Abstract
Human embryonic stem (ES) cells can be maintained in an undifferentiated state if the culture medium is first conditioned on a layer of mouse embryonic fibroblast (MEF) feeder cells. Here we show that human ES cell proliferation is coordinated by MEF-secreted heparan sulfate proteoglycans (HSPG) in conditioned medium (CM). These HSPG and other heparinoids can stabilize basic fibroblast growth factor (FGF2) in unconditioned medium at levels comparable to those observed in CM. They also directly mediate binding of FGF2 to the human ES cell surface, and their removal from CM impairs proliferation. Finally, we have developed a purification scheme for MEF-secreted HSPG in CM. Utilizing column chromatography, immunoblotting, and mass spectrometry-based proteomic analysis, we have identified multiple HSPG species in CM. The results demonstrate that HSPG are key signaling cofactors in CM-based human ES cell culture.
Keywords: Human embryonic stem cells, heparan sulfate proteoglycans, stem cell-FGF2 interactions
INTRODUCTION
Since their derivation, human embryonic stem (ES) cells have held promise as a model system for human development. Immortal and karyotypically normal, human ES cells have the potential to form any cell type in the body—though guiding specific developmental outcomes remains a core challenge. Another has been establishing reproducible culture techniques yielding homogenous populations of undifferentiated, pluripotent human ES cells. The first human ES cell lines were successfully derived and expanded in a co-culture system atop a layer of mitotically inactivated mouse embryonic fibroblast (MEF) feeder cells [1]. Since then, multiple human ES cell culture systems have been developed [2-9].
Our previous work highlighted the central importance of basic fibroblast growth factor (FGF2) in human ES cell proliferation [4]. MEF-conditioned media (CM) stabilizes over seventy five percent of the initial FGF2 dose through a standard 24-hour culture period. In contrast, less than twenty percent of the same dose of FGF2 in unconditioned media (UM) remains after overnight incubation, and UM alone cannot support long-term human ES cell culture. The effect of this FGF2 degradation can be overcome by increasing its initial dose in UM. If FGF2 remains above nanomolar concentrations, long-term, feeder-independent growth can proceed [4]. In this report we investigate the function of FGF2 stabilization during medium conditioning.
Heparan sulfate (HS) is a key cofactor for FGF signaling. FGF family members can bind their receptors, but the addition of HS increases binding affinities by more than an order of magnitude [10]. As a result, high concentrations of FGF can signal in a heparin-free environment, but high affinity signaling is limited to the full ligand/receptor/HS complex [11, 12]. Modeling studies demonstrate a wide range of flexibility in stoichiometry, but the most typical models describe a dimer of FGF ligands bound to a dimer of FGF receptors stabilized by a cell surface-anchored heparan sulfate proteoglycan (HSPG) through its HS chain [13, 14]. Common to virtually all cell types, HSPG interact with many growth factors, morphogens, and cytokines, critically influencing numerous biological processes [15]. They can be found attached to the cell surface, localized to the basement membrane, or secreted into culture media [16]. Organized as sulfated domains separated by spacers of low sulfation, the HS chains of HSPG vary in length and sulfation patterns, yielding vast potential for variability [17].
Only a limited subset of cell types can effectively condition human ES cell culture media. Therefore we hypothesized that distinct, MEF-secreted HSPG are required for human ES cell self-renewal. We show that culture systems utilizing high concentrations of FGF2 in place of media-conditioning can circumvent secreted HSPG requirements, while stimulating human ES cell proliferation.
MATERIALS AND METHODS
Human ES Cell Culture
Pluripotent human ES cell lines H1 (XY), H7 (XX), H9 (XX), or H14 (XY) from WiCell (http://www.wicell.org) were maintained in CM, UM100, or TeSR1 as previously described [4, 7, 18]. For heparin (porcine intestinal mucosa), HS (porcine intestinal mucosa), and HSPG (EHS mouse sarcoma) (all from Sigma, St. Louis, MO)-supplemented growth curve assays, CM-cultured human ES cells were plated on Matrigel-coated (Becton Dickinson, Franklin Lakes, NJ) tissue culture plates (NUNC, Rochester, NY) at a density of 5 105 cells/well in CM, and duplicate or triplicate wells were converted to test media (CM, UM, UM supplemented with 40 ng/ml FGF2 [UM40], plus 0, 10, 100, or 1000 ng/ml heparin, HS, or HSPG) after 24 hours (day 1). At the conclusion of each passage, cells in a single well were individualized with trypsin/EDTA (Invitrogen, Carlsbad, CA) and counted in triplicate to determine the overall proliferation. The remaining wells were then split with dispase (Invitrogen) and, if available, 5 105 cells/well were seeded in test media for a total of five passages.
For human ES cells cultured in heparin lyase I, II, or III (all from Sigma), cells were passaged at appropriate dilutions in CM, UM supplemented with 100 ng/ml FGF2 (UM100), or mTeSR1 (i.e., standard media). After 24 hours, wells were replaced with fresh standard media or standard media supplemented with 0.1 U/ml of enzyme just prior to incubation. For subsequent passages, wells were split at appropriate dilutions determined for standard media. Back-up wells were counted in triplicate and cell numbers were quantified by the ratio of cell numbers in supplemented versus standard media for five passages.
ELISAs
A perlecan Enzyme-Linked ImmunoSorbent Assay (ELISA) was developed using an anti-HSPG monoclonal (Millipore, Billerica, MA) directed against the perlecan core protein and a biotinylated anti-Endorepellin polyclonal (R&D, Minneapolis, MN) directed against the carboxy-terminal domain of perlecan. In 96-well culture dishes (Nunc), 400 ng/well of anti-HSPG was incubated overnight at room temperature (RT). Wells were washed four times with 300 μl Wash Buffer (0.05% Tween-20/phosphate-buffered saline [PBS]) and blocked for one hour at RT with 300 μl Blocking Buffer (1% bovine serum albumin [BSA]/PBS). After washing as above, wells were incubated for two hours at RT with 25, 50, or 100 μl of either CM or UM. Wells were washed, then incubated for two hours at RT with 25 ng/well anti-Endorepellin. After washing, wells were incubated with avidin-peroxidase conjugate (Sigma) for 30 min at RT. Wells were washed and the signal detected with ABTS solution (Sigma). Signal strength was measured at 405 nm using a Tecan GENios Pro plate reader and analyzed with Magellan5 software (Tecan, Zurich, Switzerland). FGF2 ELISAs were performed as previously described [4].
Human ES Cell-FGF2 Interactions
Aldehyde-terminated self-assembled monolayers (SAMs) on gold substrates were prepared [19]. Briefly, FGF2 was covalently attached to the aldehyde-terminated gold substrates by hand-spotting 0.7 μl followed by overnight incubation in a humid chamber at room temperature. 0.5 mg/mL FGF2 (Peprotech) was spotted in buffer H (10 mM HEPES, 1 mM CaCl2, 0.1% NaN3, pH 8.5). To reduce the imine product of the reaction between the primary amine groups on the protein and the aldehyde groups on the gold substrate to a more stable secondary amine, NaBH3CN was added (50 mM final concentration) to the solution prior to spotting [19, 20]. After overnight incubation, the FGF2-modified substrate was soaked in 1% BSA/PBS for 30 min to block the remaining amino-reactive surface sites on the substrate. Typical spots were 1.3 mm in diameter.
For binding studies, human ES cells were individualized and counted in triplicate. 3 105 cells were mixed either with CM or UM plus heparin, HS, or HSPG, and added to the FGF2-SAMs at the bottom of a 6-well tissue culture plate. For FGF2 competition studies, 3 105 cells in CM plus 0, 4, 40, 100, 250, 500, 750, 1000, or 4000 ng/ml FGF2 were preincubated for two hours at 37° C prior to binding at which point the combined medium/FGF2/human ES cell mixture was added to the slide. For heparin lyase III (HLIII) studies, CM was preincubated with 1 U/ml HLIII for one hour at 37° C prior to cell addition. Binding reactions were incubated for 30 min at 37° C followed by gentle washing with PBS. Phase contrast images (Leica, Wetzlar, Germany) of cell binding were taken at 5X magnification and binding densities were determined using cell-counting software.
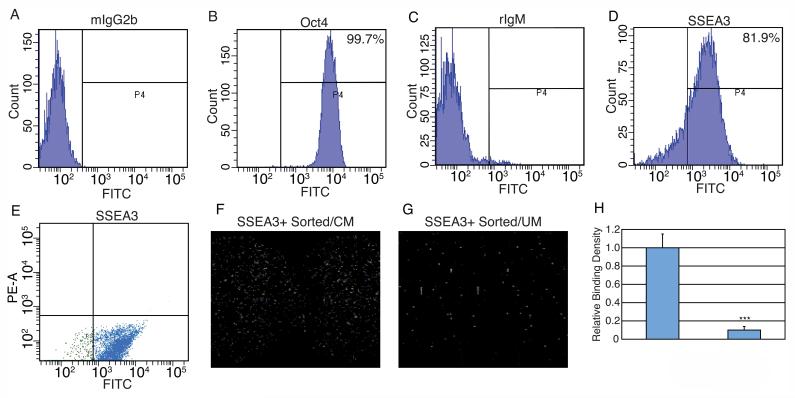
For binding studies with SSEA3 (embryonic stem cell specific cell surface antigen)-positive human ES cells, the cells were individualized in trypsin/EDTA plus 2% chick serum (Sigma) and filtered through a 100 μM screen (BD). Cells were collected and washed in Buffer F (PBS, 2% Fetal Bovine Serum [Invitrogen], 0.1% NaN3). Cells were probed with anti-SSEA3 (R&D) in Buffer F for two hours at RT and then washed in Buffer F. Cells were then probed 45 min in the dark with a FITC-conjugated goat anti-rat IgM secondary antibody (Rockland, Gilbertsville, PA). For analysis of pluripotency-specific transcription factor Oct4 expression, cells, after individualization, were fixed for ten min at 37° C in 0.5% paraformaldehyde (EMS, Hatfield, PA). Cells were then permeabilized for 30 min on ice in 90% methanol. Cells were washed in Buffer FT (Buffer F plus 0.1% Triton [Sigma]) and probed with anti-Oct4 (Santa Cruz, Santa Cruz, CA), washed in Buffer FT, and probed with a FITC-conjugated goat anti-mouse IgG2b secondary antibody (Santa Cruz). Normal rat IgM and normal mouse IgG2b served as isotype controls for SSEA3 and Oct4 respectively. Expression levels and cell sorting were performed on a FACSAria (BD) and evaluated with Diva software (BD). Sorted cells were spun down, diluted in CM or UM, and bound to FGF2-SAMs for 30 min at 37° C.
Purification of MEF-Secreted HSPG
Samples of CM were adjusted from pH 7.3 to 5.5 by drop-wise addition of 5 M HCl. A 5 mL DEAE-Sepharose Fast Flow column (GE Healthcare, Piscataway, NJ) was charged with five column volumes of Buffer B (2 M NaCl, 20 mM Piperazine pH 5.5, 0.5 mM EDTA) and equilibrated with five column volumes of Buffer A (100 mM NaCl, 20 mM Piperazine pH5.5, 0.5 mM EDTA) on an AKTA FPLC (GE Healthcare). The CM pH5.5 was then loaded and the flow-through collected. DEAE-binding species were eluted with Buffer B, pooled, and dialyzed against 100 mM NaCl/PBS.
A 1 mL FGF2-agarose affinity column (made in-house) was charged with ten column volumes of 2 M NaCl/PBS and equilibrated with ten column volumes of 100 mM NaCl/PBS. DEAE-bound fractions were loaded and the flow-through was collected. FGF2-binding species were eluted with 2 M NaCl/PBS. For mass spectrometry based protein identification, samples were precipitated directly from the elution buffer in 20% trichloroacetic acid (Pierce, Rockford, IL).
The presence of HSPG during the purification process was determined by immunoblotting. Assayed samples were concentrated with Microcon centrifugal filter units - 3000 MWCO (Millipore) according to manufacturer’s recommendations. During concentration, samples were exchanged into HLIII digestion buffer (50 mM NaCl, 20 mM Tris-HCl pH7.5, 4 mM CaCl2). Samples were digested overnight at 37° C with 50 mU HLIII and 20 mU Chondroitinase ABC (Sigma)/μg protein. Digested samples were run on SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked in 1X blocking buffer (Sigma) and probed with a “3G10” anti-HSPG antibody (Seikagaku, Tokyo, Japan) [The 3G10 antibody detects an epitope common to all HSPGs following digestion with HLIII [21]]. Filters were then probed with an HRP-conjugated goat anti-mouse IgG2b secondary antibody (Santa Cruz), washed twice, and the signal was detected with ECL reagents (GE Healthcare) and a Fuji Imager LAS-3000 (Fuji, Valhalla, NY).
Mass Spectrometry and Proteomic Analysis
Protein identifications were made following standard shotgun proteomic analysis methodology [22]. In brief, targeted FPLC fractions shown to be enriched for HSPG by 3G10 blotting were precipitated with trichloroacetic acid, resuspended in denature buffer (8M urea, 400 mM ammonium bicarbonate), reduced with DTT, alkylated with IAA and digested with sequencing grade trypsin (Promega, Madison, WI). The digests were desalted using off-line reversed-phase solid phase extraction (SPEC18, Millipore) following the manufacturer’s protocol. The cleaned digests were analyzed via nanoLC-MS/MS using LTQ or LCQ ion trap mass spectrometers (Thermo Fisher Scientific, Waltham, MA). A nanoflow LC (Eksigent, Dublin, CA) in vented column configuration was employed using trap and analytical columns (100 & 75 micron i.d. respective) packed in-house with Magic 5u, 200Å C18, (Michrom Bioresources, Inc., Auburn, CA). Data-dependent acquisition was employed over a 4 hour binary gradient elution from 98% buffer C (97:3 water:acetonitrile (B&J Brand), 0.1% formic acid (EMD Chemicals, Gibbstown, NJ), to 90% buffer D (10:90 water:acetonitrile, 0.1% formic acid). The raw data was searched against a mouse-bovine subset of the NCBI.nr or a mouse-bovine_IPI protein database using the SEQUEST algorithm included in the Bioworks Browser software package (Thermo Fisher Scientific). The database search results were filtered through the Trans-Proteomic Pipeline (TTP) open source software algorithms (Institute for Systems Biology-Seattle Proteome Center), and reported protein identifications were made from two or more distinct peptide matches with protein probability ≥ 0.9. All reagents were purchased from Sigma-Aldrich unless otherwise noted.
Statistical Analysis
To assess the statistical significance of the results, each experiment was repeated at least three times. Means and standard deviations between different groups were calculated, and error bars were included where necessary. Student’s t-test was performed for paired analysis. P values below 0.05 were considered significant.
RESULTS
Heparin, HS, and HSPG Stabilization of FGF2
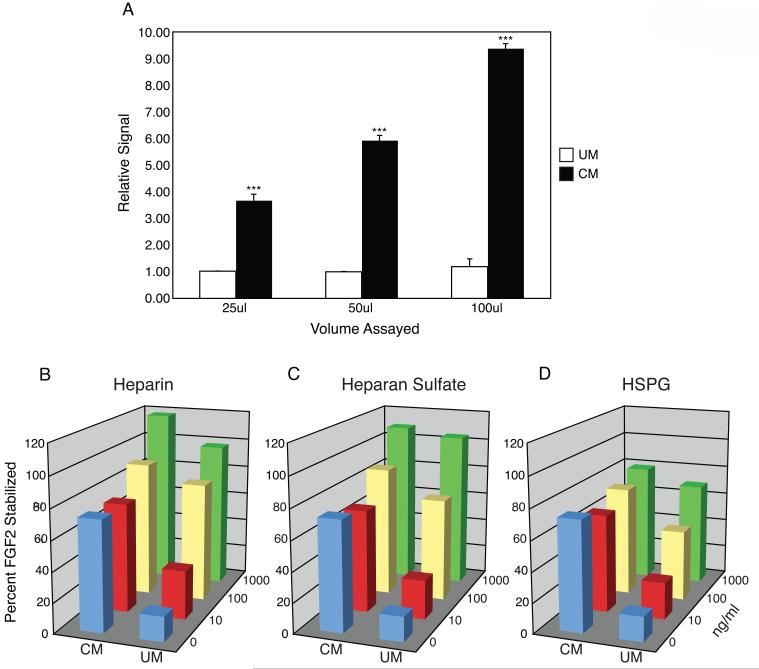
To understand the nature of FGF2 stability in CM, we examined whether MEF might secrete HSPG into culture medium during conditioning. To this end, we developed an ELISA for perlecan, employing commercial antibodies. Using a colorimetric assay, we detected an increasing and significant dose-dependent signal for CM, while UM failed to produce a signal above baseline (Figure 1A). The results clearly show that MEF secrete HSPG during medium conditioning. This observation is consistent with other reports of HSPG secretion into culture media [16].
Figure 1.
HSPG Secretion and FGF2 Stabilization. (A) ELISA for HSPG perlecan in conditioned and unconditioned media (CM or UM). Signal strength for 25ul of UM was normalized to 1.00 and relative signals for remaining samples are reported. Mean values +/- SEM, n = 3, ***p < 0.01. (B—D) Heparin, heparan sulfate, or heparan sulfate proteoglycan were added exogenously at three different concentrations or not at all. FGF2 concentration is graphed as the percent stabilization of an initial dose after 24-hour incubation at 37° C in either CM or UM.
To determine whether stem cell media are appropriate environments for heparinoids to function as stabilizing factors, we supplemented different media with a range of commercially available heparinoids. Employing a FGF2-based ELISA, we measured the stabilizing effect of heparin, HS, or HSPG at three distinct doses within CM or UM (Figure 1B-D). In UM, FGF2 stability increased in a dose-dependent manner. After an overnight incubation at 37° C the amount of FGF2 remaining in solution ranged from an average of 16 percent of the original dose in UM alone, to 91 percent at the highest concentration assayed for all heparinoids. In CM, a dose of 10 ng/ml of each heparinoid assayed consistently resulted in a slight decrease (p > 0.1) in FGF2 stability compared to CM alone, while doses of 100 or 1000 ng/ml were more effective at stabilizing FGF2 than CM alone. These data show that the levels of FGF2 stabilization observed in CM can be achieved in UM by adding exogenous heparinoids, supporting the hypothesis that MEF-secreted HSPG stabilize FGF2.
Because our culture system utilizes Matrigel as its extracellular matrix, and Matrigel is known to contain HSPG, we sought to determine whether the apparent instability of FGF2 in UM might be due to its sequestration from the media by Matrigel. A direct comparison of FGF2 levels in UM after overnight incubation either on uncoated or Matrigel-coated tissue culture plastic yielded virtually no difference in concentrations (Figure S1). Therefore we conclude that Matrigel does not significantly sequester FGF2 from media.
Heparinoids Stimulate a Mitogenic Response in Human ES Cells
It has been shown that UM supplemented with 4 ng/ml FGF2 cannot support long-term human ES cell culture. In contrast, human ES cells cultured in UM40 (UM containing 40 ng/ml FGF2) can proliferate. However, these culture conditions yield heterogeneous cell populations [8, 9]. Because MEF secrete HSPG into human ES cell media during the conditioning process, and exogenous heparinoids can stabilize FGF2 in UM, we asked whether, like FGF2, exogenous heparinoids could support long-term human ES cell culture.
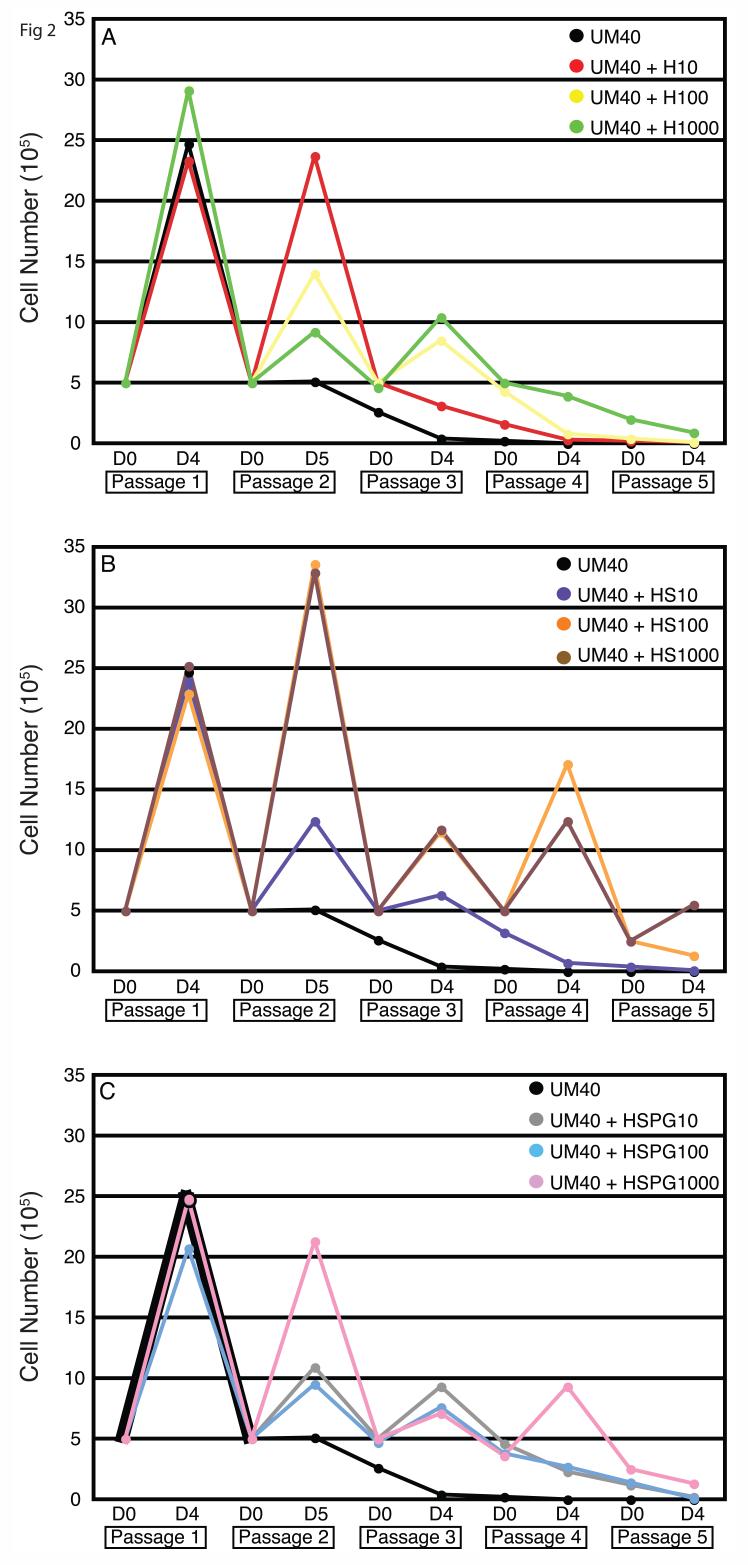
To this end, we performed growth curve assays over five passages of human ES cells in CM, UM (4 ng/ml FGF2), or UM40. Each of these media was also supplemented with exogenous heparin, HS, or HSPG at 10, 100, or 1000 ng/ml for a total of 30 experimental conditions. We found that within CM, the addition of heparinoids neither improved nor adversely affected proliferation (Figure S2). In UM, all forms and doses of heparinoids failed to support human ES cell cultures (Figure S3). However, in UM40, higher doses of the heparinoids were able to stimulate robust human ES cell growth for at least three passages, after which proliferation rates trailed off (Figure 2). It is unlikely this effect was due solely to an increase in stabilized FGF2. For example, after the addition of 100 ng/ml HS to UM40, more FGF2 remains in solution than does in non-supplemented UM100 (which supports human ES cell culture) [4]. It is more likely the proliferative boost is due to stable HS-FGF2 complexes, which have an increased affinity for cell surface receptors [10]. The inability to support long-term culture, however, suggests that specific, MEF-secreted HSPG are needed to provide the appropriate self-renewal signal. Alternatively, HSPG might be only one of multiple, critical factors secreted by MEF during conditioning.
Figure 2.
Heparinoid Supplementation of Unconditioned Medium. Growth curve assay of unconditioned medium (UM) containing 40 ng/ml FGF2 (UM40) supplemented with increasing doses of (A) heparin (H), (B) heparan sulfate (HS), or (C) heparan sulfate proteoglycan (HSPG). Human ES cells were cultured for five passages. Wells were counted in triplicate on day 4 or 5 and 5 105 cells were plated, when possible, for subsequent passages (Day 0).
HSPG Mediate Human ES Cell Binding to FGF2
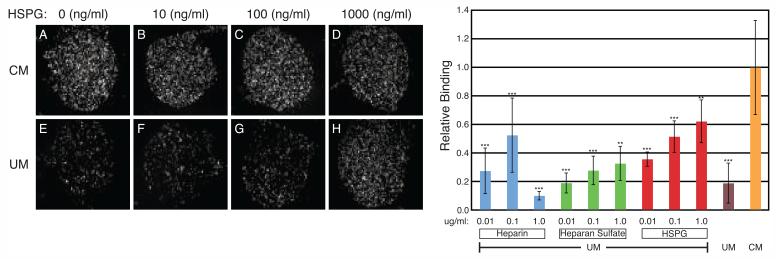
While it is clear that different heparinoids can stabilize FGF2 in human ES cell media, we sought to identify possible functional effects of MEF-secreted HSPG that could explain their role in cell culture. Therefore, we examined whether heparinoids could coordinate the interaction of FGF2 with cell surface receptors on human ES cells in different media formulations. Using self-assembled monolayer chemistry, we covalently attached FGF2 to gold-plated glass slides [19, 20]. In these experiments, the non-FGF2 bound portions of the glass slides are bound with albumin, providing an internal control for the specificity of protein-protein interactions. When human ES cells were incubated with surface-bound FGF2 in CM, robust binding was observed (Figure 3A-D, I). On the other hand, only low levels of binding were seen in UM (Figure 3E, I). However, when the concentration of exogenous heparinoid was increased, human ES cell-FGF2 binding increased as well (Figure 3F-H, I). These results support the hypothesis that secreted HSPG directly mediate high affinity receptor complex formation in CM.
Figure 3.
Heparinoids Can Mediate Human ES Cell-FGF2 Binding. (A-H) Phase contrast images of human ES cells bound to immobilized FGF2 in conditioned or unconditioned media (CM or UM). Media was supplemented with 0 (A, E), 10 (B, F), 100 (C, G), or 1000 (D, H) ng/ml HSPG. (I) The average binding densities from multiple experiments of human ES cells bound to immobilized FGF2 and the effects of heparinoid supplementation in UM. Binding is graphed relative to the average density determined for CM incubation. Mean values +/- SEM, n=4-42, **p < 0.05, ***p < 0.01.
While the degree of binding was dose-dependent for HS and HSPG, surprisingly the highest concentration of heparin assayed (1 ug/ml) consistently resulted in a large drop in human ES cell-FGF2 binding (Figure 3I). This may be due to differences between heparin and heparan sulfate chains. FGF2:heparin stoichiometry critically influences receptor binding [23]. FGF2 binds to sulfated regions and heparin is highly sulfated while HS chains are typically longer than heparin and have variably sulfated regions that may distinctly coordinate FGF2 positioning [24]. Therefore, while heparin may be effective at stabilizing FGF2, large doses could disrupt FGF receptor-FGF ligand interactions.
Even though FGF2 was significantly less effective at binding human ES cells in UM than CM, baseline levels of binding did exist. These may have been mediated through endogenous, cell-surface HSPG serving as low affinity FGF binding sites [17]. Immunocytochemistry against the HSPG perlecan confirmed human ES cells express HSPG, though confocal imaging localized perlecan to the basement membrane (Figure S4). It is likely that human ES cells, in addition to secreting HSPG, express HSPG on the cell surface (as do the vast majority of cell types), but the levels are apparently below those required to maintain signaling at picomolar concentrations of FGF2.
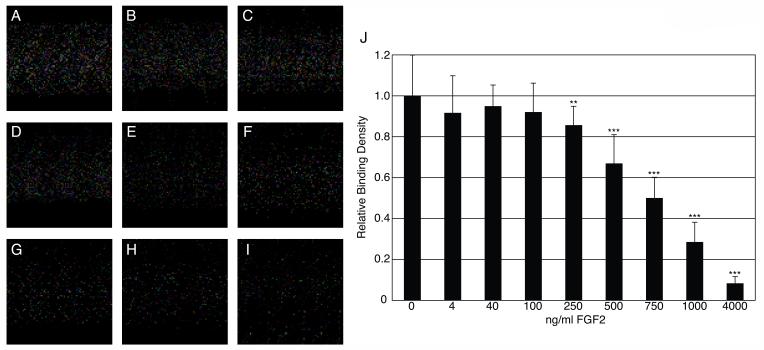
If human ES cell binding to surface-bound FGF2 is mediated through MEF-secreted HSPG, we reasoned that exogenous FGF2 could compete for these interactions. Therefore, we added increasing amounts of FGF2 into CM and analyzed binding. Indeed, we found that human ES cell binding to immobilized FGF2 decreased in a dose-dependent manner with increasing concentrations of FGF2 (Figure 4). Significant competition was observed at FGF2 concentrations of 250 ng/ml and higher. These concentrations of exogenous FGF2 are likely required to bind adequate amounts of MEF-secreted HSPG during the pre-incubation to inhibit surface-bound FGF2 from forming complexes with the secreted HSPG, which could then bind to human ES cells during the thirty minute binding reaction.
Figure 4.
Competitive Binding for Human ES Cells by Exogenous FGF2. (A-I) Phase contrast images of human ES cell binding to immobilized FGF2 in conditioned medium (CM) supplemented with 0, 4, 40, 100, 250, 500, 750, 1000, and 4000 ng/ml FGF2 respectively. (J) Relative binding densities compared to CM alone (0). Mean values +/- SEM, n=8-18, ** p < 0.05, ***p < 0.01.
Recently, it was proposed that exogenous FGF2 stimulates human ES cell self-renewal through interactions with differentiated human ES cells that persist in culture rather than directly through human ES cells themselves [25]. While our culture conditions yield highly pure human ES cell populations (>95% Oct4 positive compared to 78% in the Bendall et al. study cited above) we sought nonetheless to determine whether the binding assays might be selecting for a subpopulation of such differentiated cells present in the human ES cell population (Figure 5). We first determined that the human ES cell cultures were >80% positive for the pluripotent-specific cell surface antigen SSEA-3 (Figure 5D). We then sorted for SSEA3-positive human ES cells and incubated them with surface-bound FGF2 in either CM or UM (Figure 5E-G). Analysis of the binding densities obtained for these SSEA3-sorted human ES cells to surface-bound FGF2 showed approximately 90% more binding in CM than UM (Figure 5H), consistent with results obtained for unpurified cell cultures. These results indicate that human ES cells can directly bind FGF2 at the cell surface.
Figure 5.
SSEA3 Sorting of Human ES Cells Prior to FGF2 Binding. (A-D) Human ES cells were FACS analyzed for Oct4 (B) or SSEA3 (D) expression relative to mouse IgG2b (A) or rat IgM (C) isotype controls. (E) SSEA3 positive human ES cells were sorted with a FITC-conjugated secondary antibody following anti-SSEA3 incubation. (F, G) SSEA3 positive human ES cells bound to immobilized FGF2 in conditioned medium or unconditioned medium. (H) Average binding densities of SSEA3 positive human ES cells plotted relative to conditioned medium. Mean values +/- SEM, n = 9, ***p < 0.01.
These findings contrast with the conclusion in the Bendall et al. study that the primary mechanism of exogenous FGF2-function in human ES cell proliferation is through feeder cells. We propose three possible explanations for the differences observed between the culture systems. In the absence of fibroblast cells, cell surface receptors are upregulated. Conversely, contaminating fibroblast cells, expressing high levels of FGF receptors, result in the downregulation of FGF receptor expression on human ES cells. Finally, it may be that even relatively low expression levels of FGF receptors on human ES cells are sufficient to bind directly to FGF2 and stimulate proliferation in the media environment employed in the present work.
HSPG Are Required for Proliferation in CM with 4 ng/ml FGF2
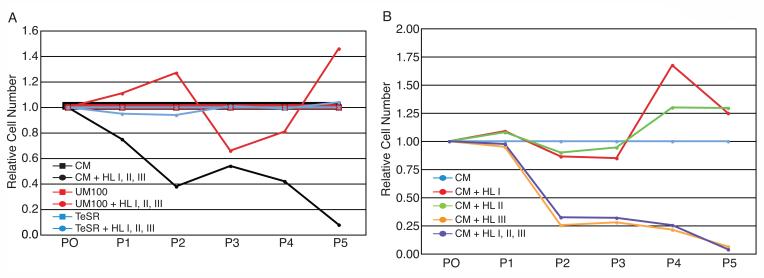
It was shown above that HSPG modulate the binding of human ES cells to FGF2. If MEF-secreted HSPG are involved in the generation of high affinity FGF2 signaling complexes, their removal should disrupt human ES cell proliferation in CM. Therefore, we supplemented CM with a cocktail of heparinoid-degrading enzymes (heparin lyase I, II, and III), and assessed the ability of human ES cells to proliferate over five consecutive passages (Figure 6A). The addition of the heparin lyase cocktail to CM abolished its ability to support human ES cell proliferation, underscoring a role for MEF-secreted HSPG in the CM culture system.
Figure 6.
Heparin Lyase Treatment of Human ES Cells. (A) The effects of heparinoid degradation in three different media formulations were investigated. Cells were passaged at equivalent dilutions for five passages. Relative cell numbers were plotted at each passage as standard media vs. media plus enzymes for each media set. The graph shows results for three typical individual experiments. Each experiment was repeated at least three times. (B) Heparinoid degrading enzymes were assayed individually in feeder-free culture conditions. Human ES cells cultured in conditioned medium (CM) plus heparin lyase I or II proliferated normally for five passages relative to CM alone. Human ES cells cultured in CM plus heparin lyase III or CM plus a cocktail of all three enzymes failed to maintain human ES cell cultures relative to CM alone. The graph shows results for a typical experiment, repeated a minimum of three times.
As an alternative to conditioning media, high concentrations of FGF2 in non-conditioned media have been shown to support human ES cell proliferation [4, 6]. If high concentrations of FGF2 circumvent the requirement of MEF-secreted HSPG for signaling, then removing heparinoids from these media should have no effect on proliferation. We tested the effects of the heparin lyase cocktail on two high-FGF2 culture systems: UM100 (UM containing 100 ng/ml FGF2) and TeSR1 (defined human ES cell medium containing 100 ng/ml FGF2). In contrast to CM, both of the high FGF2 culture systems proliferated through five passages with or without the heparin lyase cocktail (Figure 6A). Taken together, these data support a pivotal role for MEF-secreted HSPGs in human ES cell proliferation, and implicate a model of FGF2 signaling in CM that is distinct from non-conditioned, high FGF2 culture systems. These results also suggest that HSPG in Matrigel are not critical for human ES cell self-renewal.
We then cultured human ES cells with individual heparin lyases to determine whether any additional specificity could be identified for the observed effects in CM-based culture. We compared the proliferation capacity in CM alone to that in CM supplemented with each individual heparin lyase, or a cocktail of all three (Figure 6B). Only heparin lyase III activity tracked tightly with that of the full cocktail. These results are challenging to interpret. Heparin lyase I targets highly sulfated regions and therefore is described as enzymatically specific for heparin (which is highly sulfated). Because FGF2 binds to sulfated regions of heparin chains, it could be that it blocks the target regions of heparin lyase I. On the other hand, heparin lyase III digests non-sulfated regions of HS, which would allow it to degrade FGF2-bound HSPG. Because we were unable to develop an adequate activity assay, it is also possible that heparin lyase III remained active in CM while the other lyase enzymes were either inactive or limited in their activity.
HSPG Mediate FGF2-Human ES Cell Binding in CM
Because heparin lyase III can disrupt human ES cell proliferation in CM, we asked whether this might be due to disruption of FGF2-signaling complexes mediated by MEF-secreted HSPG. To address this question, we again utilized FGF2 immobilized on gold-coated slides. Before binding, CM was pre-incubated with heparin lyase III to digest MEF-secreted HSPG. Compared to CM alone, an average of 40% fewer human ES cells bound immobilized FGF2 in CM treated with heparin lyase III (Figure S5A-C).
To further assess the effects of heparin lyase III digestion on FGF2-signaling complexes, we examined the fate of FGF2 in CM after enzyme treatment. Using a FGF2 ELISA, we found that a significant portion of FGF2 became degraded after overnight incubation in hepain lyase III-supplemented CM (Figure S5D). Taken together, these findings support a direct role for MEF-secreted HSPG in stabilizing FGF2 and mediating FGF2-human ES cell interactions in CM.
Proteomic Analysis of MEF-Secreted HSPG
We wished to identify the specific MEF-secreted HSPG responsible for human ES cell proliferation in CM. The principal challenge facing a direct proteomic analysis of such factors secreted into human ES cell media is the large variation in protein abundance. The most abundant protein in the media is BSA, which is present in vast molar excess (over 6 orders of magnitude) relative to other important proteins of interest (e.g., exogenous FGF2). To overcome the masking effect of BSA, we employed a chromatography-based enrichment strategy to selectively purify HSPG from CM. The glycosylation and sulfation of HSPG confers a large negative charge to this class of proteins and thereby enables their selective enrichment via anion exchange chromatography. To reduce the affinity of BSA for the anion exchange resin, the pH of the media was lowered to the isoelectric point of albumin (∼5.5). At this pH, HSPG still carry net negative charge and can be fractionated by anion exchange chromatography. For a second round of purification, FGF2-agarose affinity chromatography was employed to enrich specifically for those protein species that interact with this cytokine. The enrichment for these HSPG was tracked by immunoblotting with 3G10 monoclonal antibody, which recognizes the desaturated uronate “stub” that remains on the protein backbone following heparin lyase III cleavage [21].
The protein fractions that showed HSPG enrichment, via 3G10 immunoblotting (Figure S6), were subjected to proteomic analysis using nanoLC MS/MS ion trap mass spectrometry [22]. With this approach, we identified five distinct species of MEF-secreted HSPG (Table 1). Based on spectral abundance, the most abundant species identified was perlecan [26]. This secreted, extracellular protein is an integral component of basement membranes and serves as a cellular attachment substrate. Perlecan has been shown to interact directly with FGF2 and to have pronounced biological effects [27]. Additionally we detected a significant amount of agrin and relatively lower amounts of glypican-4, glypican-1 and syndecan-4 [28]. A number of other murine and bovine proteins were also identified (Table S1). The bovine proteins most likely derive from the Knockout Serum Replacement (Invitrogen), which is a partial purification of albumin from bovine serum.
Table 1.
MEF-secreted heparan sulfate proteoglycans
| Protein Description | Gene symbol | IPI accession # | Swiss Prot | Unique # of Peptides* |
|---|---|---|---|---|
| Perlecan | Hspg2 | IPI00515360 | Q05793 | 89 |
| Agrin | Agrn | IPI00648309 | A2ASQ0 | 33 |
| Glypican-4 | Gpc4 | IPI00312407 | P51655 | 6 |
| Glypican-1 | Gpc1 | IPI00130391 | Q9Z1R9 | 4 |
| Syndecan-4 | Sdc4 | IPI00136382 | O35988 | 3 |
The number of unique peptides hits per protein can be used as means of evaluating relative protein abundance.
Attempts were made to evaluate the effects of these purified HSPG in human ES cell culture, but we were unable to reconstitute even a positive control of CM run through this purification scheme (i.e., recombined flow through and bound fractions). While the purification scheme serves as a powerful method for performing proteomic identifications, it is plausible that the drop in pH required to separate BSA from HSPG alters the proteoglycans (or other critical factors), resulting in a loss of biological activity.
DISCUSSION
Previous work has shown that the high doses of FGF2 utilized in UM100 are largely degraded over the standard course of incubation, while conditioning the media on MEF feeder cells has a stabilizing effect on exogenous FGF2 [4]. Here we have determined that this stabilization is due to secreted HSPG. Proteomic analysis of CM has revealed the presence of five distinct MEF-secreted HSPG. Treatment of CM with an enzyme that degrades HS chains disrupts human ES cell-FGF2 interactions and impairs proliferation, suggesting that human ES cells cultured in CM utilize MEF-secreted HSPG to stabilize FGF2, forming complexes capable of signaling at picomolar concentrations [29]. MEF-secreted perlecan, or one of the other secreted HSPG, may directly interact with FGF2 in solution, coordinating high affinity signaling complexes with appropriate FGF receptors.
Human ES cell proliferation in UM100 and TeSR1 media, which are not conditioned and lack mouse-secreted proteins, was unaffected by heparin lyase treatment. These culture systems maintain nanomolar concentrations of FGF2 throughout the incubation period, concentrations previously shown to activate FGF2 signaling in the absence of heparinoids [12]. However, for long-term proliferation it is generally held that heparinoids are required for continued FGF2 signaling. At a minimum, the HSPG perlecan can be detected by immunocytochemistry at the interface between human ES cells and matrix proteins (Figure S4). It seems likely that human ES cells, like the overwhelming majority of cell types, express HSPG at the cell surface that could serve as an endogenous binding partner in the presence of adequate FGF2 concentrations. It may be that short-term disruption of cell surface HSPG can be offset by high concentrations of FGF2 while the cell reprocesses the cofactor as enzymatic activity falls.
We have speculated that FGF2 bound to MEF-secreted HSPG might protect its target region from heparin lyase I digestion. Alternatively, some heparin lyases might be enzymatically inactive in stem cell media, a possibility we were unable to definitively address. Because heparin lyase III digests non-sulfated regions of HSPG, these portions of the sugar chains would be accessible to the enzyme. Indeed, human ES cell cultured in CM were highly sensitive to the presence of heparin lyase III, which also disrupted FGF2-ES cell interactions and destabilized FGF2 in CM (Figures 6 and S5). Our proteomic analysis of MEF-secreted proteins in CM identified other HSPG binding proteins as well (Table S1). It is possible that other factors, in addition to FGF2, support self-renewal in CM, and HSPG degradation disrupts more than one proliferative signal.
Heparin binding is a common feature of all FGF family members. Thus, it was not unexpected that commercially available heparinoids could stabilize FGF2 in standard, unconditioned human ES cell medium (i.e., UM). Stabilization, however, does not directly correlate with proliferation. Thus, UM containing suboptimal concentrations of FGF2 supplemented with exogenous heparinoids failed to establish the long-term proliferation seen with high FGF2 alone (e.g. UM100 or TeSR1). The higher concentrations of exogenous cofactors tested were able to produce a short-term (3-4 passages) proliferative boost, which might suggest a general mitogenic response that lacked a specific, critical signal. Our unsuccessful efforts to establish an assay for CM-purified HSPG highlight the difficulties of serum-based purification. Because BSA is the most abundant protein within CM, extensive biochemical manipulation was required to isolate secreted HSPG. In this case, we reduced the pH from approximately 7.4 to 5.5 and employed anion-exchange chromatography. While this enabled selective purification of HSPG from CM for identification by mass spectrometry, it is possible that the acidic environment altered the self-renewal activity of the purified cofactors.
We confirmed a central role for FGF2 in human ES cell proliferation by examining binding interactions between stem cells and surface-bound FGF2. Interestingly, human ES cells and FGF2 showed only minimal binding in UM, but strong binding in CM. By increasing the concentration of heparinoids in UM, binding densities approached levels similar to those seen in CM, supporting a role for MEF-secreted HSPG in the formation of signaling complexes. Furthermore, in CM exogenous FGF2 was able to compete with surface-bound FGF2 for human ES cell binding, highlighting the specificity of the observed, human ES cell/HSPG/FGF2 binding.
Historically, human ES cell culture systems have supplemented base media with a cocktail of growth factors to support long-term human ES cell proliferation. Present within all of these cocktails has been either FGF2 or an FGF family member [1-9]. Recently Bhatia and colleagues developed a human ES cell co-culture system in which FGF2-human ES cell interactions are not required for proliferation [25]. By differentiating a portion of human ES cells into a network of feeder cells, the resulting co-culture system was reported to support proliferation in response to feeder-secreted IGF. By presorting for SSEA3, we were able to eliminate possible feeder cell subpopulations from the human ES cell cultures. FGF2-binding experiments on these sorted cells gave the same results as unsorted cells, confirming direct FGF2 — human ES cell interactions.
CONCLUSION
Our work has shown that different types of signaling complexes are able to achieve the same outcome: human ES cell pluripotency. High doses of FGF2 can effectively signal self-renewal, likely in combination with endogenous, cell-surface HSPG. In CM-based culture, high affinity signaling complexes made up of FGF2, FGF receptors, and MEF-secreted HSPG lead to the same result. These multiple options for maintaining pluripotency offer possibilities for an expanded repertoire of protocols for manipulating human ES cells. This could lead to a significant degree of flexibility in developing differentiation protocols or approaches for reprogramming somatic cells. As shown here, a comprehensive understanding of the signaling pathways activated within human ES cells by different “self-renewal” ligands helps to provide the fundamental understanding necessary to guide cellular growth, differentiation, and development.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mitchell Probasco and Clay Glennon for technical support, Deborah J. Faupel for critical reading of the manuscript, and Dr. Xiaolin Nan for providing the cell counting software. J.A.T. is a cofounder and shareholder of Cellular Dynamics International and Stem Cell Products, Inc.
This work was supported by NIH grants P20 GM069981 (to M.E.L. and J.A.T.) and NO1 HV28182 (to J.E.L. and L.M.S.).
REFERENCES
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts Science Nov 6 199828253911145–1147. [DOI] [PubMed] [Google Scholar]
- 2.Beattie GM, Lopez AD, Bucay N, et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers Stem Cells Apr 2005234489–495. [DOI] [PubMed] [Google Scholar]
- 3.Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R.Human embryonic stem cells derived without feeder cells Lancet May 7-13 200536594711636–1641. [DOI] [PubMed] [Google Scholar]
- 4.Levenstein ME, Ludwig TE, Xu RH, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal Stem Cells Mar 2006243568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R.Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products Biotechnol Bioeng Sep 20 2005916688–698. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions Nat Biotechnol Feb 2006242185–187. [DOI] [PubMed] [Google Scholar]
- 7.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells Nat Biotechnol Oct 20011910971–974. [DOI] [PubMed] [Google Scholar]
- 8.Xu C, Rosler E, Jiang J, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium Stem Cells Mar 2005233315–323. [DOI] [PubMed] [Google Scholar]
- 9.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA.Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells Nat Methods Mar 200523185–190. [DOI] [PubMed] [Google Scholar]
- 10.Harmer NJ.Insights into the role of heparan sulphate in fibroblast growth factor signalling Biochem Soc Trans Jun 200634Pt 3442–445. [DOI] [PubMed] [Google Scholar]
- 11.Nugent MA, Edelman ER.Kinetics of basic fibroblast growth factor binding to its receptor and heparan sulfate proteoglycan: a mechanism for cooperactivity Biochemistry Sep 22 199231378876–8883. [DOI] [PubMed] [Google Scholar]
- 12.Roghani M, Mansukhani A, Dell’Era P, et al. Heparin increases the affinity of basic fibroblast growth factor for its receptor but is not required for binding J Biol Chem Feb 11 199426963976–3984. [PubMed] [Google Scholar]
- 13.Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL.Crystal structure of fibroblast growth factor receptor ectodomain bound to ligand and heparin Nature Oct 26 200040768071029–1034. [DOI] [PubMed] [Google Scholar]
- 14.Walker A, Turnbull JE, Gallagher JT.Specific heparan sulfate saccharides mediate the activity of basic fibroblast growth factor J Biol Chem Jan 14 19942692931–935. [PubMed] [Google Scholar]
- 15.Lindahl U, Kusche-Gullberg M, Kjellen L.Regulated diversity of heparan sulfate J Biol Chem Sep 25 19982733924979–24982. [DOI] [PubMed] [Google Scholar]
- 16.Saku T, Furthmayr H.Characterization of the major heparan sulfate proteoglycan secreted by bovine aortic endothelial cells in culture. Homology to the large molecular weight molecule of basement membranes J Biol Chem Feb 25 198926463514–3523. [PubMed] [Google Scholar]
- 17.Gallagher JT.Heparan sulfate: growth control with a restricted sequence menu J Clin Invest Aug 20011083357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA.Feeder-independent culture of human embryonic stem cells Nat Methods Aug 200638637–646. [DOI] [PubMed] [Google Scholar]
- 19.Peelen D, Smith LM.Immobilization of amine-modified oligonucleotides on aldehyde-terminated alkanethiol monolayers on gold Langmuir Jan 4 2005211266–271. [DOI] [PubMed] [Google Scholar]
- 20.Peelen D, Kodoyianni V, Lee J, Zheng T, Shortreed MR, Smith LM.Specific capture of mammalian cells by cell surface receptor binding to ligand immobilized on gold thin films J Proteome Res Jul 2006571580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David G, Bai XM, Van der Schueren B, Cassiman JJ, Van den Berghe H.Developmental changes in heparan sulfate expression: in situ detection with mAbs J Cell Biol Nov 19921194961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinter M, Sherman NE. Protein sequencing and identification using tandem mass spectrometry. Wiley-Interscience; New York: 2000. [Google Scholar]
- 23.Safran M, Eisenstein M, Aviezer D, Yayon A.Oligomerization reduces heparin affinity but enhances receptor binding of fibroblast growth factor 2 Biochem J Jan 1 2000345Pt 1107–113. [PMC free article] [PubMed] [Google Scholar]
- 24.Kreuger J, Spillmann D, Li JP, Lindahl U.Interactions between heparan sulfate and proteins: the concept of specificity J Cell Biol Jul 31 20061743323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bendall SC, Stewart MH, Menendez P, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro Nature Jul 11 2007 [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Sadygov RG, Yates JR., 3rdA model for random sampling and estimation of relative protein abundance in shotgun proteomics Anal Chem Jul 15 200476144193–4201. [DOI] [PubMed] [Google Scholar]
- 27.Knox S, Merry C, Stringer S, Melrose J, Whitelock J.Not all perlecans are created equal: interactions with fibroblast growth factor (FGF) 2 and FGF receptors J Biol Chem Apr 26 20022771714657–14665. [DOI] [PubMed] [Google Scholar]
- 28.Cool SM, Nurcombe V.Heparan sulfate regulation of progenitor cell fate J Cell Biochem Nov 1 20069941040–1051. [DOI] [PubMed] [Google Scholar]
- 29.Naski MC, Ornitz DM. FGF signaling in skeletal development. Front Biosci. 1998;3:d781–794. doi: 10.2741/a321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.