Abstract
Females tend to be smaller than males in woody dioecious plant species, but they tend to be larger in herbs. The smaller size of females in woody species has been attributed to higher reproductive costs, yet no satisfactory explanation has been provided for their larger size in herbs. Because herbs have higher nitrogen concentrations in their tissues than woody plants, and because pollen is particularly rich in nitrogen, we predicted that male growth would be more compromised by reproduction than female growth. To test this hypothesis, we conducted three experiments on the annual dioecious herb Mercurialis annua. First, we compared the timing of reproduction between males and females and found that males started flowering earlier than females; early flowering is expected to compromise growth more than later flowering. Second, we compared plants allowed to flower with those prevented from flowering by experimental debudding and found that males incurred a higher reproductive cost than females in terms of both biomass and, particularly, nitrogen. Third, we grew plants under varying levels of nitrogen availability and found that although sexual size dimorphism was unaffected by nitrogen, females, but not males, decreased their relative allocation to both roots and reproduction under high nitrogen availability. We propose that males deal with the high cost of pollen production in terms of nitrogen by allocating biomass to nitrogen-harvesting roots, whereas females pay for carbon-rich seeds and fruits by investing in photosynthetic organs. Sexual dimorphism would thus seem to be the outcome of allocation to above- versus below-ground sinks that supply resources (carbon versus nitrogen) limiting the female and male reproduction differentially.
Keywords: life history, Mercurialis annua, trade-off, nitrogen allocation, carbon allocation
1. Introduction
Many dioecious plants display sexual size dimorphism (SSD). In woody trees and shrubs with SSD, males are usually the larger sex, whereas females tend to be larger in herbs with SSD (Antos & Allen 1990; Delph & Meagher 1995; Obeso 2002). Hypotheses to explain these trends typically invoke a differential somatic cost of reproduction between the sexes. For example, female growth is hypothesized to be more severely compromised by allocation to reproduction than male growth, because females need to fill seeds and produce fruits whereas males only produce flowers (Lloyd & Webb 1977; Delph 1999). This hypothesis might explain the male-biased SSD that is common in woody species, but it fails to account for the female-biased SSD in dioecious herbs.
We propose that female herbs might be larger than males if the cost of male reproduction in such species is in fact higher than female reproduction. We argue that whereas females might pay a high cost for seed and fruit production in terms of carbon allocation, males must invest heavily in terms of other currencies, particularly nitrogen. Herbs tend to be more nitrogen-rich than woody plants (Kerkhoff et al. 2006), and pollen is richer in nitrogen than fruits and seeds (Ishida et al. 2005). Thus, if nitrogen limits their growth, as it often must, allocation of nitrogen by male herbs to pollen production could compromise plant growth more than the allocation of carbon to seeds and fruits by females. The idea that male and female functions might be limited by different resources or ‘currencies’ is not new (Chapin 1989; Antos & Allen 1990; Ashman & Baker 1992). However, to our knowledge the hypothesis that such differences might underlie the different direction of SSD in herbs versus woody plants has not been tested before.
The so-called ‘somatic cost of reproduction’ refers to the reduced growth in vegetative tissues which results from trade offs associated with allocation to reproduction (Williams 1966; Bell 1980; Reznick 1985). This cost has most commonly been assessed by measuring the accumulation of above-ground (shoot and leaf) biomass in plants prevented from reproduction, compared with that of plants allowed to reproduce (Tuomi et al. 1983; Silvertown 1987; Reznick 1992; Syrjanen & Lehtila 1993; Fox 1995; Ehrlen & Van Groenendael 2001). But plants must allocate resources to root growth below ground too, so that allocation trade offs really ought to be assessed between roots versus shoots and reproduction. Surprisingly, few studies have attempted to do this (Putwain & Harper 1972; Zimmerman & Lechowicz 1982; Eckhart & Chapin 1997; Poot 1997; Fujitaka & Sakai 2007), most likely because measuring below-ground allocation is logistically difficult. Finally, because roots and shoots function both as allocation sinks as well as sources of different resources (soil nutrients, such as nitrogen and carbon, respectively), an assessment of allocation trade offs ought also to differentiate between above- versus below-ground plant structures. If male and female reproductions are differently limited by nitrogen and carbon, respectively, such a decomposition of allocation sinks would seem to be particularly important for an understanding of sexual dimorphism due to costs of reproduction.
Here, we present results from three experiments on the wind-pollinated dioecious herb Mercurialis annua to test the hypothesis that female-biased SSD in herbs is due to the greater cost of reproduction for males than females; in M. annua, males are known to be smaller than females (Heyer 1884). In the first experiment, we test the hypothesis that males are smaller than females because they begin to reproduce earlier; early flowering is expected to compromise growth more than later flowering (Obeso 2002). In the second experiment, we estimate the somatic cost of reproduction in males and females by comparing control plants with those that were experimentally prevented from allocating resources to reproduction. Finally, in the third experiment, we test the prediction that if SSD in M. annua is driven by nitrogen limitation in the growth of males, then plants growing under conditions of experimentally reduced nitrogen availability should display greater SSD. In this experiment, we also decompose somatic allocation into that directed towards roots versus shoots and leaves. We conclude that the three-way trade offs among shoots, roots and reproduction are critical to an understanding of sexual dimorphism driven by somatic costs of pollen versus seed and fruit production.
2. Material and methods
(a) Study species
Mercurialis annua is a wind-pollinated annual herb that grows in disturbed habitats in western Europe and Mediterranean countries (Tutin et al. 1964). Although the species varies in its sexual system from dioecy through androdioecy to monoecy (Durand 1963; Pannell et al. 2004), in this study, we used solely dioecious material. Males of M. annua produce green staminate flowers that are held on erect axillary inflorescence stalks (peduncles). Females produce green dehiscent capsules that each contains two or, rarely, three seeds. In both males and females, flowering commences several weeks after seeds germinate and continues indeterminately during plant growth, with new inflorescences produced in each new leaf axil (Pannell 1997). Seed set in natural populations can be pollen limited when plants are more than several metres apart (E. Hesse & J. R. Pannell 2007, unpublished data), but in our experiments we grew plants in pots at a much greater density so that pollen limitation was probably not important. For all of our experiments, we used seeds bulked and well mixed from approximately 60 adult individuals from two French populations of M. annua.
(b) Experiment 1: time to reproduction
Following seed germination, we sowed 50 pre-reproductive seedlings in a seedling tray and recorded the number of males and females that had started producing inflorescences on the 25th, 27th and 35th days after seed sowing. For each time interval separately, we tested the sex ratio for bias using a binomial test with expected sex ratio equal to the final sex ratio observed when all plants had expressed their gender.
(c) Experiment 2: inflorescence bud removal
We germinated seeds in potting compost in a greenhouse. When buds of the first true leaves appeared, we transferred 70 seedlings to vermiculite in separate 10 cm diameter pots. We placed each pot on a saucer in a greenhouse maintained at approximately 24°C with 16-hour days. When inflorescence buds were produced and sex could be determined, we randomly allocated plants to either debudding or control treatments. We maintained all plants by watering them daily with tap water and, spraying weekly with 100 ml of 1 ml l−1 Phostrogen fertilizer (nitrogen, potassium and phosphorus, at equal ratios, and micronutrients; Phostrogen, Cambridge, UK). Throughout the experiment, we used fine forceps to remove inflorescence buds from plants in the debudding treatment group as soon as the buds became visible. This did not damage the apical or lateral meristems; in a pilot study, we found no difference in growth form or biomass between control plants and plants that received a small amount of damage at the base of each leaf petiole equivalent to that caused by debudding. Plants were placed randomly on the glasshouse bench and were re-randomized at weekly intervals.
Because flowering in M. annua is indeterminate, sex allocation must be assessed in terms of a plant's male and female allocation at a snapshot in time. Previous work has shown that this provides a robust estimate of a plant's total allocation (Pannell 1997). We chose to measure allocation to growth and reproduction after 40 days' growth while plants were still growing strongly and before they became pot bound. By this stage, both pollen and seeds were beginning to be dispersed from the plants, i.e. the plants had reached the size-dependent quasi-steady state in terms of their allocation, as documented by Pannell (1997).
We harvested the above-ground portions of plants and dissected them into vegetative (leaves and stems) and reproductive components. After drying at 80°C to constant mass, we recorded the biomass of each component. We randomly selected four males and four females from each treatment combination and measured the nitrogen content of vegetative and reproductive structures using mass spectrometry. We then calculated the mean absolute nitrogen allocation to reproductive and vegetative structures for plants in each treatment group by multiplying the mean biomass of the structure by the corresponding mean nitrogen concentration.
For both biomass and nitrogen in both males and females, we calculated reproductive effort as the total resource allocated to reproduction divided by the resource allocated to the plant as a whole (Thompson & Stewart 1981). We also estimated the somatic cost of reproductive allocation for each resource by comparing the biomass or absolute nitrogen content of the vegetative tissues of control plants, Af, with that of those whose reproductive investment had been prevented by debudding, An. Specifically, we calculated the cost of reproduction for both males and females as the proportion (An−Af)/An, following Bell (1980). Reproductive effort and reproductive cost can be directly compared using the methods of Tuomi et al. (1983).
For both biomass and nitrogen, we tested the effects of sex and inflorescence treatment on absolute vegetative allocation and total absolute resource allocation (i.e. vegetative plus reproductive allocations), using a two-way ANOVA in a factorial design (table 1a). We tested post hoc comparisons between means using a Tukey–Kramer procedure for multiple comparisons, with α<0.05 (Sokal & Rohlf 1995). For both biomass and nitrogen, we tested the difference in absolute reproductive allocation and in reproductive effort (proportion of resource allocated to reproduction) between control males and females using a simple ANOVA (table 1b). We tested the effects of sex and inflorescence treatment on vegetative nitrogen concentration using a two-way ANOVA in a factorial design and again tested post hoc comparisons between means using a Tukey–Kramer procedure for multiple comparisons, with α<0.05. We also tested the difference in reproductive nitrogen concentration between males and females using a one-way ANOVA.
Table 1.
F-ratios for (a) two-way and (b) one-way ANOVA for the effects of sex and debudding treatment and (c) two-way ANOVA for the effects of sex and nitrogen levels on measures of biomass and nitrogen allocation in Mercurialis annua. (Corresponding degrees of freedom for each independent variable are given under d.f. and the error degrees of freedom are given to the right of d.f. Significance levels are indicated: *Pr<0.05; **Pr<0.01; ***Pr<0.001. ANOVA was conducted on log-transformed data for all variables except reproductive effort and root allocation, for which values were transformed through arcsin(x1/2).)
| (a) | d.f. 66 | vegetative biomass | total biomass | vegetative nitrogen | total nitrogen |
|---|---|---|---|---|---|
| sex | 1 | 42.6*** | 37.3*** | 89.9*** | 25.8*** |
| treatment | 1 | 62.9*** | 7.45** | 35.1*** | 9.20** |
| sex×treatment | 1 | 8.69** | 6.92* | 18.0*** | 0.02 |
| (b) | d.f. 33 | reproductive biomass | reproductive effort (biomass) | reproductive mass of N | reproductive effort (N) |
|---|---|---|---|---|---|
| sex | 1 | 5.18* | 1.50 | 16.7*** | 139*** |
| (c) | d.f. 24 | total biomass | reproductive efforta | root allocation | |
|---|---|---|---|---|---|
| sex | 1 | 10.0** | 14.4*** | 0.71 | |
| nitrogen | 2 | 259*** | 1.48 | 206*** | |
| sex×nitrogen | 2 | 1.41 | 4.63* | 8.00** |
This analysis was carried out with 23 error degrees of freedom due to a missing data point.
Because nitrogen concentration was estimated on the basis of an assay for a sample of individuals from each experimental group, we calculated standard errors for the absolute nitrogen content of plants (i.e. the product of total biomass and nitrogen concentration on a per mass basis) and error sum of squares in the ANOVA analyses using the method of Hansen et al. (1993) for the product of variances. This accounts not only for the error associated with the biomass but also for the error associated with the nitrogen concentration. We log-transformed absolute biomass and nitrogen amounts and arcsine-transformed nitrogen concentrations and reproductive effort to satisfy ANOVA assumptions; we tested for normality of residuals using a Shapiro–Wilk test for goodness of fit (Shapiro & Wilk 1965).
(d) Experiment 3: nitrogen limitation
As in the previous experiments, we sowed seeds of M. annua into seedling trays and allowed them to germinate. Following seed germination, we transferred 10 seedlings into each of 30 aerated hydroponic containers. We then allocated each container to one of three nitrogen treatments: high-nitrogen plants were grown in full strength Hoagland solution (Hoagland & Arnon 1938); medium-nitrogen plants received 0.1 normal nitrogen concentration; and low-nitrogen plants received 0.01 normal nitrogen concentration. In the medium- and low-nitrogen treatments, nitrate ions were replaced with chloride. All other ions were maintained at the same concentrations across treatments.
When plants commenced flowering, we thinned seedlings and redistributed them among containers within each nitrogen treatment in such a way that there were only three seedlings of the same sex in each container. Every week throughout the experiment, we replaced the solutions and redistributed the containers randomly on the greenhouse bench. After 30 days, we harvested all plants and dissected them into shoots (leaves and stems), roots and reproductive structures. We recorded the dry biomass of each tissue component for each container separately, i.e. each container represented a single data point for each tissue component as the sum over three plants.
We analysed the effects of sex and nitrogen treatment on vegetative biomass, reproductive effort (reproductive biomass divided by total vegetative biomass) and root allocation (root biomass divided by total vegetative biomass) using a two-way ANOVA in a factorial design (table 1c). We tested post hoc comparisons between means using a Tukey–Kramer procedure for multiple comparisons, with α<0.05 (Sokal & Rohlf 1995). All of our analyses were conduced in JMP (v. 5, SAS Institute, Inc., Cary, NC, USA).
3. Results
(a) Experiment 1: time to reproduction
Males began flowering earlier than females. Out of 49 seedlings, of which 32 were male, the first 25 to start flowering were all male (binomial test, n=25, , p=1.00, Pr<0.001). Of the first 35 seedlings to commence flowering, 28 were male (n=35, , p=0.80, Pr=0.045). There was no significant bias in the final sex ratio (n=49, , p=0.65, Pr=0.566).
(b) Experiment 2: inflorescence bud removal
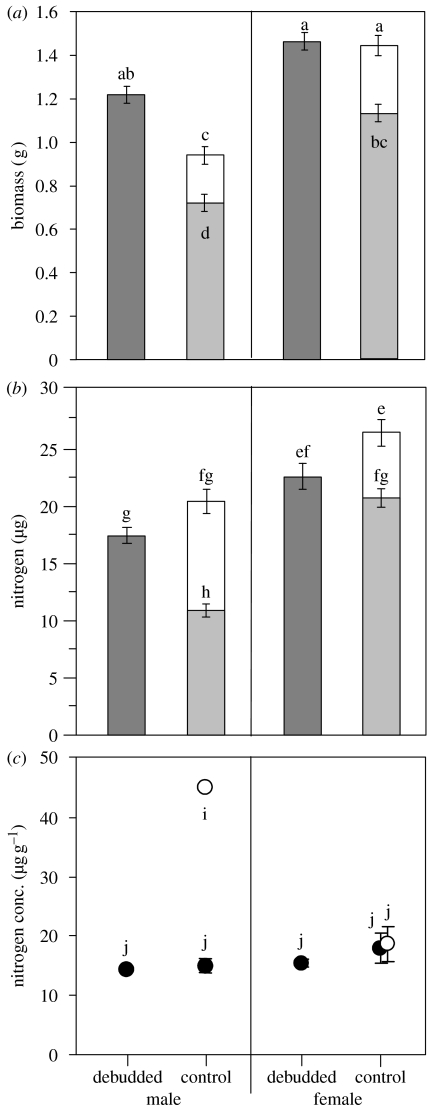
In terms of both vegetative and total biomass, control females were larger than control males and had a greater absolute reproductive biomass allocation (table 1a, figure 1a). Preventing plants from reproducing by debudding reduced this SSD in vegetative biomass substantially (sex×treatment interaction: F3,66=8.69; Pr=0.004; table 1a). In particular, debudded males were 41 per cent larger in vegetative biomass than control males, whereas debudded females were only 22 per cent larger in their vegetative biomass than control females.
Figure 1.
Effects of inflorescence removal on (a) biomass, (b) absolute nitrogen content and (c) nitrogen concentration in vegetative and reproductive structures of males and females of M. annua. Dark grey bars represent the vegetative resource in plants that were prevented from producing reproductive structures. Light grey and open bars represent, respectively, resources allocated to vegetative and reproductive structures of control plants. Filled and open circles represent the nitrogen concentration in vegetative and reproductive structures, respectively. Column and circle heights represent treatment means (±1 s.e.). Different letters next to bars and circles represent differences among groups detected using a Tukey–Kramer procedure for multiple comparisons with α<0.05 (Sokal & Rohlf 1995). Standard errors for nitrogen allocation were calculated using the method of Hansen et al. (1993) for calculating the variance of a product.
Debudded females had the same vegetative biomass as the total (vegetative+reproductive) biomass of control females (figure 1a). By contrast, the combined vegetative and reproductive biomass of control males was 23 per cent lower than that of debudded males (figure 1a). There was no difference in reproductive effort between the males and females in terms of biomass (table 1b).
The females had more absolute vegetative and total nitrogen than the males in both of the treatment groups (table 1a, figure 1b). However, in absolute and relative terms, the males allocated substantially more nitrogen to reproduction than did the females (table 1b, figure 1b). The difference in absolute vegetative nitrogen content between the sexes was significantly reduced by bud removal (sex×treatment interaction: F3,66=18.0; Pr<0.001, table 1a). Indeed, whereas debudded males had significantly more absolute vegetative nitrogen (63%) than control males, there was no significant difference in the absolute vegetative nitrogen in debudded and control females (figure 1b). Control plants had more total absolute nitrogen than debudded plants (table 1a, figure 1b). Bud removal did not significantly affect the degree of sexual dimorphism in total absolute nitrogen content (sex×treatment interaction: F3,66=0.02; Pr=0.887, table 1a).
Male inflorescences had a substantially higher nitrogen concentration (mg N g−1 biomass) than those of the females (F1,6=92.2, Pr=0.011, figure 1c). By contrast, there were no significant differences in nitrogen concentration in leaves of the males and females (figure 1c). While the nitrogen concentration of the male inflorescences was much higher than that in leaves, there was no difference in the nitrogen concentration between the inflorescences and leaves of the females (figure 1c). There was no effect of bud removal on the concentration of nitrogen in leaves (figure 1c).
(c) Experiment 3: nitrogen limitation
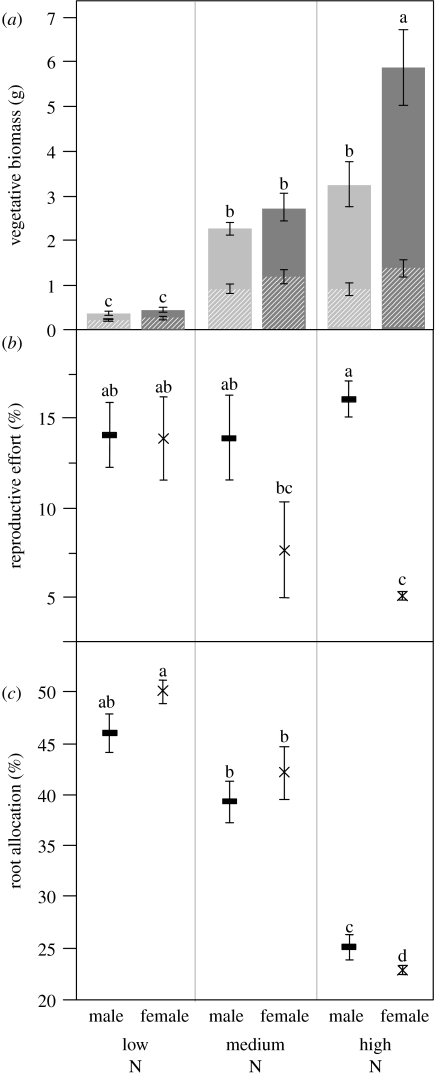
The males and females both increased their total absolute vegetative (shoot plus root) biomass with increasing nitrogen (figure 2a). Sexual dimorphism in total absolute vegetative biomass did not depend on nitrogen availability (sex×nitrogen interaction: F2,16=1.41, Pr=0.266, table 1c, figure 2a).
Figure 2.
(a) Vegetative biomass, (b) reproductive effort and (c) root allocation of males and females of M. annua grown under low-, medium- and high-nitrogen conditions. Hatched and solid portions of bars in (a) represent root and shoot biomass, respectively. All the error bars represent 1 s.e. Within each graph, means with different letters are significantly different as tested with a Tukey–Kramer test for multiple comparisons at α<0.05 (Sokal & Rohlf 1995).
Nitrogen availability affected the males and females in their reproductive effort (proportion of biomass allocated to reproductive structures) differently (sex×nitrogen interaction: F2,16=4.63, Pr=0.022, table 1c, figure 2b). Whereas the male reproductive effort was independent of nitrogen availability, the females decreased their reproductive effort under the high nitrogen treatment (figure 2b). There was no difference in reproductive effort between the sexes at low or medium nitrogen levels, but the females had significantly lower reproductive effort than males under the high nitrogen level (figure 2b).
Nitrogen availability also differentially affected the males and females in their relative allocation of biomass to roots (sex×nitrogen interaction: F2,16=8.00, Pr=0.002, table 1c, figure 2c). Although both the males and females reduced their relative root allocation with increasing nitrogen concentration, the females did so more than the males (figure 2c). Thus, while there was no significant difference in relative root allocation between the sexes at low or medium nitrogen availability, at high-nitrogen availability, the females had significantly lower relative root allocation than the males (figure 2c).
4. Discussion
(a) Time to flowering and costs of reproduction
Our results confirm that males of dioecious M. annua are smaller than females. This difference in size is probably partly due to the fact that males began flowering earlier than females, causing slower earlier growth, as suggested by Obeso (2002, see also Delph 1999). Earlier reproductive maturity in males than females has been reported in several dioecious species, including herbs (Putwain & Harper 1972; Delph 1999), woody plants (Allen & Antos 1993; Delph 1999) and many animals (Andersson 1994), so our result is not unusual. Slower growth in young plants as a result of trade offs between growth and reproduction is likely to impact on size later in life, and this probably contributed to the differences in size between mature males and mature females in M. annua. However, our other experiments indicate that SSD in M. annua is also due to the different resource requirements of males and females.
(b) Effect of bud removal on sexual dimorphism
In our bud removal experiment, males that were allowed to reproduce accumulated less total above-ground biomass than did those that were prevented from flowering by debudding, whereas females prevented from reproduction simply re-allocated the corresponding photosynthates to vegetative growth. In other words, the allocation of resources to reproduction inhibited carbon fixation in the males, but not in the females. Several other studies have found the reproductive cost (i.e. the impact of reproduction on vegetative growth) to be lower than or equal to reproductive effort (i.e. the relative allocation of biomass to reproduction), as we found for the females of M. annua (Obeso 1993; Delph & Meagher 1995; Thoren et al. 1996; Hemborg & Karlsson 1998). However, we are aware of no other study showing that reproductive plants accumulate lower total biomass than non-reproductive plants, as in the males of M. annua.
One possible explanation for this finding is that reproductive structures may be capable of photosynthesizing more effectively in females than in males, as has been found for female calyces of Silene latifolia (Gehring & Delph 2006) and the carpels of hermaphrodite Ranunculus (Galen et al. 1993). Although we cannot rule out this possibility for M. annua, it seems unlikely because both male and female flowers in M. annua are photosynthetic, and male inflorescences are held above the plant in full sunlight, whereas female inflorescence are more likely to be shaded by leaves.
A second possible explanation is that SSD in M. annua could be due partially to intrinsic physiological differences between males and females. Intrinsic SSD, e.g. in pre-reproductive individuals, is common in animals but has rarely been recorded in plants (Banks et al. 1993; Eppley 2006). In S. latifolia, males are smaller and die earlier than females owing to genetic correlations with flower number (Delph et al. 2005). More generally, females of dioecious species often have a higher photosynthetic rate, and this could compensate for their reproductive costs (Dawson & Bliss 1989; Dawson & Ehleringer 1993). In M. annua, gender-specific development appears to be mediated by the plant growth substances auxin and cytokinin (Champaul 1973; Hamdi et al. 1987), and it is possible that pleiotropic effects of these growth substances are partially responsible for sexual dimorphism in M. annua. Nevertheless, none of these possible effects can account for the large component of SSD in M. annua that was exposed by the differential effect of debudding on the size of the males versus females.
Finally, sexual dimorphism in M. annua may be explained directly by greater costs of reproduction in males in terms of a resource other than carbon. Given that males allocated 70 per cent more nitrogen, in absolute terms, to inflorescences than females (figure 1b), this other allocation currency is likely to be nitrogen. Because nitrogen limits vegetative growth in M. annua, as our nitrogen-limitation experiment showed, greater nitrogen allocation to reproduction is likely to have directly compromised the accumulation of biomass more in males than in females. If the reallocation of nitrogen from vegetative to reproductive structures reduces the photosynthetic potential of individuals of M. annua, as has been found in several other species (Field & Mooney 1986; Evans 1989; Karlsson 1994; Saulnier & Reekie 1995), then this would contribute to the smaller size of males.
Previous work on herbaceous gynodioecious species also suggests that the allocation of resources to male function significantly reduces the total accumulation of biomass. For example, in Phacelia linearis, hermaphrodites accumulated less biomass and nitrogen than did females, particularly under low nutrient availability (Eckhart & Chapin 1997). Similarly, in Plantago lanceolata, the allocation of nitrogen to pollen in hermaphrodites caused significant reductions in growth compared with females (Poot 1997). It therefore seems that the high nitrogen cost of male function may generally contribute to the reduced growth of pollen-producing morphs compared with females in herbs.
(c) Nitrogen limitation and a model for sexual dimorphism in M. annua
We predicted sexual dimorphism in vegetative biomass to decrease with increasing nitrogen availability. By contrast, there was no effect of nitrogen availability on sexual dimorphism in total vegetative biomass. However, there was a large and significant difference between males and females in where the biomass was allocated in response to nitrogen availability. Indeed, whereas male reproductive effort was independent of the nitrogen level, the females reduced their relative allocation to reproduction at higher nitrogen levels. We also found that although both males and females reduced their root allocation with increasing nitrogen availability, as expected (Davidson 1969; Buysse et al. 1993; de Groot et al. 2002), the females did so more than the males. These results contrast with those found in the gynodioecious herb P. linearis, in which sexual dimorphism in total biomass and proportional root allocation did increase with increasing nutrient limitation (Eckhart & Chapin 1997).
A number of researchers have grown males and females of dioecious plant species under varying resource levels (Zimmerman & Lechowicz 1982; Gehring & Linhart 1993; Dorken & Barrett 2004), but few studies have found sexual dimorphism to increase with increasing resource limitation, as would be expected if sexual dimorphism were the result of differential resource allocation to reproduction (Lloyd & Webb 1977; Stearns 1989; Gehring & Linhart 1993). Although these results, and those of our study, may be counterintuitive, our measurements of allocation to both above- and below-ground components suggest that three-way trade offs between roots, shoots and reproduction need to be invoked to explain sexual dimorphism in M. annua.
We suggest that if male reproduction in M. annua is nitrogen limited and female reproduction is carbon limited, selection on resource allocation to nitrogen- versus carbon-harvesting organs (roots and shoots, respectively) should differ between the sexes; this may then explain their different responses to varying nitrogen availability. For example, under low nitrogen availability, both sexes probably experienced severe nitrogen limitation and would have therefore both benefited from allocating a high proportion of their vegetative resources to roots. With increasing nitrogen availability, however, we might expect females to benefit more than males from increasing allocation to shoots and leaves (and reducing allocation to roots), because shoots and leaves harvest the carbon needed for seeds and fruits. By contrast, males might be expected to continue to invest heavily in roots, because these harvest the nitrogen needed for pollen production.
If this simple model is adequate, why might females of M. annua reduce their reproductive effort under high nitrogen? We have no firm answer to this question. However, we speculate that reduced reproductive effort may be a response to increased levels of anticipated or experienced above-ground competition when nitrogen availability is high. Although we did not manipulate or measure the intensity of above-ground competition for light, it is well known that above-ground competition increases with nutrient availability (Harper 1977). Thus, it might pay females more than males to divert resources from both roots and early reproduction to shoots during plant establishment and maturation in order to avoid being overtopped and shaded by competitive neighbours; such females should be able to produce more seeds and fruits later in the reproductive season.
5. Conclusions
Our results suggest a reason for the smaller size of males than females in dioecious herbs: males allocate a higher proportion of their biomass to roots in order to increase nitrogen acquisition for pollen production, and they pay the price in terms of reduced growth above ground and reduced carbon acquisition. If this pattern of allocation in males is indeed driven by the need to supply reproductive structures with nitrogen, then removing this sink (e.g. by debudding) might allow reduced allocation to roots, as we observed. The idea that sexual dimorphism in dioecious plants might be a result of differences between males and females in their growth both above and below ground is reminiscent of observations of allocation patterns in the herbaceous genera Phacelia, Plantago and Rumex, in which pollen-producing plants allocate a greater proportion of their biomass to below-ground structures than females (Putwain & Harper 1972; Zimmerman & Lechowicz 1982; Eckhart & Chapin 1997; Poot 1997; Fujitaka & Sakai 2007). The patterns we have observed might thus be common among dioecious herbs. Certainly, it is clear from our study that models of sexual dimorphism need to incorporate the dynamic allocation of more than just biomass, particularly when one allocation sink becomes the source for a different limiting resource.
Acknowledgments
We thank Andrew Smith for useful advice and use of equipment and Elze Hesse for valuable comments on the manuscript. We thank Peter Ditchfield of the Institute of Archaeology, Oxford, for help with nutrient analysis. M.S.H. was supported by a NERC studentship.
References
- Allen G.A, Antos J.A. Sex-ratio variation in the dioecious shrub Oemleria cerasiformis. Am. Nat. 1993;141:537–553. doi: 10.1086/285490. doi:10.1086/285490 [DOI] [PubMed] [Google Scholar]
- Andersson, M. B. 1994 Sexual selection Monographs in Behavior and Ecology. Princeton, NJ: Princeton University Press.
- Antos J.A, Allen G.A. A comparison of reproductive effort in the dioecious shrub Oemleria cerasiformis using nitrogen, energy and biomass as currencies. Am. Midl. Nat. 1990;124:254–262. doi:10.2307/2426174 [Google Scholar]
- Ashman T.L, Baker I. Variation in floral sex allocation with time of season and currency. Ecology. 1992;73:1237–1243. doi:10.2307/1940672 [Google Scholar]
- Banks J.A, Hickok L, Webb M.A. The programming of sexual phenotype in the homosporous fern Ceratopteris richardii. Int. J. Plant Sci. 1993;154:522–534. doi:10.1086/297135 [Google Scholar]
- Bell G. The costs of reproduction and their consequences. Am. Nat. 1980;116:45–76. doi:10.1086/283611 [Google Scholar]
- Buysse J, Smolders E, Merckx R. The role of free sugars and amino-acids in the regulation of biomass partitioning and plant-growth. Plant Nutr. Genet. Eng. Field Pract. 1993;45:211–214. [Google Scholar]
- Champaul A. Effects of several growth-substances observed on in-vitro cultivated nodes of Mercurialis annua L. Bulletin De La Societe Botanique De France. 1973;120:87–99. [Google Scholar]
- Chapin F.S. The cost of tundra plant structures: evaluation of concepts and currencies. Am. Nat. 1989;133:1–19. doi:10.1086/284898 [Google Scholar]
- Davidson R.L. Effects of soil nutrients and moisture on root/shoot ratios in Lolium perenne L and Trifolium repens L. Ann. Bot. 1969;33:571–577. [Google Scholar]
- Dawson T.E, Bliss L.C. Patterns of water-use and the tissue water relations in the dioecious shrub, Salix arctica: the physiological-basis for habitat partitioning between the sexes. Oecologia. 1989;79:332–343. doi: 10.1007/BF00384312. doi:10.1007/BF00384312 [DOI] [PubMed] [Google Scholar]
- Dawson T.E, Ehleringer J.R. Gender-specific physiology, carbon isotope discrimination, and habitat distribution in Boxelder, Acer negundo. Ecology. 1993;74:798–815. doi:10.2307/1940807 [Google Scholar]
- de Groot C.C, Marcelis L.F.M, van den Boogaard R, Lambers H. Interactive effects of nitrogen and irradiance on growth and partitioning of dry mass and nitrogen in young tomato plants. Funct. Plant Biol. 2002;29:1319–1328. doi: 10.1071/FP02087. doi:10.1071/FP02087 [DOI] [PubMed] [Google Scholar]
- Delph L.F. Sexual dimorphism in life history. In: Geber M.A, Dawson T.E, Delph L.F, editors. Gender and sexual dimorphism in flowering plants. Springer; Heidelberg, Germany: 1999. pp. 149–173. [Google Scholar]
- Delph L.F, Meagher T.R. Sexual dimorphism masks life-history trade-offs in the dioecious plant Silene latifolia. Ecology. 1995;76:775–785. doi:10.2307/1939343 [Google Scholar]
- Delph L.F, Gehring J.L, Arntz A.M, Levri M, Frey F.M. Genetic correlations with floral display lead to sexual dimorphism in the cost of reproduction. Am. Nat. 2005;166:S31–S41. doi: 10.1086/444597. doi:10.1086/444597 [DOI] [PubMed] [Google Scholar]
- Dorken M.E, Barrett S.C.H. Phenotypic plasticity of vegetative and reproductive traits in monoecious and dioecious populations of Sagittaria latifolia (Alismataceae): a clonal aquatic plant. J. Ecol. 2004;92:32–44. doi: 10.1111/j.1365-294X.2004.02246.x. doi:10.1111/j.1365-2745.2004.00857.x [DOI] [PubMed] [Google Scholar]
- Durand B. Le complexe Mercurialis annua L. S. L.: Une étude biosystématique. Annales des Sciences Naturelles, Botanique, Paris. 1963;12:579–736. [Google Scholar]
- Eckhart V.M, Chapin F.S. Nutrient sensitivity of the cost of male function in gynodioecious Phacelia linearis (Hydrophyllaceae) Am. J. Bot. 1997;84:1092–1098. doi:10.2307/2446152 [PubMed] [Google Scholar]
- Ehrlen J, Van Groenendael J. Storage and the delayed costs of reproduction in the understorey perennial Lathyrus vernus. J. Ecol. 2001;89:237–246. doi:10.1046/j.1365-2745.2001.00546.x [Google Scholar]
- Eppley S.M. Females make tough neighbors: sex-specific competitive effects in seedlings of a dioecious grass. Oecologia. 2006;146:549–554. doi: 10.1007/s00442-005-0026-3. doi:10.1007/s00442-005-0026-3 [DOI] [PubMed] [Google Scholar]
- Evans J.R. Photosynthesis and nitrogen relationships in leaves of C-3 plants. Oecologia. 1989;78:9–19. doi: 10.1007/BF00377192. doi:10.1007/BF00377192 [DOI] [PubMed] [Google Scholar]
- Field C, Mooney H.A. The photosynthesis–nitrogen relationship in wild plants. In: Givnish R, editor. On the economy of plant form and function. Cambridge University Press; Cambridge, UK: 1986. pp. 25–55. [Google Scholar]
- Fox J.F. Shoot demographic responses to manipulation of reproductive effort by bud removal in a willow. Oikos. 1995;72:283–287. doi:10.2307/3546230 [Google Scholar]
- Fujitaka T, Sakai S. Sexual dimorphism in clonal growth forms and ramet distribution patterns in Rumex acetosella (Polygonaceae) Ecol. Res. 2007;22:248–254. doi:10.1007/s11284-006-0020-1 [Google Scholar]
- Galen C, Dawson T.E, Stanton M.L. Carpels as leaves: meeting the carbon cost of reproduction in an alpine buttercup. Oecologia. 1993;95:187–193. doi: 10.1007/BF00323489. doi:10.1007/BF00323489 [DOI] [PubMed] [Google Scholar]
- Gehring J.L, Delph L.F. Effects of reduced source–sink ratio on the cost of reproduction in females of Silene latifolia. Int. J. Plant Sci. 2006;167:843–851. doi:10.1086/503784 [Google Scholar]
- Gehring J.L, Linhart Y.B. Sexual dimorphisms and response to low resources in the dioecious plant Silene latifolia (Caryophyllaceae) Int. J. Plant Sci. 1993;154:152–162. doi:10.1086/297100 [Google Scholar]
- Hamdi S, Teller G, Louis J.P. Master regulatory genes, auxin levels, and sexual organogenesis in the dioecious plant Mercurialis annua. Plant Physiol. 1987;85:393–399. doi: 10.1104/pp.85.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M.H, Hurwitz W.N, Madow W.G. Wiley; New York, NY: 1993. Sample survey methods and theory. [Google Scholar]
- Harper J.L. Academic Press; London, UK: 1977. The population biology of plants. [Google Scholar]
- Hemborg A.M, Karlsson P.S. Somatic costs of reproduction in eight subarctic plant species. Oikos. 1998;82:149–157. doi:10.2307/3546925 [Google Scholar]
- Heyer F. Untersuchungen über das verhältnis des geschlechts bei einhäusigen und zweihäusigen pflanzen, unter berücksichtigung des geschlechtsverhältniss bei thieren und menschen. Ber. physiolog. Lab. u. Versuchsan. landw. Inst. Univ. Halle. 1884;1:43. [Google Scholar]
- Hoagland, D. R. & Arnon, D. K. 1938 The water culture method for growing plants without soil. California Agricultural Experiment Station Circular, no. 347.
- Ishida T.A, Hattori K, Shibata S, Suzuki M, Kimura M.T. Sex allocation of a cosexual wind-pollinated tree, Quercus dentata, in terms of four currencies. J. Plant Res. 2005;118:193–197. doi: 10.1007/s10265-005-0206-6. doi:10.1007/s10265-005-0206-6 [DOI] [PubMed] [Google Scholar]
- Karlsson P.S. Photosynthetic capacity and photosynthetic nutrient-use efficiency of Rhododendron lapponicum leaves as related to leaf nutrient status, leaf age and branch reproductive status. Funct. Ecol. 1994;8:694–700. doi:10.2307/2390228 [Google Scholar]
- Kerkhoff A.J, Fagan W.F, Elser J.J, Enquist B.J. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006;168:E103–E122. doi: 10.1086/507879. doi:10.1086/507879 [DOI] [PubMed] [Google Scholar]
- Lloyd D.G, Webb C.J. Secondary sex characters in plants. Bot. Rev. 1977;43:177–216. doi:10.1007/BF02860717 [Google Scholar]
- Obeso J.R. Cost of reproduction in the perennial herb Asphodelus albus (Liliaceae) Ecography. 1993;16:365–371. doi:10.1111/j.1600-0587.1993.tb00226.x [Google Scholar]
- Obeso J.R. The costs of reproduction in plants. New Phytol. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. doi:10.1046/j.1469-8137.2002.00477.x [DOI] [PubMed] [Google Scholar]
- Pannell J. Widespread functional androdioecy in Mercurialis annua L (Euphorbiaceae) Biol. J. Linn. Soc. 1997;61:95–116. [Google Scholar]
- Pannell J.R, Obbard D.J, Buggs R.J.A. Polyploidy and the sexual system: what can we learn from Mercurialis annua? Biol. J. Linn. Soc. 2004;82:547–560. doi:10.1111/j.1095-8312.2004.00340.x [Google Scholar]
- Poot P. Reproductive allocation and resource compensation in male-sterile and hermaphroditic plants of Plantago lanceolata (Plantaginaceae) Am. J. Bot. 1997;84:1256–1265. doi:10.2307/2446050 [PubMed] [Google Scholar]
- Putwain P.D, Harper J.L. Studies in the dynamics of plant populations. V. Mechanisms governing the sex ratio in Rumex acetosa and R. acetosella. J. Ecol. 1972;60:113–129. doi:10.2307/2258045 [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. doi:10.2307/3544698 [Google Scholar]
- Reznick D. Measuring the costs of reproduction. Trends Ecol. Evol. 1992;7:42–45. doi: 10.1016/0169-5347(92)90104-J. doi:10.1016/0169-5347(92)90150-A [DOI] [PubMed] [Google Scholar]
- Saulnier T.P, Reekie E.G. Effect of reproduction on nitrogen allocation and carbon gain in Oenothera biennis. J. Ecol. 1995;83:23–29. doi:10.2307/2261147 [Google Scholar]
- Shapiro S.S, Wilk M.B. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- Silvertown J. The evolution of hermaphroditism: an experimental test of the resource model. Oecologia. 1987;72:157–159. doi: 10.1007/BF00385060. doi:10.1007/BF00385060 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. W. H. Freeman and Co; New York, NY: 1995. Biometry: the principles and practice of statistics in biological research. [Google Scholar]
- Stearns S.C. Trade-offs in life-history evolution. Funct. Ecol. 1989;3:259–268. doi:10.2307/2389364 [Google Scholar]
- Syrjanen K, Lehtila K. The cost of reproduction in Primula veris: differences between two adjacent populations. Oikos. 1993;67:465–472. doi:10.2307/3545358 [Google Scholar]
- Thompson K, Stewart A.J.A. The measurement and meaning of reproductive effort in plants. Am. Nat. 1981;117:205–211. doi:10.1086/283700 [Google Scholar]
- Thoren L.M, Karlsson P.S, Tuomi J. Somatic cost of reproduction in three carnivorous Pinguicula species. Oikos. 1996;76:427–434. doi:10.2307/3546336 [Google Scholar]
- Tuomi J, Hakala T, Haukioja E. Alternative concepts of reproductive effort, costs of reproduction, and selection in life-history evolution. Am. Zool. 1983;23:25–34. [Google Scholar]
- Tutin T.G, Heywood V.H, Burges N.A, Valentine D.H, Walters S.M, Webb D.A. Cambridge University Press; Cambridge, UK: 1964. Flora Europaea. [Google Scholar]
- Williams G.C. Natural selection, the costs of reproduction, and the refinement of Lack's principle. Am. Nat. 1966;100:687–690. doi:10.1086/282461 [Google Scholar]
- Zimmerman J.K, Lechowicz M.J. Responses to moisture stress in male and female plants of Rumex acetosella L. (Polygonaceae) Oecologia. 1982;53:305–309. doi: 10.1007/BF00389005. doi:10.1007/BF00389005 [DOI] [PubMed] [Google Scholar]