Abstract
Repeated exposure to drugs of abuse induces a variety of persistent changes in the brain and the dopamine D1 receptor plays a major role in the process. To understand intracellular mechanisms contributing to cocaine-induced neuroadaptations, we previously examined the role of the immediate early gene Fos using a mouse in which Fos is disrupted primarily in D1 receptor-expressing neurons in the brain. We found that both dendritic remodeling of medium spiny neurons and behavioral sensitization induced by repeated exposure to cocaine are attenuated in the mutant mice. Moreover, the expression of genes encoding several transcription factors, neurotransmitter receptors and intracellular signaling molecules following repeated cocaine administration is altered in the mutant mice compared to that in wild-type mice. In the present study, we have investigated the role of Fos in regulating neuronal excitability at a cellular level and found that medium spiny nucleus accumbens neurons in the mutant mice exhibit increased excitability and attenuated inhibitory responses to stimulation of D1 receptors compared to those in wild-type mice. Our findings suggest that Fos functions in D1 receptor-bearing neurons to regulate neuronal activity which may contribute to the persistence of drug-induced changes.
Keywords: nucleus accumbens, D1 receptors, Fos, neuronal excitability
Drug addiction is a brain disease that is long-lasting with a high propensity to relapse [8,10,23]. Pharmacological and genetic studies suggest that the dopamine (DA) D1 receptor plays a critical role in mediating the neurobiological effects of cocaine [2,6,9,17, 19-22,24-25,28]. The most prominent cellular responses to D1 receptor agonists and cocaine administration are a transient up-regulation of the immediate early gene Fos in the brain [1,3-4,11-15]. A functional D1 receptor is required for the Fos induction by cocaine [14,24-25,28]. To investigate intracellular mechanisms associated with the D1 receptor that may contribute to persistent changes induced by cocaine, we previously made a mouse in which Fos is mutated primarily in D1 receptor-expressing neurons (f/fc-fos-D1-cre) [26-27]. Using this mouse model, we found that both dendritic remodeling of medium spiny neurons and behavioral sensitization induced by repeated exposure to cocaine are attenuated in the mutant mice [27]. Moreover, the expression of genes encoding several transcription factors, neurotransmitter receptors and intracellular signaling molecules following repeated cocaine administration is altered in the mutant mice compared to that in wild-type mice [27]. These finding suggest that Fos expression in D1 receptor-bearing neurons contributes to cocaine-induced neuroadaptations [27].
It remains unclear, however, whether and how Fos may regulate neuronal activity of D1 receptor-bearing medium spiny neurons. In the current study, we further investigated this issue. We used f/fc-fos-D1-cre and their wild-type control littermates 7 to 28 weeks of age for all the analyses. Equal number of male and female mice was used in the study. Experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Mice were decapitated under halothane anesthesia and the brains were excised and dissected into ∼3 mm blocks containing the nucleus accumbens (NAc). The blocks were immersed in ice-cold artificial cerebrospinal fluid (aCSF). 300 μm coronal slices were cut and incubated in oxygenated aCSF for 1 hour at room temperature before recording. Brain slices were anchored in the recording chamber and perfused by gravity-fed oxygenated aCSF at 34°C. Patch pipettes (3-5 MΩ) were filled with an internal solution [5] and used for current-clamp recording in visually identified medium spiny neurons in the NAc using differential interference contrast microscopy [18]. Electrical signals were amplified in bridge mode using a SEC-05L npi amplifier (ALA Instruments), digitized by a DigiData 1200 Series (Axon Instruments), and distributed to a computer running pCLAMP 7.01 software (Axon Instruments). The current-voltage relationships were studied by injecting step constant current pulses (500 msec duration, -0.8 to +0.8 nA) through the recording electrode. Passive and active membrane properties were studied as described [29]. Only cells with a resting membrane potential of at least -70 mV, stable baseline recordings, and evoked spikes that overshot 0 mV were used for analysis and drug treatment. The D1 receptor agonist SKF38393 was applied to the medium at 1 μM and its effects on evoked action potentials of NAc neurons were studied. The current-voltage and current-evoked spike relationships were analyzed by analysis of variance (ANOVA) with repeated measures and post-hoc comparisons carried out by using Newman-Keuls test. Student's t-test was also used to compare the neuronal responses to SKF38393 between cells recorded from wild-type and f/fc-fos-D1-cre mice.
Basal Fos expression may regulate neuronal activity and mediate neurophysiological responses elicited by D1 receptor stimulation in the NAc. To investigate these possibilities, we performed current clamp recordings in medium spiny neurons in the core of the NAc of wild-type and f/fc-fos-D1-cre mice. All neurons were recorded with stabilized resting membrane potentials in the -82 to -70 mV range [7,16]. No spontaneous activity in either wild-type or f/fc-fos-D1-cre neurons was recorded in the NAc. Statistical analysis of the membrane properties indicates that there was no significant difference in the resting membrane potential, input resistance, action potential threshold, and duration measured at the half amplitude of action potential between NAc neurons in wild-type and f/fc-fos-D1-cre mice (Table 1). Injection of depolarizing current pulses evoked Na+-dependent action potentials in wild-type NAc neurons (Fig. 1A). However, the number of action potentials evoked by a 200 ms of depolarizing current pulse (firing frequency) was markedly increased in NAc neurons in f/fc-fos-D1-cre mice (Fig. 1A). The currents required for generating the initial action potential in f/fc-fos-D1-cre neurons were also significantly reduced as compared to wild-type neurons (Table 1). The current-spike response curves show that the evoked firing frequency in f/fc-fos-D1-cre neurons was significantly higher than that in wild-type neurons (F1,12=5.50, p < 0.05; post hoc Newman-Keuls test, p < 0.05) (Fig. 1B). Analysis of the characteristics of evoked action potentials indicates that the amplitude of both the spike and after-hyperpolarization was significantly reduced in f/fc-fos-D1-cre neurons as compared to wild-type neurons (Fig. 1C and Table 1). The inward rectification during membrane hyperpolarization and the subthreshold excitability during membrane depolarization were not markedly affected by the Fos mutation. Thus, the current-voltage relationship in NAc neurons was not significantly changed in f/fc-fos-D1-cre mice (Fig. 1D).
Table 1.
Comparison of membrane properties of NAc neurons in wild type and Fos mutant mice
| Group | Wild-Type | Mutant |
|---|---|---|
| Number of neurons | 10 | 12 |
| Passive Membrane properties | ||
|
| ||
| RMP (mV) | -79.02 ± 0.93 | -79.37 ± 0.41 |
| Rin (mW) | 128.65 ± 24.59 | 159.17 ± 15.90 |
| Active Membrane Properties | ||
|
| ||
| Current to generate AP (nA) | 0.29 ± 0.04 | 0.20 ± 0.01 * |
| AP threshold (mV) | -33.93 ± 1.09 | -34.27 ± 1.19 |
| AP amplitude (mV) | 74.86 ± 1.86 | 67.83 ± 1.67 * |
| ½AP duration (msec) | 1.09 ± 0.05 | 1.17 ± 0.04 |
| AHP amplitude (mV) | 13.51 ± 1.05 | 9.83 ± 0.67 * |
The passive and active membrane properties of medium spiny NAc neurons were measured by current clamp recordings from brain slices of wild-type and f/fc-fos-D1-cre mice. Values represent the mean±SEM for the number of neurons indicated (*p < 0.05; Student's t-test).
RMP: resting membrane potential; AP: action potential; AHP: after-hyperpolarization.
Figure 1.
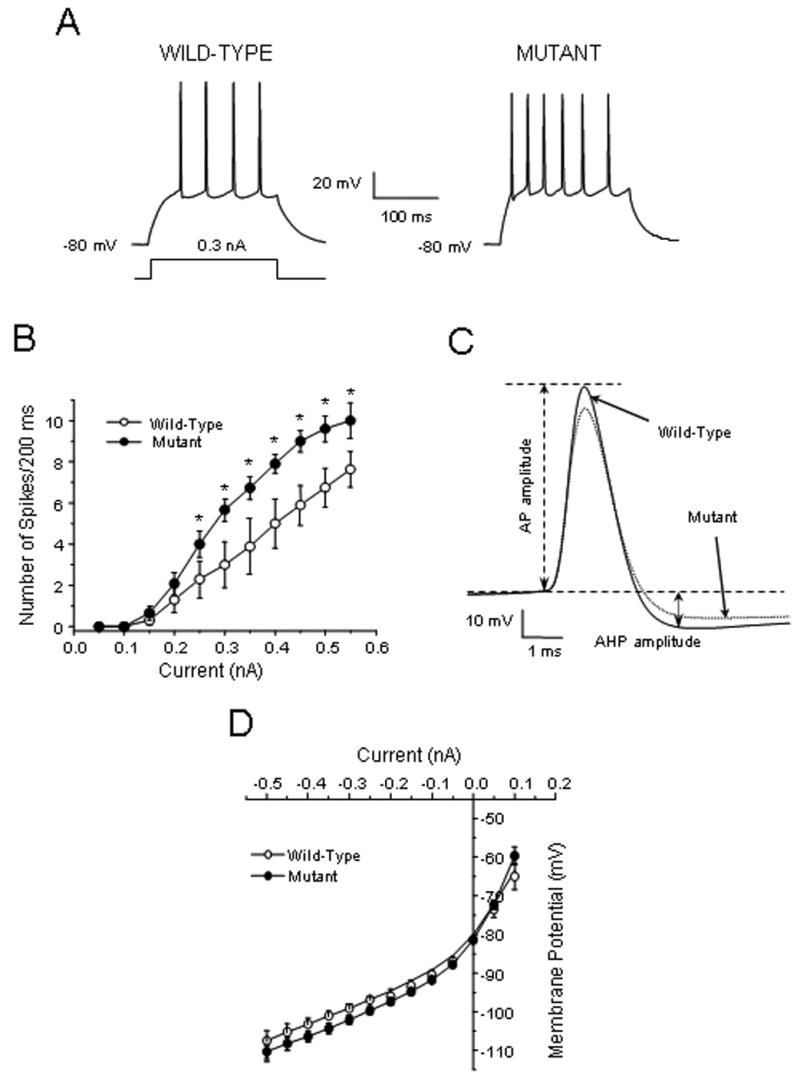
Evoked neuronal activity is enhanced in the NAc of f/fc-fos-D1-cre (mutant) mice as compared to wild-type mice. (A) Representative traces showing that depolarizing currents evoke more action potentials in NAc neurons in f/fc-fos-D1-cre mice compared to those in wild-type mice (n=10-12 cells per group). (B) There is a significant difference in current-evoked spike response curves between NAc neurons in wild-type and f/fc-fos-D1-cre mice (F1,12=5.50; p < 0.05, Newman-Keuls test, *p < 0.05). (C) Representative action potential traces indicate that the amplitude of action potentials (AP) and after-hyperpolarization (AHP) was reduced in NAc neurons in f/fc-fos-D1-cre mice compared to wild-type mice. (D) The current-voltage relationship shows that the inward rectification during membrane hyperpolarization is not affected by the Fos mutation in f/fc-fos-D1-cre mice.
D1 receptor stimulation by SKF38393 (1 μM) suppressed evoked action potentials in NAc core neurons in wild-type mice (Fig. 2A). In contrast, this inhibitory effect was abolished in f/fc-fos-D1-cre neurons (Fig. 2B). The time course of evoked spikes shows that the SKF38393-mediated inhibition of evoked action potentials in wild-type NAc neurons was time-dependent, and this effect was significantly attenuated in f/fc-fos-D1-cre neurons (F1,14=8.59, p < 0.05; post hoc Newman-Keuls test, p < 0.05) (Fig. 2C). The SKF38393-induced suppression of evoked action potentials was reversible and can be washed out by fresh medium. We previously demonstrated that the D1 receptor-modulated reduction in voltage-sensitive Na+ currents and high voltage-activated non-L type Ca2+ currents contributes primarily to decrease of action potentials [7,21,29-30]. Interestingly, D1 receptor stimulation also depolarized resting membrane potential in these NAc cells and whether this depolarization could be attributed to inhibition of the two-pore domain K+ channels remains to be determined. However, the resting membrane potential depolarization induced by SKF38393 was significantly attenuated in f/fc-fos-D1-cre neurons (wild-type vs f/fc-fos-D1-cre: 7.2 ± 1.6 vs 2.3 ± 0.9 mV; Student t-test, p < 0.05) (Fig. 2D). Based on the fact that D1 receptor stimulation facilitates PKA activity that consequently activates Fos, and that a Fos mutation is associated with significant reduction of the D1 receptor effects on depressing action potentials, we propose that D1 receptors and c-Fos proteins functionally interact to regulate neuronal activity in the NAc.
Figure 2.
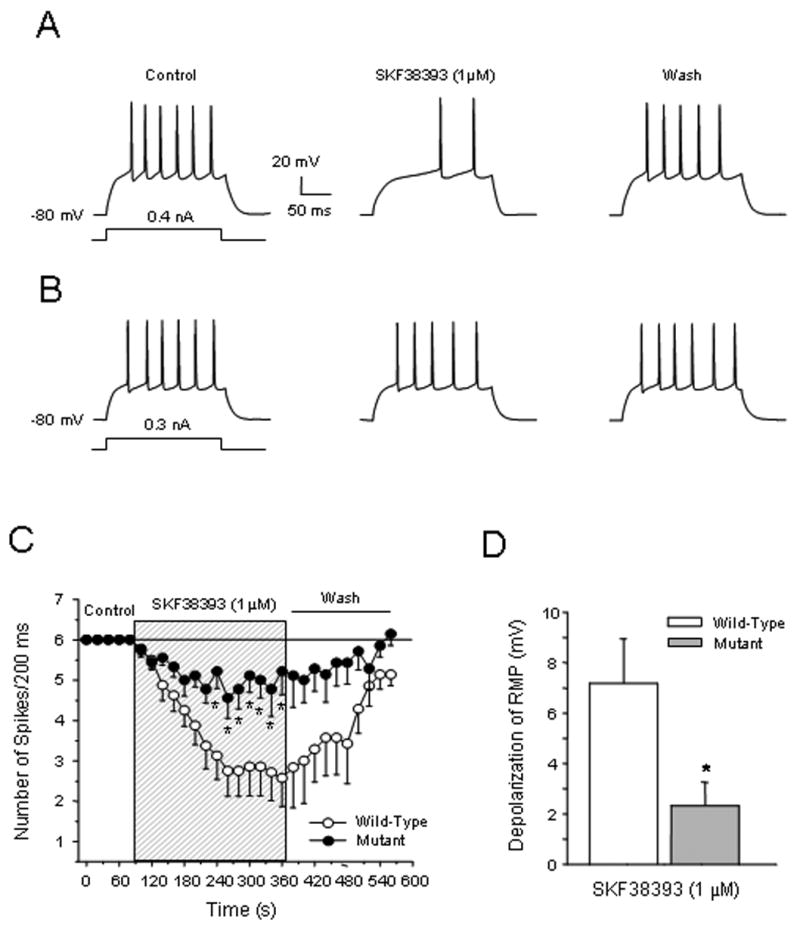
D1 receptor-mediated suppression of evoked action potentials is attenuated in NAc neurons in f/fc-fos-D1-cre (mutant) mice. (A) D1 receptor stimulation reduces evoked firing of NAc neurons (n=8) in wild-type mice. (B) The inhibitory effects of D1 receptor stimulation on evoked spikes (n=9 neurons) are attenuated in NAc neurons in f/fc-fos-D1-cre mice. (C) D1 receptor-mediated inhibition is abolished in NAc neurons of f/fc-fos-D1-cre mice. There is a significant attenuation in suppression of evoked firing by SKF38393 in NAc neurons in f/fc-fos-D1-cre mice compared to that in wild-type mice (F1,14=8.59; p < 0.05, *Newman-Keuls test, p < 0.05). (D) Depolarization of the resting membrane potential (RMP) induced by SKF38393 is significantly attenuated in NAc neurons of f/fc-fos-D1-cre mice as compared to wild-type mice (Student's t-test, *p < 0.05).
Our findings demonstrate that a Fos mutation in D1 receptor-bearing neurons results in an increase in neuronal excitability of the majority of medium spiny NAc core cells that was reflected by increased firing and reduced inhibition of evoked action potentials mediated by the D1 receptor. We previously showed that the majority of medium spiny NAc neurons responds to application of selective D1 receptor agonists [7,21,29]. Our current results indicate that modulation of neuronal activity by D1 receptors via c-Fos protein-mediated signaling is significantly attenuated in f/fc-fos-D1-cre NAc neurons. They also suggest that D1 receptor-mediated ion channel function, including but not limited to that associated with the cAMP/PKA cascade [8], are markedly altered in these neurons. The exact mechanisms that underlie this increase in excitability in f/fc-fos-D1-cre NAc neurons are unknown. It is also unclear whether the Fos mutation affects activity of neurons that primarily express D2 receptors in the NAc by a D1 receptor enabling mechanism [21]. Despite these unresolved issues, changes in resting membrane potential and firing, along with altered amplitude in evoked spikes and after-hyperpolarization, suggest involvement of multiple types of ion channels and/or ionotropic receptors in Fos-regulated neuronal activity.
The NAc is implicated in mediating drug effects and the persistence of cocaine-induced behavioral sensitization is in parallel with enhanced inhibitory responses of NAc neurons to cocaine and DA challenges [6]. In contrast to the increased excitability induced by the Fos mutation, repeated cocaine administration significantly decreases the membrane excitability of NAc neurons. This decrease results primarily from a reduction in voltage-sensitive Na+ and Ca2+ currents, along with an increase in voltage-gated K+ currents [7,29-30]. Given that cocaine indirectly potentiates D1 receptor stimulation by enhancing DA neurotransmission, and the D1 receptor-coupled cAMP/PKA cascade activates Fos induction [14,24-25,28], we anticipate that alterations in NAc neuronal activity induced by repeated cocaine administration would be eliminated or at least attenuated in f/fc-fos-D1-cre medium spiny NAc cells. Indeed, we have demonstrated that chronic cocaine-induced dendritic remodeling in the NAc [27] and behavior sensitization were significantly attenuated in f/fc-fos-D1-cre mice [27]. Therefore, it is likely that Fos plays an important role in chronic cocaine-induced neuronal and behavioral adaptations, and mutating this gene in D1 receptor-expressing medium spiny NAc neurons would diminish the functional consequences of chronic exposure to cocaine at the neuronal and behavioral levels. Future investigations are necessary to examine this assumption and underlying mechanisms.
Combined with previous results, the novel findings from the present study support a model in which c-Fos proteins may function as an intracellular regulator downstream from the D1 receptor that contributes to vulnerability to cocaine. By modulating the activity of membrane ion channels possibly via affecting their expression and/or structural conformation, basal c-Fos proteins regulate the activity and responsiveness of NAc neurons to cocaine. On the other hand, by influencing the level and composition of activator protein 1 transcription complexes after repeated exposure to cocaine, c-Fos proteins regulate the expression of target genes that can modulate neurotransmission and neuronal connectivity [27]. Together, these c-Fos-mediated events may contribute to the persistence of drug dependence.
Acknowledgments
This work was supported by USPHS grants DA004093 to X.-T.H., and DA14644 and DA17323 to M.X. We thank Dr. Francis J. White for discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caine SB, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Role of the dopamine D1 receptor in cocaine self-administration: studies with D1 receptor mutant mice. J Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 4.Graybiel AM, Moratalla R, Robertson RA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998;18:9139–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J Neurosci. 1995;15:6287–6299. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu XT, Basu S, White FJ. Repeated cocaine administration suppresses HVA-Ca2+ potentials and enhances activity of K+ channels in rat nucleus accumbens neurons. J Neurophysiol. 2004;92:1597–1607. doi: 10.1152/jn.00217.2004. [DOI] [PubMed] [Google Scholar]
- 8.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The Role of Reward-Related Learning and Memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 9.Jiao H, Zhang L, Gao F, Lou D, Zhang J, Xu M. Dopamine D1 and D3 receptors oppositely regulate NMDA- and cocaine-induced MAPK signaling via NMDA receptor phosphorylation. J Neurochem. 2007;103:840–848. doi: 10.1111/j.1471-4159.2007.04840.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharm. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 12.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 13.Moratalla R, Elibol B, Vallejo M, Graybiel AM. Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. [DOI] [PubMed] [Google Scholar]
- 14.Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13:135–160. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- 17.Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 18.Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- 19.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 20.Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- 21.Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 22.Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- 23.Wise RA. Addiction becomes a brain disease. Neuron. 2000;26:27–33. doi: 10.1016/s0896-6273(00)81134-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Zhang L, Lou D, Nakabeppu Y, Zhang J, Xu M. The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem. 2002;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M. Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharm. 2005;30:1443–1454. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang D, McQuade JS, Behbehani M, Tsien JZ, Xu M. c-fos regulates neuronal excitability and survival. Nat Genet. 2002;30:416–420. doi: 10.1038/ng859. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz J, Xu M. c-fos facilitates acquisition and extinction of cocaine-induced persistent change. J Neurosci. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci. 1998;18:488–498. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XF, Cooper DC, White FJ. Repeated cocaine treatment decreases whole-cell calcium current in rat nucleus accumbens neurons. J Phar Exp Ther. 2002;301:1119–1125. doi: 10.1124/jpet.301.3.1119. [DOI] [PubMed] [Google Scholar]


