaprost, an EP2 receptor agonist, increased synaptic transmission and decreased paired-pulses ratio (PPR) in EP2 WT mice, but failed to induce the changes in EP2 KO mice. Meanwhile, synaptic transmission was elevated by application of forskolin, an adenylyl cyclase activator, both in EP2 KO and WT animals. In addition, the PGE2-enhanced synaptic transmission was significantly attenuated by application of PKA, IP3 or MAPK inhibitors in EP2 WT animals. Our results show that hippocampal long-term synaptic plasticity is impaired in mice deficient in the EP2, suggesting that PGE2-EP2 signaling is important for hippocampal long-term synaptic plasticity and cognitive function.
Keywords: Prostaglandins, cyclooxygenase-2, long-term potentiation, hippocampus, synaptic plasticity
Introduction
Cyclooxygenase-2 (COX-2), an inducible enzyme converting arachidonic acid to prostaglandins, is a key player in inflammation and has been implicated in synaptic signaling and certain types of neurologic disorders. Inhibition of COX-2 with selective inhibitors has been demonstrated to block hippocampal LTP (Chen et al., 2002; Murray and O'Connor, 2003). COX-2 modulation of hippocampal synaptic transmission and plasticity appears to be mediated by PGE2, a predominant reaction product of COX-2 (Chen et al., 2002; Chen and Bazan, 2005; Sang et al., 2005; Sang and Chen, 2006; Yang and Chen, 2008). Similarly, COX-2 and PGE2 also contribute to LTP in visual cortex (Akaneya and Tsumoto, 2006). Actions of PGE2 in physiological and pharmacological functions are mediated via its four subtypes of receptors (EPs), designated as EP1, EP2, EP3, and EP4 (Breyer et al., 2001; Boie et al., 1997; Namba et al. 1993; Narumiya et al., 1999; Sugimoto and Narumiya 2007). EPs are seven-transmembrane-domain G protein-coupled receptors and have distinctive signal transduction profile and cellular actions. Activation of the EP1 elevates levels of intracellular inositol phosphate and Ca2+, whereas activation of the EP2 or EP4 stimulates adenylate cyclase, resulting in increased levels of intracellular cAMP (Bastien et al., 1994; Honda et al., 1993; Nishigaki et al., 1995). Conversely, EP3 inhibits adenylate cyclase via a pertussis toxin-sensitive Gi-coupled mechanism (Breyer et al., 1994, 2001). EP2 and EP3 appear in greater abundance in the hippocampus and cortex although all four subtypes of EPs are heterogeneously expressed in the brain (Zhu et al., 2005). Sang et al (2005) provided convincing evidence that PGE2 modulation of synaptic transmission is via its activation of presynaptic EP2 in hippocampal neurons, and PGE2 is likely a retrograde messenger. However, roles of the EP2 receptor in hippocampal long-term synaptic plasticity are largely unknown.
The contribution of COX-2 to long-term synaptic plasticity and cognition has been supported from in vivo experiments where administration of COX-2 inhibitors impairs memory acquisition (Rall et al., 2003), memory consolidation (Teather et al., 2002), passive avoidance memory (Holscher 1995), and spatial memory retention (Shaw et al., 2003; Sharifzadeh et al., 2006). This information suggests that COX-2 plays an important role in regulation of long-term synaptic plasticity and cognitive function (Bazan 2001; 2003; Sang and Chen, 2006; Yang and Chen, 2008). Recent study further confirms that endogenous basal levels of PGE2 resulting from COX-2 are necessary for synaptic plasticity and memory acquisition (Chen and Bazan 2005, Cowley et al., 2008). Andreasson et al. (2001) also provide evidence that transgenic mice overexpressing functional COX-2 in neurons of hippocampus, cortex, and amygdala develop cognitive deficits in an age-dependent manner. However, little is know about whether the EP2 receptor is involved in spatial learning and memory. In this study, we demonstrate that hippocampal LTP and spatial learning are impaired in mice deficient in the EP2, suggesting that PGE2-EP2 is an important signaling pathway in modulation of hippocampal synaptic transmission and plasticity.
Materials and methods
Animals
EP2 knockout mice (KO) were generated as described previously (Kennedy et al. 1999), and backcrossed for more than ten generations onto both the C57BL/6 and the BALB/c background strains. Breeding of heterozygous mice produced homozygous EP2 wild type (WT) and KO mice. Age-matched littermates (either sex) between the ages of six to ten weeks were used in all the studies. The care and use of the animals reported in this study were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center.
Hippocampal slice preparation
Hippocampal slices were prepared from EP2 knockout and their age-matched wild-type littermate Balb/c mice (Kennedy et al., 1999) as described previously (Chen et al., 2002; Chen, 2004). Briefly, after decapitation, brains were rapidly removed and placed in cold oxygenated (95% O2, 5% CO2) low-Ca2+/high-Mg2+ slice medium composed of (in mM) 2.5 KCl, 7.0 MgCl2, 28.0 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 7.0 glucose, 3.0 pyruvic acid, 1.0 ascorbic acid, and 234 sucrose. Slices were cut at a thickness of 350 μm and transferred to a holding chamber in an incubator containing oxygenated artificial cerebrospinal fluid (ACSF) composed of (in mM) 125.0 NaCl, 2.5 KCl, 1.0 MgCl2, 25.0 NaHCO3, 1.25 NaH2PO4, 2.0 CaCl2, 25.0 glucose, 3 pyruvic acid, and 1 ascorbic acid at 36 °C for 0.5 to 1 h, and maintained in an incubator containing oxygenated ACSF at room temperature (22−24 °C) for > 1.5 h before recordings. Slices were then transferred to a recording chamber where they were continuously perfused with 95% O2, 5% CO2-saturated standard ACSF at 32−34 °C. Individual dentate granule and pyramidal neurons were viewed with an upright microscope (Olympus BX51WI) fitted with a 60× water-immersion objective and differential interference contrast (DIC) optics.
Electrophysiological recordings
Field EPSP (fEPSP) recordings were made in response to the perforant path or Schaffer-collateral stimuli at a frequency of 0.05 Hz using an Axoclamp-2B patch-clamp amplifier (Molecular Devices, CA) in bridge mode (Yang et al., 2008). The external solution contained (in mM): 130.0 NaCl, 2.5 KCl, 1.0 MgCl2, 10.0 HEPES, 1.25 NaH2PO4, 2.0 CaCl2, 25.0 glucose (pH 7.4 with NaOH). Recording pipettes were pulled from borosilicate glass with a micropipette puller (Sutter Instrument), filled with artificial ACSF (2−4 MΩ) and placed at the middle one third of the molecular layer of the dentate gyrus and the stratum radiatum of the CA1 region of the hippocampus. LTP was induced by theta burst stimulation (TBS), consisting of 10 bursts of five pulses at 100 Hz, with each burst separated by 200 ms via a bipolar tungsten electrode. The baseline stimulation strength was set to provide fEPSP with an amplitude of 30% from the subthreshold maximum. Paired-pulse stimulation was induced by delivering two pulses with an inter-pulse interval of 30 ms. Paired-pulse ratio was calculated as P2/P1 (P1, the amplitude of the first EPSP; P2, the amplitude of the second EPSP). The bath-perfused solutions contained 10 μM bicuculline to block ionotropic GABA receptors.
Whole cell patch-clamp recordings were made using an Axoclamp-2B amplifier in bridge mode. The pipette solution contained (in mM): 120 potassium gluconate, 20 KCl, 4 NaCl, 10 HEPES, 0.5 EGTA, 0.28 CaCl2, 4 Mg2ATP, 0.3 Tris2GTP, and 14 phosphocreatine (pH 7.25 with KOH). For voltage clamp experiments, an Axopatch 200B amplifier was used to record inhibitory postsynaptic currents (IPSCs). The bath solution contained 10 μM DNQX and 50 μM AP5 to isolate GABAergic synaptic transmission.
Water maze spatial task
C57BL/6 EP2 KO and EP2 WT mice were tested in the Morris water maze (120 cm diameter) filled with opaque water (26 ± 1°C) as described previously with a slight modification (Yau et al., 2007). As the testing room supplied the visual cues, great care was taken not to disturb any object in the room during testing, and the experimenters maintained a consistent appearance and posture throughout the trials. Mice were trained to swim to a 15 cm diameter hidden platform located in the center of one of four quadrants of the pool 1 cm below the water surface. This task is one of the most widely used tests of spatial memory in mice. First, the mice were given 3 days of nonspatial training (three trials per day) to find the submerged platform marked with a visible tower block protruding 10 cm on top to test for visual, motivational, or motor deficits that may influence their performance in spatial learning. Mice unable to reach the platform within 60 s on day 3 were excluded. Spatial learning was tested next by using the available spatial cues in the room. The mice were given 4 trials (20 min intertrial interval) over seven consecutive days. Trials started with the mouse placed into the pool facing the wall at one of four start locations chosen randomly across trials and allowed to swim until they found the platform. Any mouse that failed to find the platform within 60 s was guided onto the platform. The animal was then left on the platform for 30 s before being returned to its home cage. Measures of latency in each quadrant of the pool were recorded and analyzed.
Data analysis
Data are presented as mean ± S.E.M. unless stated otherwise, Student's t-test and analysis of variance (ANOVA) with Student-Newman-Keuls test were used for statistical comparison when appropriate. Differences were considered significant when P< 0.05.
Results
Hippocampal LTP is impaired at perforant path in mice deficient in the EP2 receptor
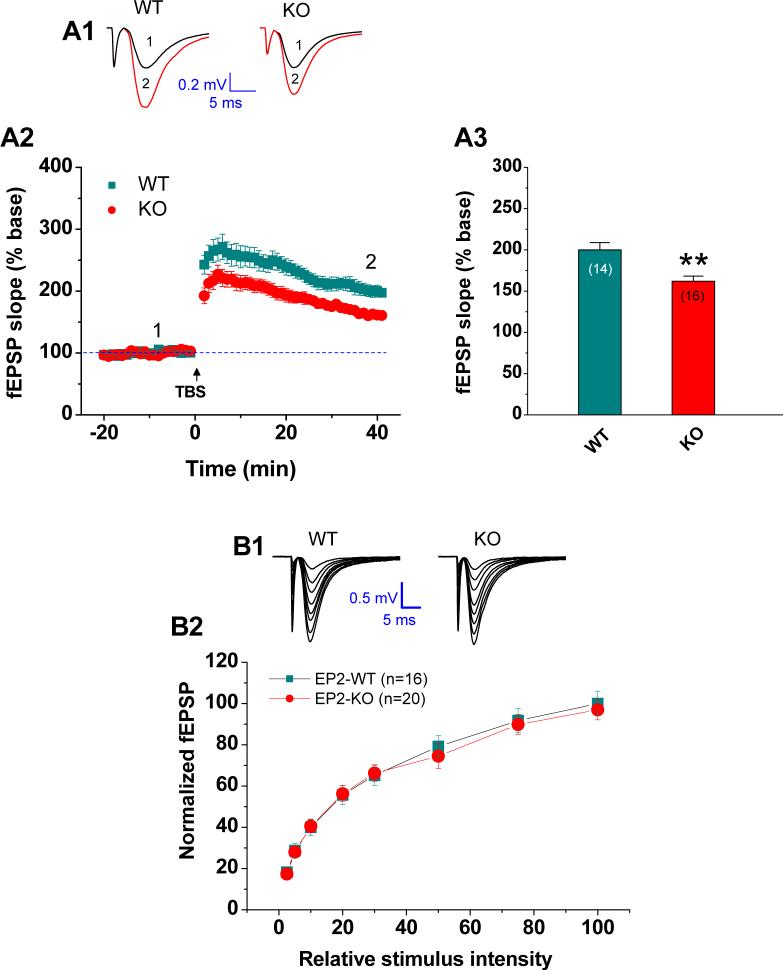
Our previous study demonstrated that the action of PGE2 in modulation of synaptic transmission is via its activation of presynaptic EP2 in hippocampal neurons (Sang et al., 2005). However, it is still not clear whether the EP2 receptor is involved in hippocampal long-term synaptic plasticity. To assess the roles of the EP2 receptor in hippocampal LTP, we recorded fEPSPs in response to perforant path or Schaffer collateral stimuli in EP2 KO mice and their WT littermates at a frequency of 0.05 Hz via a bipolar tungsten electrode in hippocampal slices. LTP was induced by theta-burst stimulation (TBS), consisting of 3 episodes at 20 sec intervals. As shown in Figure. 1A, LTP at hippocampal perforant synapses was significantly reduced in EP2 KO when compared to that in EP2 WT (161.9 ± 6.2%, n = 16 versus 199.9 ± 8.9%, n = 14, p<0.01). The duration of LTP presented in Figure 1A was monitored for 40 min following TBS. To determine whether the difference in LTP between EP2 WT and KO remains for a prolonged period, we did another set of experiments where LTP was monitored for 120 min following TBS. As shown in supplemental Figure 1, LTP is still reduced in EP2 KO when compared to that of EP WT animals. These results support the notion that the EP2 receptor contributes to hippocampal LTP.
Fig. 1.
LTP at hippocampal perforant path synapses is impaired in mice lacking the EP2. (A1) Representative traces of fEPSPs recorded at hippocampal perforant path synapses before (black) and after TBS (red) in EP2 WT and KO mice (red). (A2) Time course of the TBS-induced changes in fEPSP slopes. (A3) Mean values of the potentiation of fEPSPs averaged from 36 to 40 min following TBS at hippocampal perforant path synapses. **P< 0.01 compared with the WT control. (Numbers in the bar graphs denote the number of observations). (B1) Representative traces of fEPSPs in EP2 WT and KO mice. (B2) Input-output function curves at perforant path in EP2 WT and KO.
As we showed previously (Sang et al., 2005), we did not see changes in amplitude and frequency of mEPSCs in hippocampal neurons in culture where the EP2 was knocked down with the RNA interference technique, indicating that disruption of the EP2 does not alter basal synaptic transmission. To determine whether basal synaptic transmission is altered in EP2 null mice, we measured input-output function at hippocampal perforant path in slices from EP2 WT and KO animals. As shown in Figure 1B, the input-output function is normal in EP2 KO mice, suggesting that basal synaptic transmission is normal in EP2 KO mice.
Impaired spatial learning in EP2 KO mice
Since LTP is a cellular model of learning and memory (Bliss and Collingridge, 1993), thus we asked whether there are changes in behavioral performance in EP2 KO mice. We used a Morris water maze commonly used to test hippocampus dependent spatial memory (Morris, 1984). The protocol for the behavioral analysis was adopted from Yau et al., (2007) with a small modification. In the water maze task, both EP2 KO and their WT mice learned the location of the hidden platform as shown by a decrease in escape latencies over days of training (Figure 2). EP2 KO mice were significantly impaired in spatial learning compared to EP2 WT mice, showing longer escape latencies for each day of testing. EP2 KO mice were worse at finding the hidden platform than WT controls across the days of training with longer mean latencies, which reached significance on day four (Figure 2), suggesting that spatial learning is impaired in mice deficient in the EP2 receptor. This is consistent with the findings of impaired hippocampal LTP as shown in Figure 1.
Fig. 2.
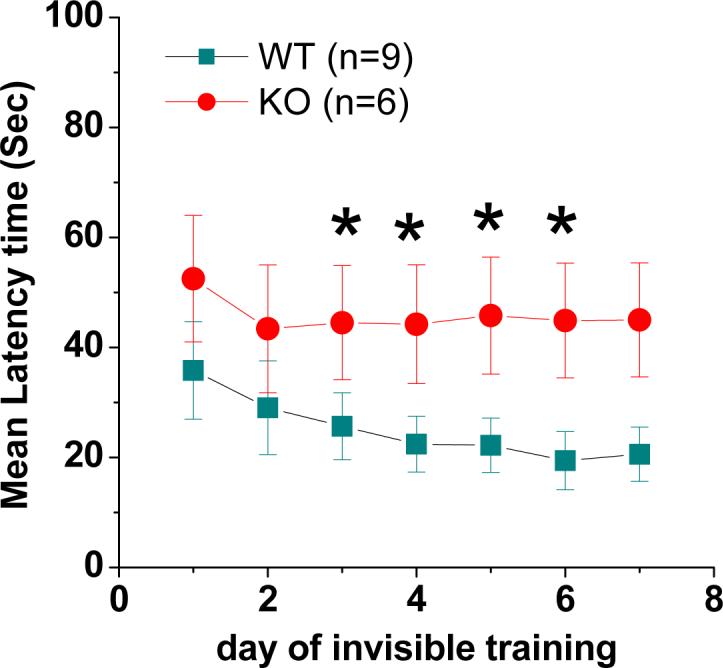
Spatial learning is impaired in EP2 KO mice. The escape latency (the time required for mice to locate the platform) is expressed as means ± S.E.M. *P< 0.05, compared with EP2 WT controls.
Deletion of the EP2 does not affect intrinsic membrane properties in hippocampal granule neurons
Activation or inhibition of EP2 receptors modulates synaptic transmission and plasticity, which may involve changes in intrinsic membrane excitability. To examine this possibility, membrane excitability and passive properties of granule neurons in the dentate gyrus were characterized in EP2 KO and their WT mice. As shown in supplemental figure 2, resting membrane potential, input resistance and firing properties in granule neurons are normal in EP2 KO mice when compared to that in EP2 WT mice. These results indicate that the impaired hippocampal LTP and cognition in EP2 KO mice are not associated with intrinsic membrane properties in hippocampal neurons.
EP2 receptors are important for the COX-2 mediated synaptic modification
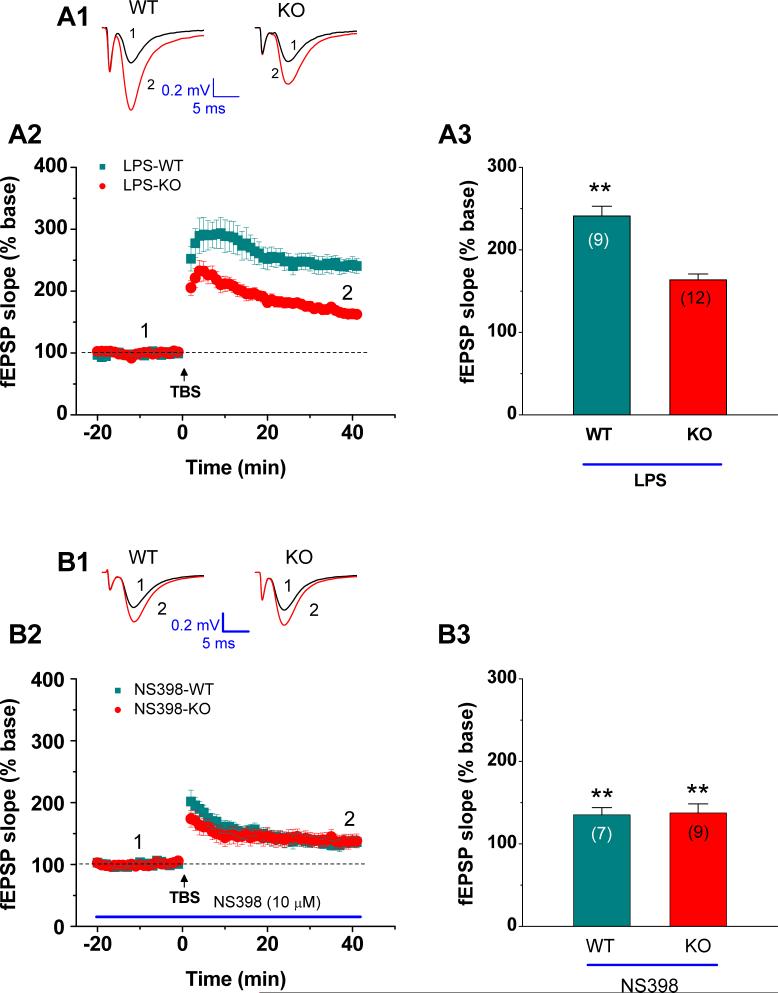
Our previous studies have showed that inhibition or elevation of COX-2 suppresses or enhances excitatory glutamatergic neurotransmission and LTP (Chen et al., 2002; Chen and Bazan, 2005; Sang et al., 2005; Yang et al., 2008). Systemic LPS administration has been shown to elevate COX-2 expression and activity in the hippocampus (Sang et al., 2005; Yang et al., 2008; Zhang & Chen, 2008). To determine whether EP2 contributes to COX-2 elevation-enhanced LTP, EP2 KO and WT mice were injected with LPS, a commonly used COX-2 inducer. As shown in Figure 3A, LTP at perforant path was significantly elevated in WT animals that received LPS (3 mg/kg, i.p.) for 12 hours (241.0 ± 11.9%, n = 9, p<0.01), but not in LPS-treated EP2 KO mice (163.6± 7.1%, n = 12, p>0.05). These results indicated that EP2 is required in COX-2 elevation-induced augmentation of LTP.
Fig. 3.
LPS fails to elevate LTP at hippocampal perforant path-dentate granule cell synapses in EP2 KO mice. (A1) Representative fEPSP recorded before (black) and after TBS (red) in the dentate gyrus in EP2 WT and KO mice injected with LPS (3 mg/kg) for 12 h. (A2) Time course of the TBS-induced changes in fEPSP slopes in EP2 WT and KO mice. (A3) Mean values of the potentiation of fEPSPs averaged from 36 to 40 min following TBS at hippocampal perforant path synapses in EP2 WT and KO mice injected with LPS for 12 h. **P<0.01 compared with vehicle control. (B1) Representative fEPSP recorded before (black) and after TBS (red) in hippocampal slices from EP2 WT and KO treated with NS398 (10 μM). Slices were treated with NS398 for at least 90 min before recording. (B2) Time course of the TBS-induced changes in fEPSP slopes in slices from EP2 WT and KO treated with NS398. (B3) Mean values of the potentiation of fEPSPs averaged from 36 to 40 min following TBS at hippocampal perforant path synapses in slices from EP2 WT and KO treated with NS398. **P<0.01 compared with vehicle control.
To determine whether inhibition of COX-2 still elicits an effect on LTP in EP2 KO, we have performed the experiments to determine perforant path LTP in EP2 KO and WT animals. As shown in Figure 3B, NS398 (10 μM) reduced LTP from 199.9 ± 8.9% (n=14) to 135.2 ± 8.7% (n=7) in EP2 WT animals and from 161.9 ± 6.2% (n=16) to 137.4 ± 11.1% (n=9) in EP2 KO animals, respectively. These results indicate that there may be other factors related to COX-2 that are involved in LTP induction. For instance, inhibition of COX-2 has been shown to augment endocannabinoid-mediated synaptic inhibition (Kim & Alger, 2004), and inhibition of the CB1 receptor prevents the COX-2 inhibitor-induced decrease of LTP (Slanina et al., 2005). It is likely that inhibition of COX-2 may reduce oxidative metabolism of endogenous cannabinoids, which contributes to LTP. Also we can not exclude out the contribution of other COX-2-related metabolites to LTP, while COX-2 is inhibited.
Both PGE2 and EP2 agonist fail to induce changes in synaptic activity in EP2 KO mice
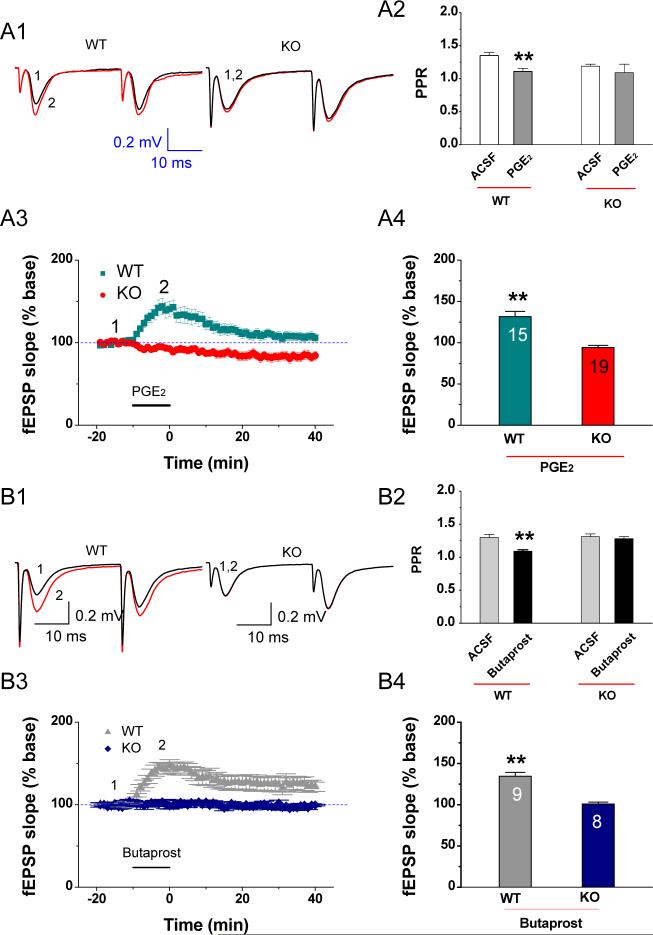
PGE2 has been proposed to act as an important signaling molecule in COX-2-mediated modulation of hippocampal synaptic transmission and plasticity (Chen et al., 2002, 2005; Sang et al., 2005; Akaneya and Tsumoto 2006). To determine whether EP2 mediates the PGE2-induced synaptic response, we recorded fEPSPs in the dentate gyrus in response to perforant path stimulus in hippocampal slices from EP2 KO and WT mice in the absence and presence of PGE2. As illustrated in Figure 4A, bath application of PGE2 (5 μM) increased fEPSP slopes to 131.6 ± 6.49 % (averaged from 10 min application; n = 15) at perforant path-dentate granule cell synapses in EP2 WT mice and to 94.3 ± 2.6 % (n = 19) in EP2 KO mice, respectively. Since application of an EP2 agonist (butaprost) did not induce any changes in fEPSPs (Figure 4B), we speculated that this small decrease may be associated with activation of the EP3 receptor. In fact, we did observe a similar small reduction of fEPSPs by application of sulprostone, an EP3 agonist, in EP2 KO animals (Data not shown). Because application of sulprostone did not induce significant changes in EPSPs and mEPSCs in normal wild-type control animals (Sang et al., 2005), it is likely that the EP2-mediated effect on synaptic activity is predominant, whereas the EP3-mediated small response is masked under normal conditions. Deletion of the EP2 unmasks the EP3-mediated small response in EP2 null mice. Our results support our speculation.
Fig. 4.
PGE2 or butaprost, an EP2 agonist, fails to enhance synaptic transmission at hippocampal perforant path in EP2 KO mice. (A1) Representative traces of fEPSP recorded before (black) and after PGE2 (red) in the dentate gyrus in EP2 WT and KO mice. (A2) Mean values of paired-pulse ratio (PPR) averaged from 6−10 min following bath application of PGE2 (5 μM) in slices from EP2 WT and KO mice. (A3) Time courses of PGE2-induced changes in fEPSP slopes in EP2 WT and KO mice. (A4) Mean values of the changes in slope of fEPSPs averaged from 10 min during bath application of PGE2. (B1) Representative traces of fEPSP recorded before (black) and after butaprost (red) in the dentate gyrus in EP2 KO and WT mice. (B2) Mean values of paired-pulse ratio (PPR) averaged from 6−10 min following bath application of butaprost (5 μM) in slices from EP2 WT and KO mice. (B3) Time courses of butaprost-induced changes in fEPSP slopes in EP2 WT and KO mice. (B4) Mean values of the changes in slope of fEPSPs averaged from 10 min during bath application of butaprost. **P< 0.01 compared with vehicle control.
Meanwhile, we used a paired-pulse protocol to determine whether there was a change in paired-pulse ratio (PPR). As indicated in Figure 4A1 & 2, PGE2 significantly reduced PPR (baseline: 1.35 ± 0.04 to PGE2: 1.1 ± 0.05, p < 0.01) at perforant path-granule cell synapses in EP2 WT mice, while it did not induce significant changes (baseline of 1.19 ± 0.03 to PGE2: 1.09 ± 0.13, p>0.05) in EP2 KO mice. These results suggested that PGE2-induced potentiation of fEPSPs may be mediated via a presynaptic EP2. To confirm this, we used butaprost, an EP2 agonist to determine whether it elicits an effect on fEPSPs in EP2 wt and KO mice. As shown in Figure 4B, bath application of butaprost (5 μM) produced a significant enhancement of the slopes of fEPSP (134.6 ± 4.6 % baseline; n = 9, P< 0.01) in EP2 WT mice, but not in EP2 KO mice (100.9 ± 2.3 % baseline; n = 8, p>0.05). Butaprost robustly decreased PPR (baseline: 1.30 ± 0.05 versus butaprost: 1.09 ± 0.02 (p<0.01) in EP2 WT mice, but failed to change the PPR in KO mice (1.31 ± 0.04% versus 1.28 ± 0.03%, p>0.05). These results thus provide conceivable evidence that PGE2 increases synaptic transmission, and this enhancement is mediated via a presynaptic EP2 receptor (Sang et al., 2005).
We demonstrated before that PGE2 inhibits GABAergic mediated spontaneous miniature IPSCs (mIPSCs) in hippocampal neurons in culture (Sang et al., 2006). To determine whether this effect is also mediated via the EP2 receptor, we recorded synaptically evoked IPSCs in granule neurons. We observed that PGE2 (5 μM) did not elicit a detectable change in IPSCs both in EP2 KO and WT animals (Supplemental figure 3), indicating that PGE2 does not have an effect on evoked GABAergic inhibitory synaptic transmission.
Downstream signaling pathways of the EP2 are still intact in EP2 KO mice
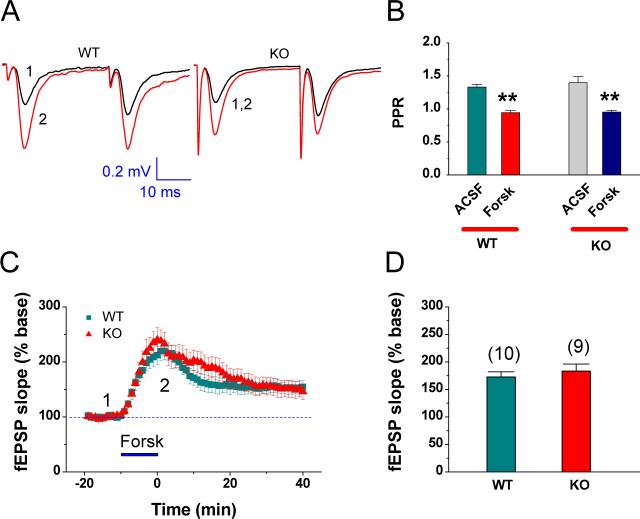
Because EP2 is linked to the Gs-cAMP/PKA pathway (Sang et al., 2005; Sang and Chen, 2006; Yang and Chen, 2008), we decided to examine whether the deletion of the EP2 results in alterations in downstream signaling pathways of the EP2. To this end, we employed forskolin (Forsk), an activator of adenylyl cyclase, to detect whether Forsk is still able to induce a change in synaptic activity in EP2 KO mice. As indicated in Figure 5, bath application of Forsk (20 μM) led to an increase in the slopes of fEPSP to 172.6 ± 9.40% (n= 10) in EP2 WT and 183.2 ± 13.0% (n=9) in EP2 KO mice. Meanwhile, Forsk significantly reduced PPR both in EP2 WT and KO mice. These data suggest that the downstream signaling pathways of the EP2 are still intact in EP2 KO mice.
Fig. 5.
Downstream signaling pathway of the EP2 is still intact in EP2 KO mice. (A) Representative traces of fEPSP recorded before (black) and after forskolin (Forsk, red) in the dentate gyrus in EP2 WT and KO mice. (A2) Mean values of paired-pulse ratio (PPR) averaged from 6−10 min following bath application of Forsk (5 μM). (B) Time courses of Forsk-induced changes in fEPSP slopes in EP2 WT and KO mice. (C) Mean values of the changes in slope of fEPSPs averaged from 10 min during bath application of Forsk in slices from EP2 WT and KO mice. **P< 0.01 compared with vehicle control.
PKA, ERK and IP3 pathways are involved in the PGE2-enhanced synaptic transmission
As we mentioned above, EP2 is linked to the Gs-cAMP/protein kinase A (PKA) pathway, we decided to use PKA inhibitors to examine whether the PGE2-induced response could be inhibited or attenuated. We used H-89, a relatively selective PKA inhibitor. As shown in Figure 6, the PGE2-induced increase in fEPSPs was significantly attenuated in the presence of 5 μM H-89 (PGE2: 131.6 ± 6.5 of baseline, n=15 versus PGE2 + H-89:110.2 ± 4.5% of baseline; n=6; p< 0.05). To test whether ERK and IP3 signaling pathways are also involved in the PGE2-mediated response, we used PD 98059 and 2-APB, ERK and IP3 inhibitors. As indicated in Figure 6, the PGE2-induced increase in fEPSP slopes was significantly reduced by bath application of PD 98059 (20 μM) or 2-APB (20 μM). These results suggest that multiple signal transduction pathways are involved in the PGE2-induced synaptic response.
Fig. 6.
PGE2-induced potentiation of synaptic transmission is mediated via multiple signaling transduction pathways. (A) PGE2-induced enhancement of fEPSP in hippocampal slices from EP2 WT mice is attenuated by H89 (5 μM). (B) PGE2-induced enhancement of fEPSP in hippocampal slices from EP2 WT mice is attenuated by 2-APB (20 μM). (C) PGE2-induced enhancement of fEPSP in hippocampal slices from EP2 WT mice is attenuated by PD98059 (10 μM). (D) Mean values of the changes in slope of fEPSPs averaged from 10 min during application of PGE2 (5 μM) in the presence of H89 (5 μM), 2-APB (20 μM) and PD (20 μM), respectively. Inset panels showing representative traces of fEPSPs under the different treatments. *P<0.05, **P<0.01 compared with PGE2 alone.
Discussion
In the present study, we show that Hippocampal LTP is impaired both at perforant path synapses, while membrane passive and firing properties of granule neurons in the dentate gyrus are still normal in EP2 KO mice. It is of interest that spatial learning in the water maze is also impaired in EP2 KO mice when compared to WT mice. Lack of efficacy in response to PGE2 and EP2 agonist in EP2 null mice suggests that EP2 mediates the PGE2-induced synaptic activity. Decrease PPR in response to PGE2 and EP2 agonist in EP2 WT means that PGE2-induced response is via a presynaptic mechanism. Failure to enhance hippocampal LTP in EP2 KO mice that received LPS further supports the notion that COX-2 elevation-induced augmentation of LTP is mediated via PGE2-EP2 signaling. Furthermore, we have identified that downstream signaling pathways of the EP2 are still intact in EP2 KO mice and the PGE2-enhanced synaptic transmission is mediated via PKA, ERK and IP3 pathways.
COX-2 is the rate-limiting enzyme catalyzing AA into prostaglandins (Simmons et al., 2004). It has been shown that PGE2 is mainly derived from the COX-2 pathway (Brock et al., 1999; Chen and Bazan, 2005; Vidensky et al., 2003; Sang et al., 2005). Increasing evidence has revealed that COX-2 and PGE2 participate in synaptic transmission and plasticity (Sang and Chen, 2006; Yang and Chen, 2008). Chen et al. (2002) provided the first direct evidence that COX-2 is involved in hippocampal long-term synaptic plasticity. The involvement of COX-2 in hippocampal LTP has been confirmed by studies performed by others who show that the inhibition of COX-2 suppresses both LTP (Murray and O'Connor, 2003; Slanina and Schweitzer, 2005) and long-term depression in the hippocampus (Murray and O'Connor, 2003). Several studies have demonstrated that elevation of COX-2 induced by LPS enhanced the frequency of miniature excitatory postsynaptic currents (mEPSCs) in hippocampal neurons in culture and significantly facilitates basal synaptic transmission (Sang et al., 2005) and mEPSCs and induces a time-dependent augmentation of LTP at hippocampal perforant path (Yang et al., 2008). Previous studies showed that COX-2 modulation of hippocampal synaptic transmission and plasticity is mediated via PGE2 (Chen et al., 2002; Chen and Bazan, 2005). We also demonstrated that all four G-protein-coupled EP1−4 receptors are heterogeneously expressed both in neurons and astroglial cells in the hippocampus and cortex (Zhu et al. 2005). Our results, which show impaired hippocampal LTP both at perforant path synapses in mice deficient in the EP2 reveal the functional role of the EP2 in hippocampal long-term synaptic plasticity. Recent study using the in vivo siRNA technique also supports the role of the EP2 in synaptic plasticity in visual cortex (Akaneya and Tsumoto, 2006).
Previously, we demonstrated that both constitutive and inducible COX-2 contribute to activity-dependent hippocampal LTP (Chen et al., 2002; Chen & Bazan, 2005; Yang et al., 2008). The results shown in Figure 1 indicate that both constitutive and inducible COX-2 likely contributes to TBS-induced LTP. Yamagata, et al. (1993) reported that COX-2 expression is up-regulated by high-frequency stimulation (HSF) that is associated with LTP induction. An increase in mRNA of COX-2 was detected 30 min after HFS. This means that LTP stimulation-induced elevation of COX-2 contributes to the maintenance of LTP. As shown in Figure 3, elevation or inhibition of COX-2 significantly modulates LTP, which provides further evidence of the involvement of both constitutive and inducible COX-2 in hippocampal LTP.
Increasing evidence suggests that COX-2 participates in cognition, which is supported from the experiments where administration of selective COX-2 inhibitors impairs passive avoidance task (Holscher 1995; Sato et al., 2007), memory acquisition, memory retention (Rall et al., 2003; Sharifzadeh et al., 2005 ;Teather et al., 2002), and spatial memory consolidation (Sharifzadeh et al., 2006;Shaw et al., 2003). Recent study further confirms that endogenous basal levels of PGE2 resulting from COX-2 are necessary for synaptic plasticity and memory acquisition (Cowley et al., 2008). Our results show that spatial learning in the water maze is significantly impaired in EP2 KO when compared to that in WT mice, indicating that the EP2 receptor is involved in spatial learning. Taken together, the EP2 receptor may be the downstream target of COX-2 in brain cognitive function. Although both hippocampal LTP and spatial learning are impaired in mice deficient of the EP2 receptor, membrane passive and firing properties in granule neurons of dentate gyrus are still normal in EP2 KO mice. Previous study in our lab revealed that endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons (Chen and Bazan, 2005).
Sang et al. (2005) reported that PGE2 increases excitatory postsynaptic potentials (EPSPs) and decreases the paired-pulse ratio (PPR) at perforant path-granule cell synapses in the dentate gyrus and Schaffer-collateral synapses in the CA1 area in hippocampal slices and enhances the frequency of mEPSCs in hippocampal neurons in culture. They further demonstrated that EP2 agonist mimics the PGE2 effect, whereas EP1 or EP3 agonist fails to enhance the synaptic transmission both in hippocampal slices and neurons in culture. We further confirmed that both PGE2 and EP2 agonist produce an increase in synaptic transmission and decrease PPR in slices from EP2 WT, but not in EP2 KO mice. These findings suggest that presynaptic EP2 receptors mediate the PGE2-induced response. Since the PGE2-elevated synaptic activity is mainly mediated by the EP2, which is linked to the Gs-cAMP/PKA pathway, it is believed that the PGE2-potentiate neurotransmission is mediated via the EP2–Gs–cAMP/PKA pathway (Sang et al., 2005). In the present study, we show that the PGE2-enhanced synaptic transmission was not only attenuated by a PKA inhibitor, but also by ERK and IP3 inhibitors, indicating that multiple pathways are involved in the PGE2-induced synaptic activity.
In this report, we provide evidence that EP2 receptor plays an important role in hippocampal LTP and spatial learning. We also confirm that the PGE2-induced synaptic response is via its action on a presynaptic EP2 receptor (Sang et al., 2005). This means that PGE2-EP2 signaling is important for hippocampal long-term synaptic plasticity and cognitive function.
Supplementary Material
Acknowledgement
Authors’ work was supported by National Institutes of Health grants R01NS054886 and P20RR16816 (to CC) and P50GM15431 (to RMB), and the Alzheimer's Association grant IIRG-05-13580 (to CC).
References
- Akaneya Y, Tsumoto T. Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex. J. Neurosci. 2006;26:10209–10221. doi: 10.1523/JNEUROSCI.3028-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson KI, Savoneko A, Vidensky S, Goellner JJ, Yang Y, Shaffer A, Kaufmann WE, Worley PF, Isakson P, Markowska AL. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J. Neurosci. 2001;21:8198–8209. doi: 10.1523/JNEUROSCI.21-20-08198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien L, Sawyer N, Grygorczyk R, Metters KM, Adam M. Cloning, functional expression, and characterization of the human prostaglandin E2 receptor subtype. J. Biol. Chem. 1994;269:11873–11877. [PubMed] [Google Scholar]
- Bazan NG. COX-2 as a multifunctional neuronal modulator. Nat. Med. 2001;7:414–415. doi: 10.1038/86477. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J. Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Boie Y, Stocco R, Sawyer N, Slipetz DM, Ungrin MD, Neuschäfer-Rube F, Püschel GP, Metters KM, Abramovitz M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur. J. Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Bagdassarian CK, Myers SA. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- Breyer RM, Emeson RB, Tarng JL, Breyer MD, Davis LS, Abromson RM, Ferrenbach SM. Alternative splicing generates multiple isoforms of a rabbit prostaglandin E2 receptor. J. Biol. Chem. 1994;269:6163–6169. [PubMed] [Google Scholar]
- Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J. Biol. Chem. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- Chen C. ZD7288 inhibits postsynaptic glutamate receptor-mediated responses at hippocampal perforant path-granule cell synapses. Eur. J. Neurosci. 2004;19:643–649. doi: 10.1111/j.0953-816x.2003.03174.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J. Neurophysiol. 2005;93:929–941. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- Cowley TR, Fahey B, O'Mara SM. COX-2, but not COX-1, activity is necessary for the induction of perforant path long-term potentiation and spatial learning in vivo. Eur. J. Neurosci. 2008;27:2999–3008. doi: 10.1111/j.1460-9568.2008.06251.x. [DOI] [PubMed] [Google Scholar]
- Holscher C. Inhibitors of cyclooxygenases produce amnesia for a passive avoidance task in the chick. Eur. J. Neurosci. 1995;7:1360–1365. doi: 10.1111/j.1460-9568.1995.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Honda A, Sugimoto Y, Namba T, Watabe A, Irie A, Negishi M, Narumiya S, Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin E2 receptor to induce wakefulness in rats. J. Neurosci. 1993;23:5975–5983. [Google Scholar]
- Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat. Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat. Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Murray HJ, O'Connor JJ. A role for COX-2 and p38 mitogen activated protein kinase in long-term depression in the rat dentate gyrus in vitro. Neuropharmacol. 2003;44:374–380. doi: 10.1016/s0028-3908(02)00375-1. [DOI] [PubMed] [Google Scholar]
- Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, Ito S, Ichikawa A, Narumiya S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993;365:166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structure, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nishigaki N, Negishi M, Honda A, Sugimoto Y, Namba T, Narumiya S, Ichikawa A. Identification of prostaglandin E receptor “EP2” cloned from mastocytoma cells EP4 subtype. FEBS Lett. 1995;364:339–341. doi: 10.1016/0014-5793(95)00421-5. [DOI] [PubMed] [Google Scholar]
- Rall JM, Mach SA, Dash PK. Intrahippocampal infusion of a cyclooxygenase-2 inhibitor attenuates memory acquisition in rats. Brain Res. 2003;968:273–276. doi: 10.1016/s0006-8993(03)02248-0. [DOI] [PubMed] [Google Scholar]
- Sang N, Chen C. Lipid signaling and synaptic plasticity. Neuroscientist. 2006;12:425–434. doi: 10.1177/1073858406290794. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Chen C. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. J. Physiol. 2006;572:735–745. doi: 10.1113/jphysiol.2006.105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J. Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Ishida T, Irifune M, Tanaka K, Hirate K, Nakamura N, Nishikawa T. Effect of NC-1900, an active fragment analog of arginine vasopressin, and inhibitors of arachidonic acid metabolism on performance of a passive avoidance task in mice. Eur. J. Pharmacol. 2007;560:36–41. doi: 10.1016/j.ejphar.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Sharifzadeh M, Naghdi N, Khosrovani S, Ostad SN, Sharifzadeh K, Roghani A. Post-training intrahippocampal infusion of the COX-2 inhibitor celecoxib impaired spatial memory retention in rats. Eur. J. Pharmacol. 2005;511:159–166. doi: 10.1016/j.ejphar.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Sharifzadeh M, Tavasoli M, Soodi M, Mohammadi-Eraghi S, Ghahremani MH, Roghani A. A time course analysis of cyclooxygenase-2 suggests a role in spatial memory retrieval in rats. Neurosci. Res. 2006;54:171–179. doi: 10.1016/j.neures.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O'Mara SM. Deficits in spatial learning and synaptic plasticity induced by the rapid and competitive broadspectrum cyclooxygenase inhibitor ibuprofen are reversed by increasing endogenous brain-derived neurotrophic factor. Eur. J. Neurosci. 2003;17:2438–2446. doi: 10.1046/j.1460-9568.2003.02643.x. [DOI] [PubMed] [Google Scholar]
- Slanina KA, Schweitzer P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacol. 2005;49:653–659. doi: 10.1016/j.neuropharm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Slanina KA, Roberto M, Schweitzer P. Endocannabinoids restrict hippocampal long-term potentiation via CB1. Neuropharmacol. 2005;49:660–668. doi: 10.1016/j.neuropharm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin Prostaglandin E receptors. J. Biol. Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn. Mem. 2002;9:41–47. doi: 10.1101/lm.43602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidensky S, Zhang Y, Hand T, Goellner J, Shaffer A, Isakson P, Andreasson K. Neuronal overexpression of COX-2 results in dominant production of PGE2 and altered fever response. Neuromol. Med. 2003;3:15–28. doi: 10.1385/NMM:3:1:15. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen C. Cyclooxygenase-2 in synaptic signaling. Curr. Pharm. Des. 2008;14:1443–1451. doi: 10.2174/138161208784480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang J, Andreasson K, Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol. Cell. Neurosci. 2008;37:682–695. doi: 10.1016/j.mcn.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, McNair KM, Noble J, Brownstein D, Hibberd C, Morton N, Mullins JJ, Morris RG, Cobb S, Seckl JR. Enhanced hippocampal long-term potentiation and spatial learning in aged 11beta-hydroxysteroid dehydrogenase type 1 knock-out mice. J. Neurosci. 2007;27:10487–10496. doi: 10.1523/JNEUROSCI.2190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J. Biol. Chem. 2008;283:22601–22611. doi: 10.1074/jbc.M800524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PM, Genc A.i., Zhang X, Zhang J, Bazan NG, Chen C. Heterogeneous expression and regulation of hippocampal prostaglandin E2 receptors. J. Neurosci. Res. 2005;81:817–826. doi: 10.1002/jnr.20597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.