Abstract
Species range boundaries often form along environmental gradients that dictate the success of the phenotypes present in each habitat. Sociality may allow colonization of environments where related species with a solitary lifestyle cannot persist. Social spiders in the genus Anelosimus appear restricted to low- and mid-elevation moist environments in the tropics, while subsocial spiders, common at higher elevations and latitudes, appear to be absent from the lowland tropical rainforest. Here, we seek factors that may simultaneously prevent subsocial Anelosimus species from colonizing the lowland rainforest while favouring species with large social groups in this habitat. To this end, we transplanted small groups of a subsocial species, which contain the offspring of a single female, from cloud forest habitat in the centre of its natural range to lower montane rainforest on the range margin and to lowland rainforest outside of the species range. Groups transplanted at the range margin and below their range limit were less likely to disperse and experienced increased mortality. This was correlated with greater rainfall intensity and ant abundance. We show that protection from rainfall enhances the performance of small groups of spiders in the lowland rainforest, and suggest that predation or disturbance by ants may influence the geographical range limits of this species.
Keywords: cooperation, dispersal, local adaptation, predation, rain exclosure, Theridiidae
1. Introduction
Social traits can change the way an organism interacts with its environment, often by allowing groups to access a niche not available to solitary individuals (Wilson 1975; Slobodchikoff 1984; Avilés 1999). As a result, social behaviours may differ across environmental gradients, both within species (e.g. Eickwort et al. 1996; Liebert et al. 2005; Purcell & Avilés 2007) and among related species (e.g. Mori & Saito 2005; Avilés et al. 2007). By investigating factors influencing the distribution of social organisms across such environmental gradients, we simultaneously seek to understand the factors maintaining species ranges and the selective forces favouring particular social phenotypes in different environments.
Theoretical studies have demonstrated the importance of both environmental and intrinsic factors in delimiting the geographical range of a species (e.g. Gaston 2003; Holt et al. 2005). Kirkpatrick & Barton (1997), for instance, modelled a hypothetical species with simple quantitative fitness determination along an environmental gradient. Populations in the centre of the range were well adapted to the local environment, but those on the range edge performed poorly, as genotypes from the range centre swamped locally favourable mutations through migration. Empirical studies are still needed, however, to test the hypotheses generated by this and other theoretical models of range boundaries in real systems (Bridle & Vines 2007). Transplant experiments can provide insight into the role of both intrinsic and environmental factors at a range boundary by facilitating a direct comparison of life-history traits in native and foreign habitats with measurable environmental differences (e.g. Riechert & Hall 2000; Angert & Schemske 2005; Geber & Eckhart 2005). Here, we use the transplant experimental approach to investigate the factors, both intrinsic and extrinsic (environmental), that may prevent subsocial Anelosimus spiders from colonizing the lowland tropical rainforest in Ecuador (e.g. Avilés et al. 2007). We focus on intrinsic aspects of the biology of these organisms that relate to their social system, such as colony size and dispersal tendencies, although we cannot exclude the possibility that physiological factors (e.g. temperature and humidity preferences) may also play a role in determining the observed patterns.
Several studies have noted that social (non-territorial permanently social) spiders are concentrated in tropical regions of the world, and occupy only a subset of the habitats used by members of their phylogenetic lineages (Avilés 1997; Avilés et al. 2007). Just as striking, but less frequently noted, is the absence of related subsocial or solitary species from some areas where social species are present. This pattern is especially clear in the genus Anelosimus where subsocial species are absent from lowland tropical rainforest habitats in eastern Ecuador (Agnarsson 2006; Avilés et al. 2007). In subsocial species, colonies typically contain the offspring of a single female, usually up to several dozen individuals, which cooperate early in their life cycle, but disperse prior to sexual maturity. Social species, thought to be derived from subsocial-like ancestors (Avilés 1997; Agnarsson 2006; Lubin & Bilde 2007), cooperate in prey capture, nest maintenance and brood rearing and extend their group living period for multiple generations, with some colonies containing tens of thousands of spiders. In discussing the mechanisms that may lead to the distinct geographical distribution of social and subsocial Anelosimus species, Avilés et al. (2007) suggested that two separate patterns need to be addressed: first, the absence of social spiders from higher altitudes and latitudes; and second, the absence of subsocial spiders from the lowland tropical rainforest. The former may result from an insufficient abundance of large insect prey outside of the lowland rainforest to meet the nutritional requirements of large spider groups (Guevara & Avilés 2007; Powers & Avilés 2007; Yip et al. in press; see also Uetz & Hodge 1990; Rypstra & Tiery 1991). With both large and small prey available in the lowland rainforest, however, this mechanism cannot explain the absence of subsocial species from this habitat.
Using a transplant experiment, here we test the hypotheses, based on Avilés et al. (2007), that subsocial species may instead be excluded from the lowland rainforest by factors that increase mortality during dispersal (H1) or lead to the complete failure of nests with solitary foundresses and their brood (H2). We also explore the possibility that intrinsic benefits of remaining in the natal group may disfavour dispersal in this habitat (H3). To test these hypotheses we transplanted nests of a subsocial spider from its native upper elevation cloud forest habitat to lower montane and lowland rainforest. We explore the following specific predictions derived from these hypotheses. If subsocial species have not simply failed to disperse to lowland rainforest, but rather are maladapted to this habitat, we expect (i) increased extinction of small groups and (ii) higher individual mortality as distance to the rainforest decreases; moreover, if spiders in the lowland rainforest are better off living in groups than solitarily, we would expect (iii) decreased dispersal tendencies of individuals (i.e. fewer dispersers) and (iv) decreased survival of dispersers at lower elevations compared to the cloud forest control habitat.
We simultaneously explore potential candidate factors that may contribute to the failure of solitary dispersers or small groups in the lowland rainforest, namely rainfall intensity (H4) and predation (H5). Anelosimus spiders build three-dimensional webs requiring active maintenance and a large volume of thread, which would be costly to repair when damaged by intense rain showers or falling branches (Avilés 1997). Riechert et al. (1986) observed that larger groups of the social spider Agelena consociata recovered more rapidly from extreme weather disturbance events than small groups. In subsocial species, with fewer individuals present to maintain and rebuild the web, nest damage resulting from intense rain could render some habitats inhospitable. We test this hypothesis by sheltering half of the nests transplanted in the lowland rainforest from rain. We expect (i) greater survival rates and (ii) larger nests in the sheltered compared to the exposed treatment. We also shelter small groups of the native social species and test the same predictions. Predation was suggested as a mechanism selecting for sociality in Stegodyphus dumicola (Henschel 1998) and colonial aggregations in Metepeira incrassata spiders (Uetz et al. 2002), and in other social organisms (London & Jeanne 2003; Smith et al. 2003; Mori & Saito 2005). We hypothesize that small subsocial groups may be excluded from areas where potential predators are abundant, because they are not able to repulse or evade predators as effectively as large social groups. We test our prediction that predators may be more abundant in the lower elevation habitats by comparing the presence/absence of two likely spider predators, ants and jumping spiders (J. Purcell 2006, personal observation), in nests at all three transplant habitats.
2. Material and methods
We transplanted colonies of the subsocial spider species Anelosimus baeza Agnarsson (2006) within the centre (2100 m elevation) and to the edge of its native habitat (1000 m) and to a low elevation habitat beyond the species range boundary (400 m). Along the Andes' eastern slope, elevation appears to be correlated with several environmental factors that may be relevant in this system, including rainfall intensity and potential predator abundance, so we use elevation as a proxy to describe the position of each habitat along the transition from cloud forest to lowland tropical rainforest.
(a) Species description
Anelosimus baeza, a subsocial spider, is widespread in South America, extending from Panama to Peru and Brazil (Agnarsson 2006). Like other subsocial spiders, A. baeza lives in single-family groups—a female and her brood or groups of pre-reproductive siblings—with individuals dispersing to live solitarily in their late juvenile to young adult stages (Powers 2004). On the eastern slopes of the Ecuadorian Andes, this species occurs primarily in cloud forest habitat (900–2500 m above sea level), with the occasional nest found down to approximately 600 m elevation. It is absent from elevations below 600 m in the tropical rainforest (Avilés et al. 2007). We used this species for our studies because it has a body size comparable to that of Anelosimus eximius Keyserling (1884), a common social species in the lowland rainforest (adult female total length: 4 mm for A. baeza versus 4.6 mm for A. eximius; Agnarsson 2006) and because, among the Ecuadorian subsocial species, it occurs in habitats closest to the rainforest (Avilés et al. 2007). The latter reduces the possibility of physiological maladaptation to the transplant environments.
(b) Habitat descriptions
Transplanted groups were placed at three locations within 65 km of one another along an altitudinal gradient on the eastern slope of the Andes in Ecuador's Napo province: at its native cloud forest habitat near the Yanayacu Biological Station (0.061° S, 77.893° W, 2000–2200 m), at an intermediate elevation of lower montane rainforest area adjacent to the Hollin River near Sumaco National Park (0.695° S, 77.731° W, 950–1100 m), and at lowland tropical rainforest habitat located in the Jatun Sacha Reserve along the Napo River, east of Tena (1.072° S, 77.617° W, 380–450 m). Both the upper and intermediate elevation habitats have been termed ‘lower montane rainforest’ or ‘cloud forest’ habitats in the literature (Neill 1999a). Here, we distinguish between them by referring to the upper elevation habitat as cloud forest and the intermediate habitat as lower montane rainforest, because the plant composition differs greatly between these two habitats (see Neill 1999a,b for floral and climatic characteristics of all three habitats). The focal subsocial species A. baeza occurred naturally in open and disturbed habitats in the two upper elevation locations. The social A. eximius, the subsocial Anelosimus elegans and an undescribed subsocial species were also present in the lower montane habitat. Anelosimus eximius and two additional social species, Anelosimus domingo and Anelosimus rupununi, occurred naturally in the lowland rainforest (Avilés et al. 2007).
At all three transplant locations, we measured rainfall intensity by collecting rain for 30 min during heavy showers with a plastic precipitation gauge placed in an open field (at least three measurements per habitat). We also estimated average daily rainfall by collecting rain for 24 hour periods in a second plastic gauge (at least seven measurements per habitat, throughout the course of the study).
(c) Transplant methods
For transplantation, in June 2006 we collected relatively large A. baeza nests (median 50, range 20–325 spiders) from seven distinct sites—separated by 500 m or more—near the Yanayacu Biological Station. We dissected each nest and sorted the occupants by age and sex. At the time of collection, nests in the area contained primarily late-instar juveniles to young adults. We transplanted only female spiders (late-instar juvenile to adult) in order to avoid inadvertently introducing the species into foreign habitat. We placed the spiders from each transplant group together in containers with a small plastic plant to encourage initial nest construction and to provide identical structures to support the nests at all habitats. All groups built webbing on this artificial substrate. The number of spiders transplanted per colony varied from 12 to 100 individuals. We matched colonies by size, and randomly assigned them to treatments to ensure an even distribution of group sizes in each treatment. Three large groups were divided into two or three transplant groups and placed in separate treatments to avoid pseudoreplication.
Within each transplant location, we selected four to five sites that were at least 1 km apart, except for two sites at the intermediate elevation that were separated by 500 m on a steep hillside. We transplanted 20 nests to five sites within the source habitat (2100 m); 19 nests to four sites at the intermediate habitat (1000 m); and 50 nests to five sites in the lowlands (400 m; see appendix table 1 in the electronic supplementary material). At each of the sites, we placed transplanted nests at least 10 m from each other and at least 5 m from any natural Anelosimus nests.
Within the source habitat, we placed transplanted nests on their preferred plant substrate, a Baccharis and a Monochaetum species (Asteraceae and Melastomataceae, respectively; J. Purcell 2005, personal observations). At the other two locations, where these plant species were not common, we sought sites that resembled the characteristics (leaf size, shape and texture, branch architecture, and the openness of the habitat) of the preferred nest locations in the source habitat as much as possible. At all sites, we avoided areas where ants were already present on the substrate.
To transplant nests, we attached the artificial plant with the incipient nest to the selected branch and enclosed the branch in a mesh tube. We removed the mesh tubes after at least 4 days, when new webbing was observed within the enclosure (the spiders were fed with insect prey every 2–3 days while enclosed). After removing the mesh tubes, we closely monitored the incipient nests to prevent any premature dispersal attempts. Although some spiders tried to disperse immediately after the mesh tubes were removed, at the two upper elevation habitats most spiders soon settled in their newly transplanted nest. In the lowlands, on the other hand, we were forced to suspend the mesh removal process after it became clear that the spiders would not remain at their prescribed location. We were nonetheless able to obtain partial data for the rain exclosure experiment from the 35 nests that were recollected, without the mesh tubes having been removed, approximately 15 days after the start of the experiment, and from some of the 15 nests for which the mesh removal process was completed (nine nests for number of spiders remaining and one for amount of webbing built; see §3 and appendix table 1 in the electronic supplementary material). We then monitored all nests that became established at least once per week for up to two months (see appendix table 1 in the electronic supplementary material), noting the size and condition of the nest, approximate number of occupants and of dead spiders (carcasses), presence within the nests of potential predators (including ants or jumping spiders) and occurrence of dispersal, as evidenced by the presence of newly founded nests (here termed as ‘propagules’) within an approximately 5 m radius of the surveyed nest. Previous studies have demonstrated that the majority of subsocial Anelosimus propagules, which are easily distinguishable by the fresh webbing and clear appearance, become established within 5 m of the source nest (Avilés & Gelsey 1998; Powers & Avilés 2003). Therefore, we feel confident in our ability to detect the presence or absence of dispersal. All propagules were flagged and monitored with the same criteria as the transplanted nests for the rest of the experiment. When no propagules were observed, we ascribed the disappearance of spiders to predation or mortality from other causes. At the end of the experimental period, all nests, both transplants and propagules, were recollected and the spiders sorted and counted.
(d) Rain exclosure
In order to test the effect of intense rainfall on the survival and web-building ability of the transplanted nests in the lowland rainforest habitat (400 m), we sheltered 25 of the 50 transplanted A. baeza nests with semipermeable tarpaulin sheets (approx. 150×150 cm) placed approximately 1 m above the nest. We repeated this test using small groups (100 spiders) of the locally occurring social species, A. eximius. We established 22 such A. eximius groups at three sites chosen to match the preferred location and substrate characteristics of the species, with half the nests at each location sheltered from the rain and half exposed. For the experimental period, the colony dynamics of a small social group should resemble a subsocial group prior to dispersal. We monitored the nests as above, additionally noting the size of the nest and the number of webbing threads present at the end of the experiment.
(e) Analysis
We used hierarchical linear models (HLMs) to investigate differences between our treatment and control groups, taking into account the variation across different transplant sites within a habitat. For presence–absence and non-normally distributed outcome data, we used a generalized linear mixed model via the GLIMMIX macro in SAS v. 9.1 (SAS Institute, Cary, NC) to compare habitat as a fixed effect, with a random intercept clustered around transplant sites to control for within habitat variation. For normally distributed data, we used the MIXED procedure in SAS, with a similar model structure (Singer 1998). For the comparisons of colony survival and propagule survival, we used the survival actuarial analysis in StatView v. 4.1 (Abacus Concepts, Inc. Berkeley, CA), which allows variables to be censored. We tested both the effect of habitat, and of site within habitat, on colony survival. The remaining tests were performed using JMP v. 5.1 (SAS Institute, Cary, NC). For the comparisons of predator presence in nests across all three habitats, we constructed a nested model with one fixed effect (habitat) and one random effect (site within habitat). The effect of rain exclosure on small social groups was assessed using t-tests for normally distributed data and Wilcoxon rank-sum tests for non-normal data. Rainfall measurements were compared using ANOVA. We transformed proportions and nest size measurements using the arcsine square root and natural log, respectively.
3. Results
(a) Survival and dispersal
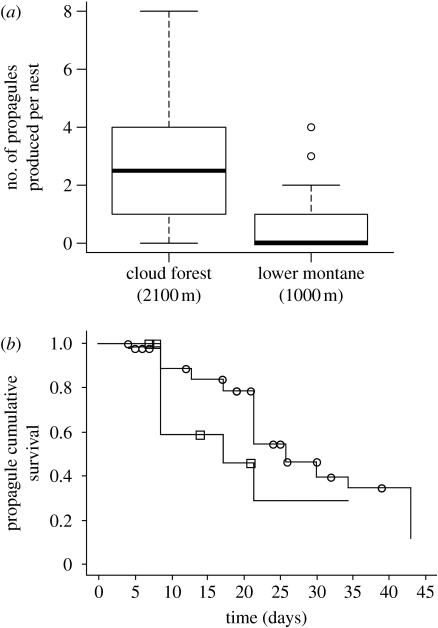
Following our initial expectation, A. baeza colony survival decreased with decreasing elevation (figure 1, table 1). In the cloud forest (2100 m), 93 per cent of transplanted colonies survived the initial 30 days following mesh tube removal (13 out of 14 nests), whereas 50 per cent survived in the lower montane rainforest (1000 m, 9 out of 18 nests). In the lowland rainforest, all colonies for which the mesh tubes were removed went extinct or disbanded within 4 days (400 m, 15 out of 15 nests, figure 1a). Within the species range, nests in lower montane habitat had a significantly smaller proportion of spiders remaining at the end of the experiment, but also produced fewer propagules, compared to the source habitat (figures 1b and 2a). We found no survival differences between transplant sites within habitats. The propagules that became established at the lower montane habitat (1000 m) went extinct faster than those in the cloud forest (figure 2b, table 1). We were unable to measure propagule formation and survival in the lowland rainforest because none of the transplanted colonies yielded propagules.
Figure 1.
(a) The greatest colony survival (circles, cloud forest; squares, lower montane; triangles, lowland rainforest) and (b) number of individuals remaining occurred in the native cloud forest habitat. Subsocial colonies did not persist in the lowland rainforest.
Table 1.
Summary of statistical method and result for each comparison. (Habitats are abbreviated according to their relative elevation; high refers to cloud forest (2100 m), mid- to lower montane rainforest (1000 m) and low to lowland rainforest (400 m).)
| species | treatments compared | comparison | approach (one-sided unless stated) | no. observations per habitat high, mid, low/closed, open | test statistic | model | direction | |
|---|---|---|---|---|---|---|---|---|
| test statistic | p-value | |||||||
| survival results | ||||||||
| A. baeza | high, mid and low | nest extinction | logrank (Mantel-Cox) test | H: 14, M: 18, L: 15 | Χ2 | 41.37 | <0.0001 | as predicted: high>mid>low |
| A. baeza | high and mid | proportion of spiders remaining | HLM (mixed) | H: 12, M: 9 | F | 11.74 | 0.007 | as predicted: high>mid |
| dispersal results | ||||||||
| A. baeza | high and mid | number of propagules | HLM (mixed) | H: 18, M: 17 | F | 7.96 | 0.009 | as predicted: high>mid |
| A. baeza | high and mid | propagule survival | logrank (Mantel–Cox) test | H: 47, M: 18 | Χ2 | 2.88 | 0.045 | as predicted: high>mid |
| rain exclosure | ||||||||
| A. baeza | low, exposed versus covered | proportion of spiders remaining | HLM (GLIMMIX) | C: 22, O: 22 | F | 3.68 | 0.032 | as predicted: covered>exposed |
| A. baeza | low, exposed versus covered | number of web strands on plant | HLM (mixed) | C: 17, O: 19 | F | 5.58 | 0.05 | as predicted: covered>exposed |
| A. eximius | low, exposed versus covered | proportion of spiders remaining | Wilcoxon test | C: 11, O: 10 | Χ2 | 0.24 | 0.31 | as predicted: covered>exposed |
| A. eximius | low, exposed versus covered | nest volume | t-test | C: 11, O: 11 | T | 1.85 | 0.04 | as predicted: covered>exposed |
| predation | ||||||||
| A. baeza | high, mid and low | presence/absence of ants | two-sided nested model | H: 18, M: 17, L: 50 | Χ2 | 26 | 0.038 | as predicted: high<mid<low |
| A. baeza | high, mid and low | presence/absence of spiders | two-sided nested model | H: 18, M: 17, L: 50 | Χ2 | 42.9 | 0.0002 | opposite of predicted: high>mid>low |
Figure 2.
(a) Transplanted subsocial spider groups yielded more propagules (new colonies founded by dispersers) per nest and (b) those propagules survived longer in their native cloud forest (circles, 2100 m) habitat compared to the lower montane (squares, 1000 m) transplant habitat. Propagule survival was measured from the first day we observed a new propagule, and was censored if the propagule survived until the end of the experiment, and uncensored if the propagule went extinct during the experiment.
(b) Mechanisms
(i) Rain exclosure
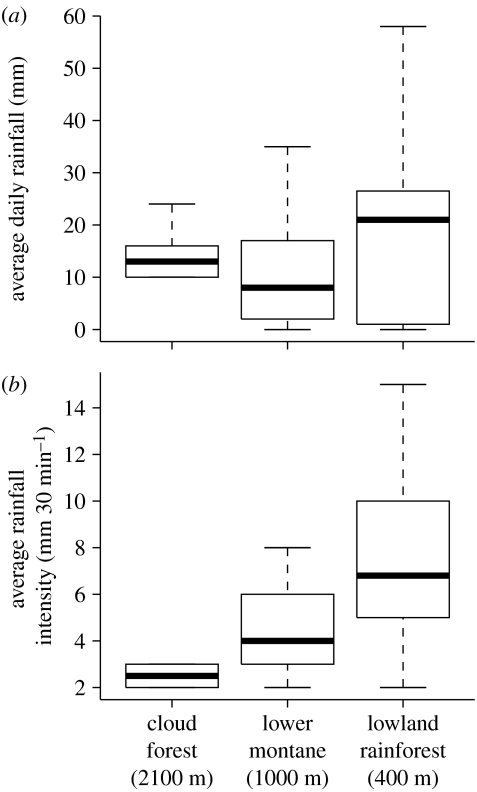
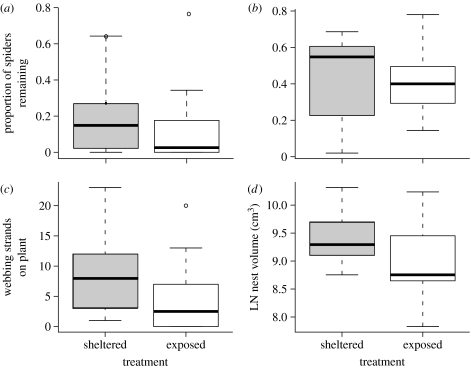
Rainfall intensity (figure 3b) tended to decrease with increasing elevation (ANOVA: F13=3.23; p=0.079), but daily rainfall (figure 3a) did not change linearly with elevation (ANOVA F29=1.08, p=0.35). Our measurements suggest that June–August 2006 received more rainfall than average, based on monthly averages recorded in Baeza, near Yanayacu (approx. 8 mm day−1, 1900 m) and Tena, near Jatun Sacha (approx. 13 mm day−1, 500 m; Grubb & Whitmore 1966; Neill 1999b). Although A. baeza survival was quite low in nests transplanted to the lowland rainforest even when the mesh tube was never removed, six times more spiders remained at the time of mesh removal or nest recollection in nests that were sheltered from the rain compared to those that were exposed (figure 4a; table 1). Spiders in the rain protection treatment built significantly more webbing than those in the unsheltered treatment (figure 4c). The native social species, A. eximius, also built significantly larger nests (nest volume compared; figure 4d; table 1) and showed a non-significant trend (1.5-fold increase) towards greater survival (figure 4b) when protected from the rain.
Figure 3.
(a) Average daily rainfall did not decrease linearly with elevation. However, (b) average rainfall intensity increased with decreasing elevation as expected.
Figure 4.
In rain-sheltered nests (grey) in the lowland rainforest, small groups of both the foreign ((a,c) A. baeza (subsocial)) and native ((b,d) A. eximius (social)) species showed improved survival and a greater amount of webbing compared to nests exposed to the rain (white). Our subsocial measurements were taken upon mesh tube removal or recollection of nests still enclosed in mesh (see §2). Both (a,c) comparisons were significant for the subsocial species, while only (d) nest volume was significantly greater for the social species (table 1).
(ii) Predator presence
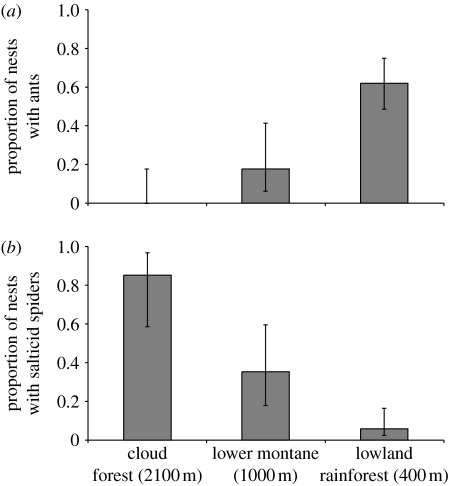
Significantly more nests had ants present within them as we approached the lowland rainforest (figure 5a, table 1). In the lowlands, at least 62 per cent of the A. baeza nests contained ants, some even before removal of the mesh tubes. Jumping spiders (family Salticidae), another potential spider predator, by contrast, occurred more frequently in nests at higher elevations (figure 5b, table 2). During this study, we observed predation of Anelosimus spiders by ants six times and by jumping spiders twice.
Figure 5.
(a) The presence of ants in transplanted spider nests was highest in the lowland rainforest, while (b) the reverse pattern was observed for salticid spiders. Error bars show 95% CI.
4. Discussion
(a) Survival and dispersal in native versus foreign habitat
In some scenarios, sociality may be an adaptation that allows organisms to colonize habitats where solitary individuals are unable to replace themselves (Wilson 1975; Slobodchikoff 1984; Avilés 1999). Along these lines, Avilés & Tufiño (1998) showed that females of the highly social spider A. eximius produce, on average, less than one surviving offspring per capita in lowland tropical rainforest habitats when attempting to raise their brood solitarily. This suggests that the lowland tropical rainforest may be a sink environment for non-social species in the genus, a suggestion further supported by the absence of subsocial Anelosimus species in this habitat (Avilés et al. 2007). The idea that sociality is favoured in environments where solitary foundresses cannot replace themselves underlies the ‘assured fitness returns hypothesis’ developed by Gadagkar (1990) to explain the evolution of worker castes in eusocial organisms. This hypothesis has been generalized to explain selection for sociality in conditions that are unfavourable to solitary living in a range of organisms, including the subsocial spider Anelosimus studiosus (Jones et al. 2007). The present study investigates the hypothesis that solitary dispersers and their brood cannot replace themselves in the lowland tropical rainforest, thus preventing colonization of that habitat by subsocial species.
Consistent with our predictions, the subsocial A. baeza suffered a dramatic decrease in colony and individual survival when transplanted to two lower elevation rainforest areas relative to control transplants within the native cloud forest habitat (figure 1). Mortality increased in severity with decreasing elevation, such that all groups went extinct within 4 days of mesh removal at the lowest transplant location (400 m). The strength of the effect suggests that lowland rainforest is unsuitable for this species, although we cannot yet tease apart the extent to which this effect may be due to the social phenotype (i.e. small groups containing the offspring of a single female being unable to maintain their web or ward off predators) versus physiological maladaptation to this environment. Some factors, such as higher temperatures (Neill 1999b) or diseases not present in the native range of the species, could lead to greater mortality independent of social phenotype. We found, for instance, a greater number of moulding A. baeza carcasses in the rainforest than at the two higher elevation habitats, demonstrating that predation was not the only source of mortality in the lowlands. The apparent reluctance of A. baeza spiders to settle in incipient nests in the lowland rainforest (see §2) further suggests that the spiders found local conditions inhospitable.
Anelosimus baeza groups yielded significantly fewer propagules in the lower montane habitat, where temperature, rain intensity and ant abundance were intermediate between cloud forest and lowland habitats (figure 2a). This finding supports our third prediction that spiders should attempt to remain in groups by dispersing less frequently as we approach the rainforest. This result suggests that at least one key subsocial trait that appears adaptive in the species' native habitat—early dispersal—may be less so in areas approaching lowland rainforest conditions. Previous studies have demonstrated that subsocial spiders will delay dispersal if greater food abundance or other factors favour remaining in groups (e.g. Krafft et al. 1986; Ruttan 1990). Such behavioural plasticity may explain the decreased dispersal behaviour in the lower montane habitat. Newly established propagules also went extinct faster in the lower montane habitat (1000 m) relative to native cloud forest habitat (2100 m), further supporting the notion that a solitary lifestyle is maladaptive in areas closer to the rainforest.
(b) Mechanisms preventing subsocial species from colonizing the lowland rainforest
We identified two environmental factors—rain intensity and incidence of ants—that were positively correlated with environments where greater mortality of solitary dispersers and small groups occurred. While other environmental parameters may also influence the success of the spiders in this system, we suggest that these two factors, in particular, could discourage small groups or solitary spiders relative to large groups in lowland rainforest and lower montane habitats. The same factors may have played a historical role selecting for large groups in the lowland rainforest, thus contributing to the repeated evolution of sociality in this genus (Agnarsson 2006; Agnarsson et al. 2006).
Rain intensity (mm per 30 min) increased linearly as we approached the lowland rainforest from higher elevation cloud forest areas (figure 3b), though average rainfall did not (figure 3a). Rain-sheltered nests of both the social and subsocial species transplanted in the lowlands had increased survival and more webbing than exposed nests (figure 4). These results support the hypothesis that intense rainfall can adversely affect the web maintenance abilities and survival of spiders with dense and potentially expensive webs. Anelosimus baeza individuals living in smaller groups contribute more webbing per capita to maintain their nests (Powers 2004). By extension, small groups might be particularly susceptible to nest damage. Our experiment demonstrated that a release from intense rainfall allowed groups containing the same number of individuals to build more webbing or larger nests (figure 4). Historically, intense rainfall in the rainforest may have exerted strong selective pressure on individuals to remain in the natal nest and share the cost of web maintenance and rebuilding, as was suggested for the social spider A. consociata (Riechert et al. 1986). Our finding that shelter from rain had a proportionally larger effect on survival in subsocial than in social groups (see §3) suggests that the social species may be better adapted to cope with heavy rains. An experiment testing whether larger social groups experience a reduced benefit from rain protection compared to the small groups tested here would further support the idea that cooperative web care is an important benefit of group living. However, because the rain-sheltered subsocial groups still went extinct, rain is clearly not the only factor preventing this subsocial species from colonizing the lowland rainforest.
Greater predation rates may also contribute to the absence of subsocial species from the lowland rainforest. We observed ants inside nests most frequently in the lowland rainforest (400 m), where A. baeza nests failed to establish (figures 1 and 5a). Ants are known to be important predators in terrestrial habitats (Hölldobler & Wilson 1990), and recently have been shown to reduce the survival of solitary allodapine bees, thus driving an increased cooperative nesting (Zammit et al. 2008). Our finding matched previous studies of ant abundance, which showed that ants are most common in lowland tropical rainforests in South America (e.g. Janzen et al. 1976; Guevara & Avilés in press), the Phillipines (Samson et al. 1997) and Malaysia (Bruhl et al. 1999). Previous studies of social spiders and our preliminary observations (see §3) also suggest that ants can cause rapid reduction in colony size or colony extinction (Henschel 1998). In response to ants, we observed Anelosimus spiders moving away from plant surfaces, attacking small numbers of ants (J. Purcell 2006, personal observations) or building a web retreat (J. Guevara 2005, personal communication). Through cooperation and greater nest volume, larger groups would have an advantage over small groups and solitary foundresses in all three responses. Thus increased predation or disturbance by ants in the lowland rainforest may exclude A. baeza from this habitat. However, further experiments are needed to directly demonstrate that predation by ants hinders the establishment of Anelosimus species with exclusively small groups and solitary females in the lowland tropical rainforest. By contrast, though spiders may be ecologically important Anelosimus predators (Perkins et al. 2007), our findings suggest that jumping spiders may not threaten the survival of Anelosimus spiders in the lowland rainforest (figure 5b).
Jones et al. (2007) suggested that colder temperatures favour multi-female groups in the otherwise subsocial species A. studiosus by lengthening the juvenile development period. This, in turn, provides cooperative nesters an increased probability of surrogate offspring care in the event of maternal death. Although not driven by temperature, we suggest that a similar mismatch between offspring development and maternal lifespan may occur in the lowland rainforest. Here, the mismatch may result from predation increasing the risk of maternal mortality or intense rains hindering the ability of single individuals or small groups to maintain their prey capture webs and feed their offspring.
(c) Conclusion
We found higher mortality both of transplanted groups and of propagules at the range boundary of A. baeza compared with the source population, thus matching the findings of theoretical predictions about species ranges (e.g. Kirkpatrick & Barton 1997) and previous empirical studies (e.g. Angert & Schemske 2005). Our example is novel in suggesting social behaviour as an adaptive phenotypic axis. While further work is still required to elucidate the relative influence of individual versus social traits to the adaptive limits of A. baeza, this study has identified at least two factors—intense rainfall and presence of potential predators in nests—that are correlated with an absence of subsocial Anelosimus in the lowland rainforest. These factors, combined with the availability of an abundant supply of large insects in this habitat (Guevara & Avilés 2007; Powers & Avilés 2007; Yip et al. in press), may have selected for larger social groups during the initial colonization of the lowland rainforest. This system thus presents an ideal opportunity to simultaneously investigate the role of interacting environmental factors driving the distinctive pattern of social distribution in spiders, and to pursue several of the unanswered questions about range margins, as enumerated by Bridle & Vines (2007).
Acknowledgments
This research was financed by a Canadian NSERC Discovery grant to L.A. J.P. was funded by a USA NSF Graduate Research fellowship. We thank the Corporación Sociedad para la Investigación y Monitoreo de la Biodiversidad Ecuatoriana for sponsorship and the Instituto Ecuatoriano de Recursos Naturales y Vida Silvestre for research permits. Thanks to L. Neame, K. Zeron, the Vazquez family, Yanayacu and Jatun Sacha Biological Stations for field support. Thanks also to J. Myers, M. Vellend, A. Brelsford, J. Guevara and N. Brown for their comments on this manuscript and to A. Shui for statistical advice.
Supplementary Material
Summary of methods, experimental treatments, and transplant duration for all nests. In the lower montane rainforest habitat, we transplanted 9 nests that had been recollected from Jatun Sacha. We transplanted these to see if they would be able to recover in a more favourable habitat. These nests performed as well as the 10 nests that were transplanted directly from the source habitat
References
- Agnarsson I. A revision of the New World eximius lineage of Anelosimus (Araneae, Theridiidae) and a phylogenetic analysis using worldwide exemplars. Zool. J. Linn. Soc. Lond. 2006;146:453–493. doi:10.1111/j.1096-3642.2006.00213.x [Google Scholar]
- Agnarsson I, Avilés L, Coddington J.A, Maddison W.P. Sociality in theridiid spiders: repeated origins of an evolutionary dead end. Evolution. 2006;60:2342–2351. doi:10.1554/06-078.1 [PubMed] [Google Scholar]
- Angert A.L, Schemske D.W. The evolution of species' distributions: reciprocal transplants across the elevation ranges of Mimulus cardinalis and M. lewisii. Evolution. 2005;59:1671–1684. doi:10.1111/j.0014-3820.2005.tb01817.x [PubMed] [Google Scholar]
- Avilés L. Causes and consequences of cooperation and permanent sociality in spiders. In: Choe J.C, Crespi B, editors. The evolution of social behaviour in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 476–498. [Google Scholar]
- Avilés L. Cooperation and non-linear dynamics: an ecological perspective on the evolution of sociality. Evol. Ecol. Res. 1999;1:459–477. [Google Scholar]
- Avilés L, Gelsey G. Natal dispersal and demography of a subsocial Anelosimus species and its implications for the evolution of sociality in spiders. Can. J. Zool. 1998;76:2137–2147. doi:10.1139/cjz-76-12-2137 [Google Scholar]
- Avilés L, Tufiño P. Colony size and individual fitness in the social spider Anelosimus eximius. Am. Nat. 1998;152:403–418. doi: 10.1086/286178. doi:10.1086/286178 [DOI] [PubMed] [Google Scholar]
- Avilés L, Agnarsson I, Salazar P, Purcell L, Iturralde G, Yip E, Powers K.S, Bukowski T. Altitudinal patterns of spider sociality and the biology of a new mid-elevation social Anelosimus species in Ecuador. Am. Nat. 2007;170:783–792. doi: 10.1086/521965. doi:10.1086/521965 [DOI] [PubMed] [Google Scholar]
- Bridle J.R, Vines T.H. Limits to adaptation at range margins: when and why does adaptation fail? Trends Ecol. Evol. 2007;22:140–147. doi: 10.1016/j.tree.2006.11.002. doi:10.1016/j.tree.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Bruhl C.A, Mohamed M, Linsenmair K.E. Altitudinal distribution of leaf litter ants along a transect in primary forests on Mount Kinabalu, Sabah, Malaysia. J. Trop. Ecol. 1999;15:265–277. doi:10.1017/S0266467499000802 [Google Scholar]
- Eickwort G.C, Eickwort J.M, Gordon J, Eickwort M.A. Solitary behavior in a high altitude population of the social sweat bee Halictus rubicundus (Hymenoptera: Halictidae) Behav. Ecol. Sociobiol. 1996;38:227–233. doi:10.1007/s002650050236 [Google Scholar]
- Gadagkar R. Evolution of eusociality: the advantage of assured fitness returns. Phil. Trans. R. Soc. B. 1990;329:17–25. doi:10.1098/rstb.1990.0146 [Google Scholar]
- Gaston K.J. Oxford University Press; Oxford, UK: 2003. The structure and dynamics of geographic ranges. [Google Scholar]
- Geber M.A, Eckhart V.M. Experimental studies of adaptation in Clarkia xantiana. II. Fitness variation across a subspecies border. Evolution. 2005;59:521–531. doi:10.1111/j.0014-3820.2005.tb01012.x [PubMed] [Google Scholar]
- Grubb P.J, Whitmore T.C. A comparison of montane and lowland rain forest in Ecuador: II. The climate and its effects on the distribution and physiognomy of the forests. J. Ecol. 1966;54:303–333. doi:10.2307/2257951 [Google Scholar]
- Guevara J, Avilés L. Multiple sampling techniques confirm differences in insect size between low and high elevations that may influence levels of spider sociality. Ecology. 2007;88:2015–2023. doi: 10.1890/06-0995.1. doi:10.1890/06-0995.1 [DOI] [PubMed] [Google Scholar]
- Guevara, J. & Avilés, L. In press. Elevational changes in insect and other arthropod composition at tropical latitudes: a comparison of multiple sampling methods and social spider diets. Insect Conserv. Div.
- Henschel J.R. Predation on social and solitary individuals of the spider Stegodyphus dumicola (Araneae, Eresidae) J. Arachnol. 1998;26:61–69. [Google Scholar]
- Hölldobler B, Wilson E.O. Belknap Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Holt R.D, Keitt T.H, Lewis M.A, Maurer B.A, Taper M.L. Theoretical models of species' borders: single species approaches. Oikos. 2005;108:18–27. doi:10.1111/j.0030-1299.2005.13147.x [Google Scholar]
- Janzen D.H, Ataroff M, Farinas M, Reyes S, Rincon N, Soler A, Soriano P, Vera M. Changes in the arthropod community along an elevational transect in the Venezuelan Andes. Biotropica. 1976;8:193–203. doi:10.2307/2989685 [Google Scholar]
- Jones T.C, Riechert S.E, Dalrymple S.E, Parker P.G. Fostering model explains variation in levels of sociality in a spider system. Anim. Behav. 2007;73:195–204. doi:10.1016/j.anbehav.2006.06.006 [Google Scholar]
- Keyserling E. Die Spinnen Amerikas. II. Theridiidae. Nürnberg. 1884;1:1–222. [Google Scholar]
- Kirkpatrick M, Barton N. Evolution of a species' range. Am. Nat. 1997;150:1–23. doi: 10.1086/286054. doi:10.1086/286054 [DOI] [PubMed] [Google Scholar]
- Krafft B, Horel A, Julita J.M. Influence of food supply on the duration of the gregarious phase of a maternal–social spider, Coelotes terrestris (Araneae, Agelenidae) J. Arachnol. 1986;14:219–226. [Google Scholar]
- Liebert A.E, Nonacs P, Wayne R.K. Solitary nesting and reproductive success in the paper wasp Polistes aurifer. Behav. Ecol. Sociobiol. 2005;57:445–456. doi:10.1007/s00265-004-0875-5 [Google Scholar]
- London K.B, Jeanne R.L. Effects of colony size and stage of development on defense response by the swarm-founding wasp, Polybia occidentalis. Behav. Ecol. Sociobiol. 2003;57:445–456. doi:10.1007/s00265-004-0875-5 [Google Scholar]
- Lubin Y, Bilde T. The evolution of sociality in spiders. Adv. Study Behav. 2007;37:83–145. doi:10.1016/s0065-3454(07)37003-4 [Google Scholar]
- Mori K, Saito T. Variation in social behavior within a spider mite genus, Stigmaeopsis (Acari: Tetranychidae) Behav. Ecol. 2005;16:232–238. doi:10.1093/beheco/arh157 [Google Scholar]
- Neill D.A. Vegetation. In: Jørgensen P.M, León-Yánez S, editors. Catalogue of the vascular plants of Ecuador. Monographs in systematic botany from the Missouri Botanical Garden. vol. 75. Missouri Botanical Garden; St. Louis, MO: 1999a. pp. 13–25. [Google Scholar]
- Neill D.A. Climates. In: Jørgensen P.M, León-Yánez S, editors. Catalogue of the vascular plants of Ecuador. Monographs in systematic botany from the Missouri Botanical Garden. vol. 75. Missouri Botanical Garden; St. Louis, MO: 1999b. pp. 8–13. [Google Scholar]
- Perkins T.A, Riechert S.E, Jones T.C. Interactions between the social spider Anelosimus studiosus (Araneae, Theridiidae) and foreign spiders that frequent its nests. J. Arachnol. 2007;35:143–152. doi:10.1636/T06-43.1 [Google Scholar]
- Powers, K. S. 2004 Prey abundance and the evolution of sociality in the social spider genus Anelosimus PhD thesis, University of Arizona.
- Powers K.S, Avilés L. Natal dispersal patterns of a subsocial spider Anelosimus cf. jucundus (Theridiidae) Ethology. 2003;109:725–737. doi:10.1046/j.1439-0310.2003.00918.x [Google Scholar]
- Powers K.S, Avilés L. The role of prey size and abundance in the geographic distribution of spider sociality. J. Anim. Ecol. 2007;76:995–1003. doi: 10.1111/j.1365-2656.2007.01267.x. doi:10.1111/j.1365-2656.2007.01267.x [DOI] [PubMed] [Google Scholar]
- Purcell J, Avilés L. Smaller colonies and more solitary living mark higher elevation populations of a social spider. J. Anim. Ecol. 2007;76:590–597. doi: 10.1111/j.1365-2656.2007.01228.x. doi:10.1111/j.1365-2656.2007.01228.x [DOI] [PubMed] [Google Scholar]
- Riechert S.E, Hall R.F. Local population success in heterogeneous habitats: reciprocal transplant experiments completed on a desert spider. J. Evol. Biol. 2000;13:541–550. doi:10.1046/j.1420-9101.2000.00176.x [Google Scholar]
- Riechert S.E, Roeloffs R, Echternacht A.C. The ecology of the cooperative spider Agelena consociata in equatorial Africa (Araneae, Agelenidae) J. Arachnol. 1986;14:175–191. [Google Scholar]
- Ruttan L.M. Experimental manipulations of dispersal in the subsocial spider, Theridion pictum. Behav. Ecol. Sociobiol. 1990;27:169–173. doi:10.1007/BF00180300 [Google Scholar]
- Rypstra A.L, Tirey R.S. Prey size, prey perishability and group foraging in a social spider. Oecologia. 1991;86:25–30. doi: 10.1007/BF00317384. doi:10.1007/BF00317384 [DOI] [PubMed] [Google Scholar]
- Samson D.A, Rickart E.A, Gonzales P.C. Ant diversity and abundance along an elevational gradient in the Philippines. Biotropica. 1997;29:349–363. doi:10.1111/j.1744-7429.1997.tb00436.x [Google Scholar]
- Singer J.D. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J. Educ. Behav. Stat. 1998;24:323–355. [Google Scholar]
- Slobodchikoff C.N. Resources and the evolution of social behaivor. In: Price P.W, Slobodchikoff C.N, Gaud W.S, editors. A new ecology: novel approaches to interactive systems. Wiley; New York, NY: 1984. pp. 227–251. [Google Scholar]
- Smith A.R, Wcislo W.T, O'Donnell S. Assured fitness returns favor sociality in a mass-provisioning sweat bee, Megalopia genalis (Hymenoptera: Halictidae) Behav. Ecol. Sociobiol. 2003;54:14–21. doi:10.1007/s00265-003-0589-0 [Google Scholar]
- Uetz G.W, Hodge M.A. Influence of habitat and prey availability on spatial organization and behavior of colonial web-building spiders. Natl Geogr. Res. 1990;6:22–40. [Google Scholar]
- Uetz G.W, Boyle J, Hieber C.S, Stimson Wilcox R. Antipredator benefits of group living in colonial web-building spiders: the ‘early warning’ effect. Anim. Behav. 2002;63:445–452. doi:10.1006/anbe.2001.1918 [Google Scholar]
- Wilson E.O. Belknap Press; Cambridge, MA: 1975. Sociobiology. [Google Scholar]
- Yip, E. C., Powers, K. C. & Avilés, L. In press. Cooperative capture of large prey solves the problem of a declining surface area to volume ratio of large social spider colonies. Proc. Natl Acad. Sci. USA [DOI] [PMC free article] [PubMed]
- Zammit J, Hogendoorn K, Schwarz M.P. Strong constraints to independent nesting in a facultatively social bee: quantifying the effects of enemies-at-the-nest. Insect Soc. 2008;55:74–78. doi:10.1007/s00040-007-0972-3 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of methods, experimental treatments, and transplant duration for all nests. In the lower montane rainforest habitat, we transplanted 9 nests that had been recollected from Jatun Sacha. We transplanted these to see if they would be able to recover in a more favourable habitat. These nests performed as well as the 10 nests that were transplanted directly from the source habitat