Abstract
Collagen is the structural molecule that is most correlated with strength in blood vessels. In this study, we compared the properties of collagen in engineered and native blood vessels. Transmission electron microscopy (TEM) was used to image sections of engineered and native arteries. Band periodicities of engineered and native collagen fibrils indicated that spacing between collagen molecules was similar in engineered and native tissues. Engineered arteries, however, had thinner collagen fibrils and fibers than native arteries. Further, collagen fibrils were more loosely packed within collagen fibers in engineered arteries than in native arteries. The sensitivity of TEM analysis allowed measurement of the relative frequency of observation for alignment of collagen. These observations showed that collagen in both engineered and native arteries was aligned circumferentially, helically, and axially, but that engineered arteries had less circumferential collagen and more axial collagen than native arteries. Given that collagen is primarily responsible for dictating the ultimate mechanical properties of arterial tissue, future efforts should focus on using relative frequency of observation for alignment of collagen as a descriptive input for models of the mechanical properties of engineered or native tissues.
Keywords: Tissue engineering, Arteries, Blood vessels, Collagen, Ultrastructure, Collagen alignment
INTRODUCTION
Engineered arteries may one day serve as vascular grafts for patients who need coronary artery or peripheral bypass surgeries, but who lack healthy autologous replacement artery or vein. Engineered grafts must be strong enough to resist rupture and to endure surgical handling, in order to achieve clinical success. In this report, we present an ultrastructural study of collagen, which is primarily responsible for the ultimate mechanical properties of arterial tissue,1,23 in tissue engineered arteries.
Collagen is synthesized intracellularly and excreted into the extracellular space, where it becomes organized spatially.8 Smooth muscle cells synthesize triple helical procollagen molecules, which are cleaved to form mature collagen molecules as they are secreted from cells. In the extracellular space, collagen molecules form fibrils in a self-assembly process controlled by the distribution of polar charged and hydrophobic amino acid residues in the collagen molecule.13,18 Band periodicity is a measure of the spacing between collagen molecules within a collagen fibril.17 Band periodicities of collagen fibrils containing both types I and III collagens, such as those found in arteries, are 54–65 nm.3,30 Diameters of collagen fibrils range from 16 to 500 nm.30 Collagen fibrils pack together to form collagen fibers.
Theoretical models suggest that the alignment of collagen impacts a tissue's mechanical properties.15,20,26 The predominant alignment of arterial collagen fibers is often thought to be helical,4,28 which allows collagen fibers to reinforce arteries both in the circumferential and axial directions. For example, as the pressure inside an artery increases, helical collagen fibers become more circumferentially oriented, and contribute to the artery's circumferential mechanical properties.28
Previous methods used to measure collagen alignment in arteries, such as polarized light microscopy,4 X-ray diffraction,2 and measurement of nuclei orientation,14 lack the sensitivity required to detect accurately the alignment of all collagen fibrils and fibers within a tissue. Rather, these methods have been used to measure the predominant alignment of collagen within native arterial tissue. Previous reports have shown that the predominant alignment of collagen in native arteries may be circumferential,5 helical,28 or axial.4 Small angle light scattering (SALS), which is significantly more sensitive than techniques traditionally used to determine collagen orientation, has recently been used to measure alignments of collagen in porcine heart valves and bovine pericardium.26,31 SALS provides a significantly more accurate picture of collagen orientation than traditional techniques, but, as shown herein, the resolution of SALS is not sensitive enough to fully characterize the alignment of sparse or loosely packed collagen fibrils in engineered vessels. A highly sensitive ultrastructural method to measure collagen alignment is described in this report.
METHODS
Culture of Porcine Tissue Engineered Vessels
Engineered arteries having dimensions of 3 mm in diameter and 7 cm in length were grown as previously described.23 Briefly, porcine carotid smooth muscle cells (SMCs; 5 × 106 cells/mL) were seeded onto a polyglycolic acid (PGA) scaffold (Albany International, Mansfield, MA) that was sewn into a cylindrical construct with 6-0 PGA suture (US Surgical, Norwalk, CT) around a silicone tube for support. Vessels were cultured in a bioreactor connected to a peristaltic pump (vessels were strained 1.5% at 2.8 Hz) for 7–8 weeks in low glucose Dulbecco's Modified Eagle's Medium (JRH Biosciences, Lenexa, Kansas) with 10% fetal bovine serum (FBS, GibcoBRL, Grand Island, NY), 10% porcine serum (PS, GibcoBRL, Grand Island, NY), basic fibroblast growth factor (10 ng/mL), platelet derived growth factor (10 ng/mL), l-ascorbic acid, copper sulfate, HEPES, l-proline, l-alanine, l-glycine, and Penicillin G (all remaining supplements were from Sigma, St. Louis, MO). No endothelial cells were seeded onto tissue engineered arteries in this study because they would not contribute to fibrillar collagen synthesis during culture.
Native Porcine Arteries
Common carotid arteries were excised in sterile fashion from anesthetized 25–30 kg Duroc swine (Lee Brothers, Four Oaks, NC). All animals were treated in accordance with standard NIH protocols, and animal procedures were approved by the Institutional Animal Care and Use Committee at Duke University. Adventitial tissue was dissected from porcine arteries prior to ultrastructural analysis of the medial layer.
Transmission Electron Microscopy
Vessel segments were fixed for transmission electron microscopy (TEM) in 4% glutaraldehyde (EMD Chemicals Inc, Gibbstown, NJ) and 0.1 M sodium cacodylate trihydrate (Sigma, St. Louis, MO). After dehydration, samples were infiltrated with resin (Poly/Bed 812, Polysciences, Warrington, PA) and polymerized. Fixed vessel segments were thin-sectioned (90 nm thickness) into circumferential cross-sections and axial cross-sections (Figs. 1a–c). Sections were embedded in a copper grid, stained with uranyl acetate and lead, and carbon coated. A Phillips EM 400T transmission electron microscope was used to view vessel sections.
FIGURE 1.
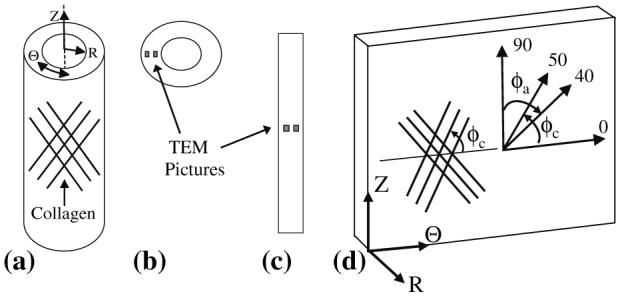
(a) Arteries with collagen aligned in the Θ–Z plane were sliced into (b) circumferential cross-sections and (c) axial cross-sections for TEM analysis. (d) In this example of an artery sliced axially and opened, collagen that is aligned in the Θ–Z plane is shown. The alignment of collagen is described by the pitch angle (φc) made with the Q-axis (φc = 0 indicates that collagen is aligned circumferentially; φc = 90 indicates that collagen is aligned axially); in this hypothetical example, a helical alignment of collagen fibrils is shown. Collagen at pitch angles of 0–50° was measured from circumferential cross-sections (φc shows pitch angles of collagen fibrils with respect to the circumferential axis), and collagen at pitch angles of 40–90° (φc) was measured from axial cross-sections (φa shows the range of angles of collagen fibrils measured from axial sections).
Ultrastructural Analysis
Sections were photographed to yield 8″ × 10″ print images with magnifications of 10,008× (used to measure diameters of collagen fibers), 36,140× (used to determine the orientation of collagen fibrils within engineered vessels), and 157,410× (used to measure diameters of collagen fibrils and banding periodicities within collagen fibrils), respectively. Photographs were taken at regular spatial intervals across the width of the vessel walls (Fig. 1).
Relative Frequency of Observation for Alignment of Collagen
TEM images (each 36,140×, which covered a 90 nm × 5.3 μm × 6.8 μm section of tissue) sampled 10–20% of the wall thickness of the circumferential and axial cross-sectional planes of each artery (Figs. 1b, c). We observed very few radially aligned collagen fibrils, and thus our study focused on collagen aligned in the Θ–Z plane (Fig. 1d). Collagen fibrils were projected onto the sectioned plane in each photographed image (Fig. 2). The angle, φ, between each collagen fibril and the sliced plane was calculated as the inverse tangent of the thickness of the TEM section (90 nm) divided by the projected length of the collagen fibril onto the sliced plane (Fig. 3). The full path of each engineered (∼40 nm in diameter; see results) and native (∼80 nm in diameter) collagen fibril could be identified within each 90 nm TEM section.
FIGURE 2.
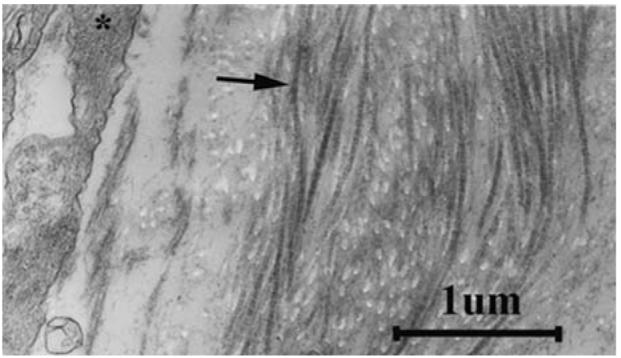
A small portion of a representative TEM micrograph reveals individual collagen fibrils (arrow) and part of a cell (*) in a tissue engineered artery.
FIGURE 3.
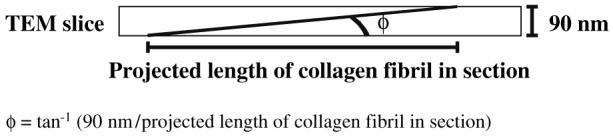
Measurement of φ, the angle that a collagen fibril makes with the sliced axis, from a TEM micrograph. All sections were 90 nm in thickness.
For each series of images, the angle of every collagen fibril in the range of 0–50° was measured with respect to the circumferential (φc, Fig. 1d) or axial axis (φa); fibrils passing through either the circumferential or axial plane at angles of greater than 50° were ignored because they were more accurately measured in the orthogonal plane. Angles measured relative to the axial axis were converted to angles relative to the circumferential axis by subtracting the axial angles from 90°. As it was impossible to know whether an individual fibril passed through the plane at +φ or −φ, we assumed that collagen fibrils were oriented symmetrically across circumferential planes, and across axial planes. Alignment of collagen was binned into nine groups of collagen pitch angles (φc, Fig. 1d): 0–10°, 10–20°, 20–30°, 30–40°, 40–50°, 50–60°, 60–70°, 70–80°, and 80–90°, with respect to the circumferential axis. The observed number of collagen fibrils within each bin of angles was normalized by the number of TEM images taken in the relevant cross-sectional plane (circumferential or axial) for each vessel. The relative frequency of observation for alignment of collagen was computed for each vessel by dividing the number of normalized collagen fibrils in each bin by the total number of normalized collagen fibrils, such that the relative frequency of observation summed across all nine bins equaled unity. The mean angle of each bin was used as the angle to represent each bin (e.g., 5° represented the bin of 0–10°).
Statistics
All values are presented as the mean ± the standard error of the mean. For comparison of two groups, statistical significance was determined by a Student's two-sample t-test, assuming unequal variances. The reported p values are one-sided. For comparison of many groups (relative frequency of observation), statistical significance was determined by one-way ANOVA and the Tukey–Kramer post-hoc multiple comparison test, which accounts for unequal sample sizes.
RESULTS
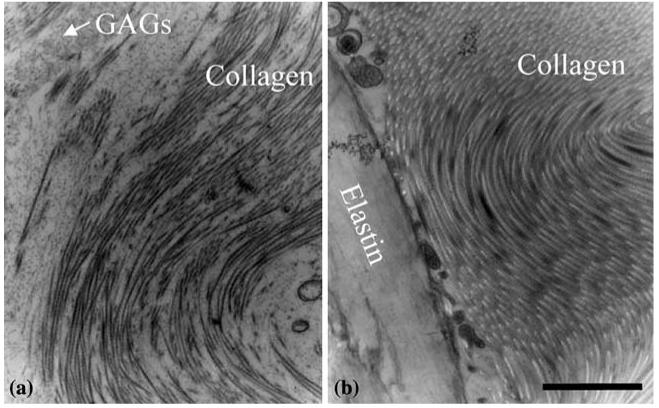
TEM images showed that collagen was prevalent in both engineered and native arteries. Collagen fibrils in engineered and native vessels had similar band periodicities (Fig. 4; Table 1). However, diameters of collagen fibrils and fibers in engineered vessels were significantly smaller than those in native vessels (Table 1). Further, packing of collagen fibrils into collagen fibers was less dense in engineered vessels (Fig. 5). In engineered arteries, collagen was frequently surrounded by glycosaminoglycans (GAGs) and SMCs (Fig. 5). In native arteries, collagen was frequently surrounded by elastin or by more collagen. No elastin fibers were seen in TEMs of engineered arteries (Fig. 5).
FIGURE 4.
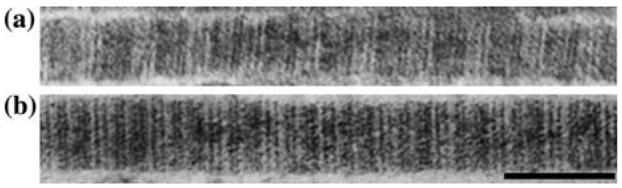
Representative collagen fibrils show similar band periodicities in (a) engineered and (b) native porcine arteries (scale bar = 100 nm).
TABLE 1.
Ultrastructural properties of collagen in engineered and native vessels.
| Engineered vessels | Native vessels | p-Value | |
|---|---|---|---|
| Collagen band periodicity (nm) | 59.2 ± 0.7 (n = 12) | 59.3 ± 2.1 (n = 2) | NS |
| Diameters of collagen fibrils (nm) | 43 ± 1 (n = 13) | 80 ± 11 (n = 2) | <0.05 |
| Diameters of collagen fibers (μm) | 1.8 ± 0.3 (n = 10) | 5.1 ± 1.6 (n = 2) | <0.05 |
NS, not significant.
FIGURE 5.
Representative TEM images show that collagen fibrils pack less densely to form collagen fibers in (a) engineered vessels than in (b) native vessels (scale bar = 2 μm).
Overall observations indicated that the spatial organization of collagen differed between engineered and native tissues. Specifically, from the lumen to the outer edge of engineered vessels, we observed that collagen was consistently arranged either as sparse fibrils or as loosely packed fibrils within a GAG-rich matrix. In contrast, native vessels had few collagen fibers near their lumens, where SMCs were most prominent. Yet, collagen fibers and elastin were prominent in the outer half of the medial layers of native arteries.
Relative frequency of observation for the orientation of collagen displayed the same trends of circumferentially, helically, and axially aligned collagen fibrils in both engineered and native vessels (Fig. 6). In both engineered and native vessels, slightly less than half of the collagen was present at helical pitches of 30–60°. Although trends in alignment of collagen were similar, engineered vessels had significantly less circumferentially aligned collagen (5°) and significantly more axially aligned collagen (85°) than native vessels.
FIGURE 6.
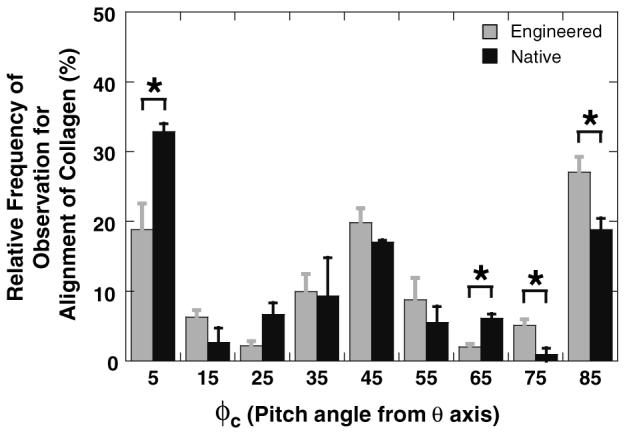
Relative frequency of observation for the alignment of collagen fibrils in porcine engineered vessels (n = 8) and porcine carotid arteries (n = 2). φc is the angle shown in Fig. 1 (φc = 5 indicates that collagen is oriented circumferentially; φc = 45 indicates that collagen is oriented helically; φc = 85 indicates that collagen is oriented axially). The frequency of collagen in circumferential or axial alignments were significantly different (*, p < 0.05) between engineered and native vessels.
DISCUSSION
Similar collagen band periodicities suggest that engineered and native collagen molecules have comparable distributions of polar charged and hydrophobic amino acid sequences to control molecular packing during collagen fibril formation. Further, given that band periodicities are larger for fibrils with only Type I collagen, such as tendon,30 and smaller for fibrils with a mix of Types I and III collagen, such as skin and vascular tissue,24 similar band periodicities may indicate that engineered and native arteries contain a comparable ratio of collagen Type I to collagen Type III.
Engineered vessels consistently had thinner collagen fibrils and fibers than their native counterparts, likely either because their collagen is “young” (i.e., recently synthesized, within the previous 8 weeks), or because GAGs in engineered vessels inhibit growth of collagen fibrils.21 A longer culture time (> 52 weeks to mimic adult porcine arteries) may allow collagen fibrils in engineered tissues to widen and fuse with other fibrils.30 However, as a practical matter, the increased cost and risk of contamination associated with longer culture times may outweigh the small potential increase in strength that might be conferred with thicker collagen fibers.
In this study, an ultrastructural approach was utilized to evaluate the alignment of collagen in native and engineered vessels. To the best of our knowledge, this is the first report that collagen fibrils in native arteries are aligned simultaneously in circumferential, helical, and axial directions, which supports the combined results of other investigators.4,5,28 Thus, our results convey a more complete picture of alignments of collagen fibrils than what has been reported previously for arterial tissues.
Our results indicate that ultrastructural measurement of collagen alignment is more sensitive than detection methods such as polarized light microscopy, X-ray diffraction, and SALS. The most precise technique previously used to measure alignments of collagen was SALS. Although SALS had the two distinct advantages of time-efficiency and providing real-time information on tissues as they were strained, the resolution of SALS (254 μm)27 was inferior to that of TEM (TEM resolution is on the order of angstroms, which is 6 orders of magnitude smaller than the resolution of SALS). Given that the diameters of collagen fibrils and fibers in engineered vessels (approximately 43 nm and 1.8 μm, respectively) and in native vessels (approximately 80 nm and 5.1 μm, respectively) were significantly smaller than the resolution of SALS, SALS may have been unable to detect alignments of individual collagen fibrils and fibers in vessels. Further, the thicknesses of porcine engineered vessels (150–300 μm) were frequently smaller than the resolution of SALS. TEM analysis, however, provided extremely sensitive measurements of ultrastructural properties and alignments of collagen fibrils in vessels.
Although an ultrastructural technique, which can be used to detect the alignment of individual collagen fibrils, is more sensitive than many other techniques, TEM analysis has several limitations. First, the alignment of undulated collagen may be misrepresented. Routine errors in the measurements of collagen fibrils would be revealed by a base level of collagen at all possible angles, or “background noise” in relative frequency of observation measurements. Given that observed alignment was minimal at 60–70° for engineered vessels, and at 70–80° for native vessels, routine incorrect measurement of collagen alignment was unlikely. Second, both engineered and native tissues were fixed in an unloaded configuration without axial strain or physiological pressures. Given that both tissue types were fixed similarly, their relative assessments are likely accurate. However, these samples may not accurately represent the circumferential or axial recruitment of collagen fibers that results from exposure to physiological luminal pressures or axial strains, respectively. Thus, the relative frequency of observation for alignment of collagen presented herein may underestimate circumferential or axial alignments of collagen, and thereby overestimate the amount of helically aligned collagen. Finally, ultrastructural analyses are excessively time consuming, and therefore, are not practical for routine assessments of tissue properties. Even with these limitations, TEM analysis provided valuable detailed insights into the properties of collagen in engineered and native arteries.
In this study, the sensitivity provided by ultrastructural analysis allowed measurement of the relative frequency of observation for alignment of collagen. These novel frequency observations revealed that engineered vessels, like native vessels, contained collagen aligned simultaneously in circumferential, helical, and axial directions. Engineered vessels, however, had less circumferentially aligned collagen and more axially aligned collagen than native vessels. Engineered vessels were cultured with circumferential cyclic strains of 1.5%. Given that native arteries may experience strains of up to 35% in the physiological pressure range (70–120 mmHg),7 and that collagen alignment increases with strain,9,11 it is not surprising that native vessels contain more circumferentially aligned collagen than engineered vessels. Thus, applying larger circumferential cyclic strains during culture may increase circumferential alignment of collagen in engineered vessels.
The theory of composite materials states that a material is stiffest when fibers within a matrix are aligned in the direction of tension.25 Thus, an artery with more circumferentially aligned collagen would likely be stronger (circumferentially) than an artery with less circumferential collagen. Further, collagen fibers aligned between 0° and 45° become circumferentially recruited as luminal pressures increase.28 Thus, generating engineered vessels with a higher percentage of circumferential or helical collagen may increase circumferential strength. We have shown previously that engineered arteries cultured with circumferential strains are stronger than those cultured in static conditions.22,23 Circumferential straining during culture likely improves the strength of engineered tissues primarily by promoting increased collagen synthesis.23,29 However, circumferential strains during culture also may promote increased circumferential and helical alignment of collagen, which in turn, may increase circumferential strengths.
Scaffold choice may also impact collagen ultrastructure in engineered tissues. First, scaffold composition may impact levels of collagen synthesis. For example, fibrin gels support more collagen synthesis than collagen gels.12 Material geometry may guide cellular alignment,10 which in turn, may guide collagen fibril alignment.6 The PGA scaffold used in this study had more axial fibers than circumferential fibers. Thus, preferential axial alignment of PGA fibers may have guided axial cell attachment, and therefore, axial collagen alignment to a greater extent than an isotropic scaffold or a scaffold with preferential circumferential alignment.
Thus, the detailed ultrastructural comparisons presented in this study suggest that molecular collagen synthesis was similar in engineered and native tissues, but that collagen fibrils and fibers were thinner in engineered vessels than in native arteries. Further, collagen fibrils were more loosely packed within collagen fibers in engineered arteries than in native arteries. The relative frequency of observation for alignment of collagen fibrils revealed similarities between alignments of collagen in engineered and native vessels, but also revealed differences in the amounts of collagen aligned circumferentially and axially. These ultrastructural measurements of collagen alignment were significantly more sensitive and thorough than measurements made with other techniques. Future studies should focus on using the relative frequency of observation for alignment of collagen presented herein as a descriptive microstructural input for models of arterial mechanical properties, which previously have utilized only one primary theoretical or measured dominant alignment of collagen.14,16,19
ACKNOWLEDGMENTS
This work was funded by NIH R01 HL63766. Many thanks to the Duke Cancer Center Electron Microscopy Facility for preparing samples for transmission electron microscopy, and for training the authors to use the transmission electron microscope. The authors also wish to thank Frank Baaijens and Niels Driessen for thought-provoking discussions about collagen alignment.
REFERENCES
- 1.Armentano RL, Levenson J, Barra JG, Cabrera Fischer EI, Breitbart GJ, Pichel RH, Simon A. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am. J. Physiol. 1991;260:H1870–H1877. doi: 10.1152/ajpheart.1991.260.6.H1870. [DOI] [PubMed] [Google Scholar]
- 2.Aspden RM, Bornstein NH, Hukins DW. Collagen organisation in the interspinous ligament and its relationship to tissue function. J. Anat. 1987;155:141–151. [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky B, Eikenberry EF, Cassidy K. An unusual collagen periodicity in skin. Biochim. Biophys. Acta. 1980;621(1):162–166. doi: 10.1016/0005-2795(80)90072-0. [DOI] [PubMed] [Google Scholar]
- 4.Canham PB, Finlay HM, Boughner DR. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovasc. Res. 1997;34(3):557–567. doi: 10.1016/s0008-6363(97)00056-4. [DOI] [PubMed] [Google Scholar]
- 5.Canham PB, Talman EA, Finlay HM, Dixon JG. Medial collagen organization in human arteries of the heart and brain by polarized light microscopy. Connect. Tissue Res. 1991;26(1–2):121–134. doi: 10.3109/03008209109152168. [DOI] [PubMed] [Google Scholar]
- 6.Canty EG, Starborg T, Lu Y, Humphries SM, Holmes DF, Meadows RS, Huffman A, O'toole ET, Kadler KE. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J. Biol. Chem. 2006;281(50):38592–38598. doi: 10.1074/jbc.M607581200. [DOI] [PubMed] [Google Scholar]
- 7.Cox RH. Differences in mechanics of arterial smooth muscle from hindlimb arteries. Am. J. Physiol. 1978;235(6):H649–H656. doi: 10.1152/ajpheart.1978.235.6.H649. [DOI] [PubMed] [Google Scholar]
- 8.Dahl SLM, Rucker RB, Niklason LE. Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant. 2005;14(10):861–868. [PubMed] [Google Scholar]
- 9.Driessen NJ, Peters GW, Huyghe JM, Bouten CV, Baaijens FP. Remodelling of continuously distributed collagen fibres in soft connective tissues. J. Biomech. 2003;36(8):1151–1158. doi: 10.1016/s0021-9290(03)00082-4. [DOI] [PubMed] [Google Scholar]
- 10.Engelmayr GCJ, Papworth GD, Watkins SC, Mayer JEJ, Sacks MS. Guidance of engineered tissue collagen orientation by large-scale scaffold microstructures. J. Biomech. 2006;39(10):1819–1831. doi: 10.1016/j.jbiomech.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Finlay HM, McCullough L, Canham PB. Three-dimensional collagen origanization of human brain arteries at different transmural pressures. J. Vasc. Res. 1995;32:301–312. doi: 10.1159/000159104. [DOI] [PubMed] [Google Scholar]
- 12.Grassl ED, Oegema TR, Tranquillo RT. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J. Biomed. Mater Res. 2002;60(4):607–612. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann H, Fietzek PP, Kuhn K. The role of polar and hydrophobic interactions for the molecular packing of type-I collagen: a three-dimensional evaluation of the amino acid sequence. J. Mol. Biol. 1978;125:137–165. doi: 10.1016/0022-2836(78)90342-x. [DOI] [PubMed] [Google Scholar]
- 14.Holzapfel GA, Gasser TC, Stadler M. A structural model for the viscoelastic behavior of arterial walls: continuum formulation and finite element analysis. Eur. J. Mech. A/Solids. 2002;21:441–463. [Google Scholar]
- 15.Holzapfel GA, Gasser TC. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elasticity. 2000;61:1–48. [Google Scholar]
- 16.Humphrey JD. Remodeling of a collagenous tissue at fixed lengths. J. Biomech. Eng. 1999;121:591–597. doi: 10.1115/1.2800858. [DOI] [PubMed] [Google Scholar]
- 17.Kadler K. Matrix loading: assembly of extracellular matrix collagen fibrils during embryogenesis. Birth Defects Res. C Embryo Today. 2004;72(1):1–11. doi: 10.1002/bdrc.20002. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn K, Glanville RW. Molecular structure and higher organization of different collgaen types. In: Viidik A, Vuust J, editors. Biology of Collagen. Academic Press Inc.; London: 1980. pp. 1–14. [Google Scholar]
- 19.Lanir Y. Constitutive equations for the lung tissue. Trans. ASME. 1983;105:374–380. doi: 10.1115/1.3138435. [DOI] [PubMed] [Google Scholar]
- 20.Lanir Y. Constitutive equations for fibrous connective tissues. J. Biomech. 1983;16(1):1–12. doi: 10.1016/0021-9290(83)90041-6. [DOI] [PubMed] [Google Scholar]
- 21.Merrilees MJ, Tiang KM, Scott L. Changes in collagen fibril diameters across artery walls including a correlation with glycosaminoglycan content. Connect. Tissue Res. 1987;16:237–257. doi: 10.3109/03008208709006979. [DOI] [PubMed] [Google Scholar]
- 22.Niklason LE, Abbott WA, Gao J, Klagges B, Hirschi KK, Ulubayram K, Conroy N, Jones R, Vasanawala A, Sanzgiri S, Langer R. Morphologic and mechanical characteristics of engineered bovine arteries. J. Vasc. Surgery. 2001;33(3):628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 23.Niklason LE, Gao J, Abbott WM, Hirschi K, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 24.Rothenburger M, Volker W, Vischer JP, Berendes E, Glasmacher B, Scheld HH, Deiwick M. Tissue engineering of heart valves: formation of a three-dimensional tissue using porcine heart valve cells. ASAIO J. 2002;48(6):586–591. doi: 10.1097/00002480-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Roylance D. Mechanics of Materials. John Wiley & Sons, Inc.; New York: 1996. [Google Scholar]
- 26.Sacks MS. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar tissues. Trans. ASME. 2003;125:280–287. doi: 10.1115/1.1544508. [DOI] [PubMed] [Google Scholar]
- 27.Sacks MS, Smith DB, Hiester ED. A small angle light scattering device for planar connective tissue micro-structural analysis. Ann. Biomed. Eng. 1997;25:678–689. doi: 10.1007/BF02684845. [DOI] [PubMed] [Google Scholar]
- 28.Shadwick RE. Mechanical design in arteries. J. Exp. Biol. 1999;202:3305–3313. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 29.Solan A, Mitchell S, Moses M, Niklason L. Effect of pulse rate on collagen deposition in the tissue-engineered blood vessel. Tissue Eng. 2003;9(4):579–586. doi: 10.1089/107632703768247287. [DOI] [PubMed] [Google Scholar]
- 30.Torp S, Baer E, Friedman B. Effects of age and of mechanical deformation on the ultrastructure of tendon. In: Atkins EDT, Keller A, editors. Structure of Fibrous Biopolymers. Butterworths; London: 1975. [Google Scholar]
- 31.Wells SM, Sellaro T, Sacks MS. Cyclic loading response of bioprosthetic heart valves: effects of fixation stress state on the collagen fiber architecture. Biomaterials. 2005;26(15):2611–2619. doi: 10.1016/j.biomaterials.2004.06.046. [DOI] [PubMed] [Google Scholar]