Abstract
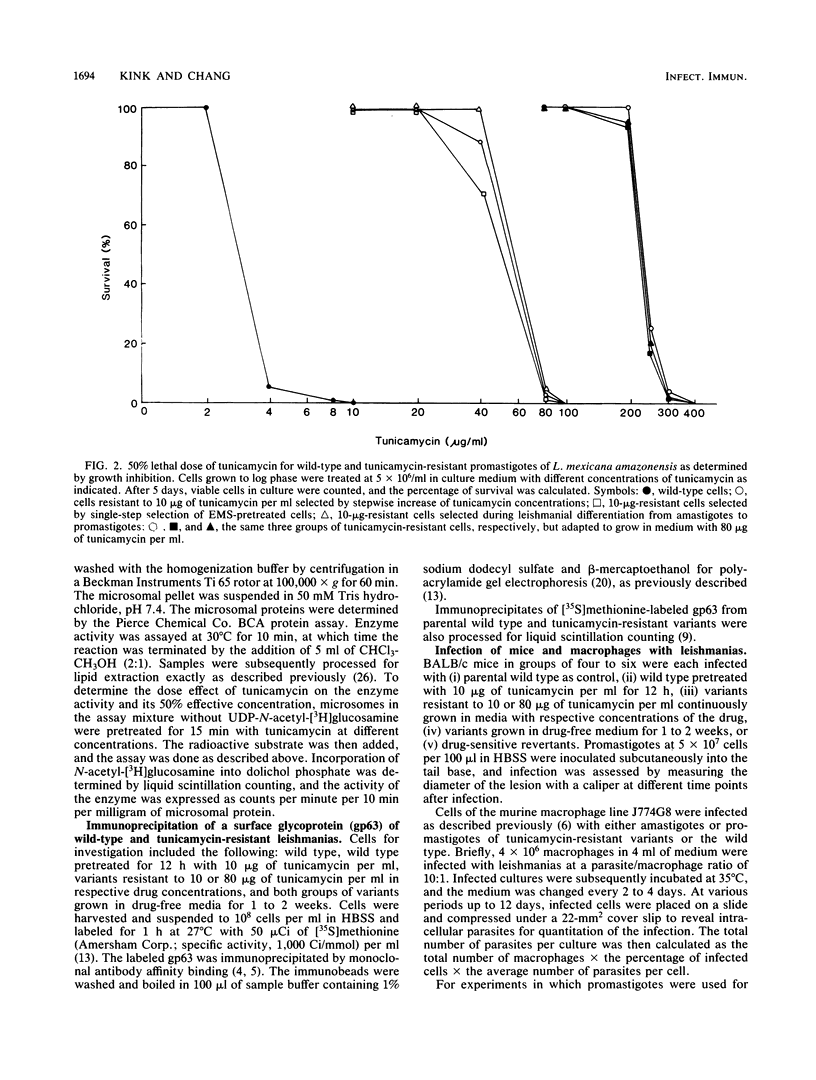
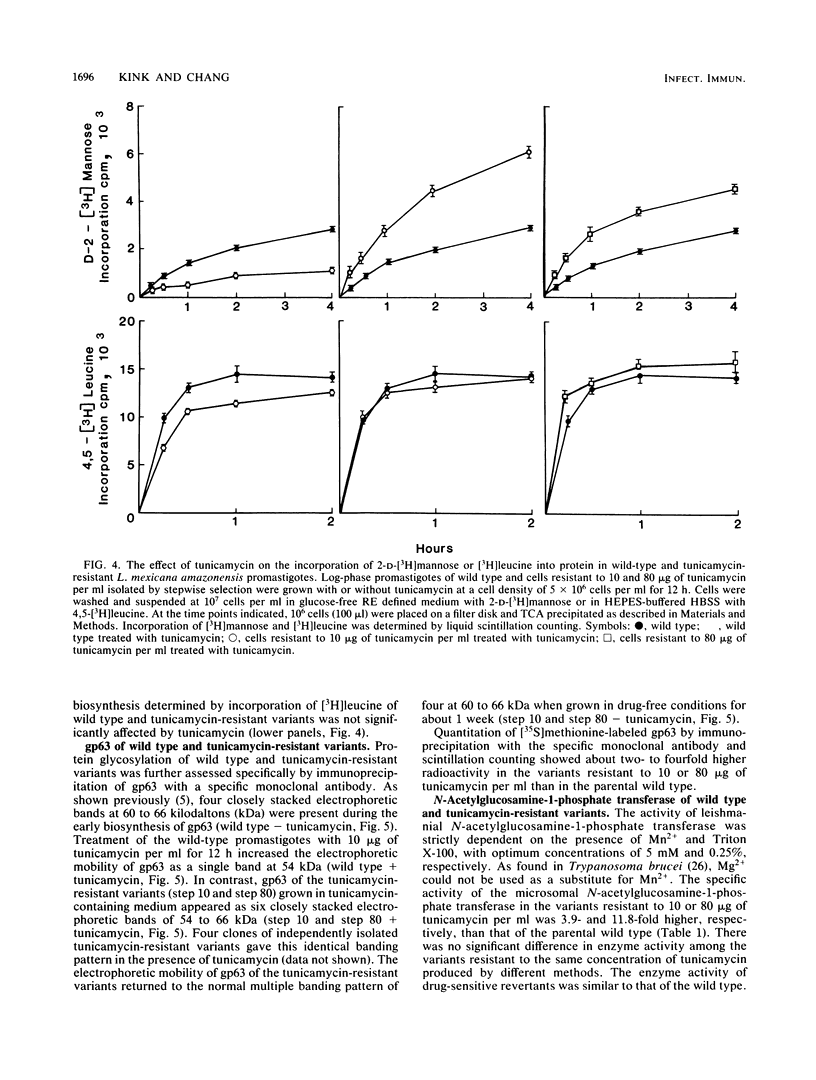
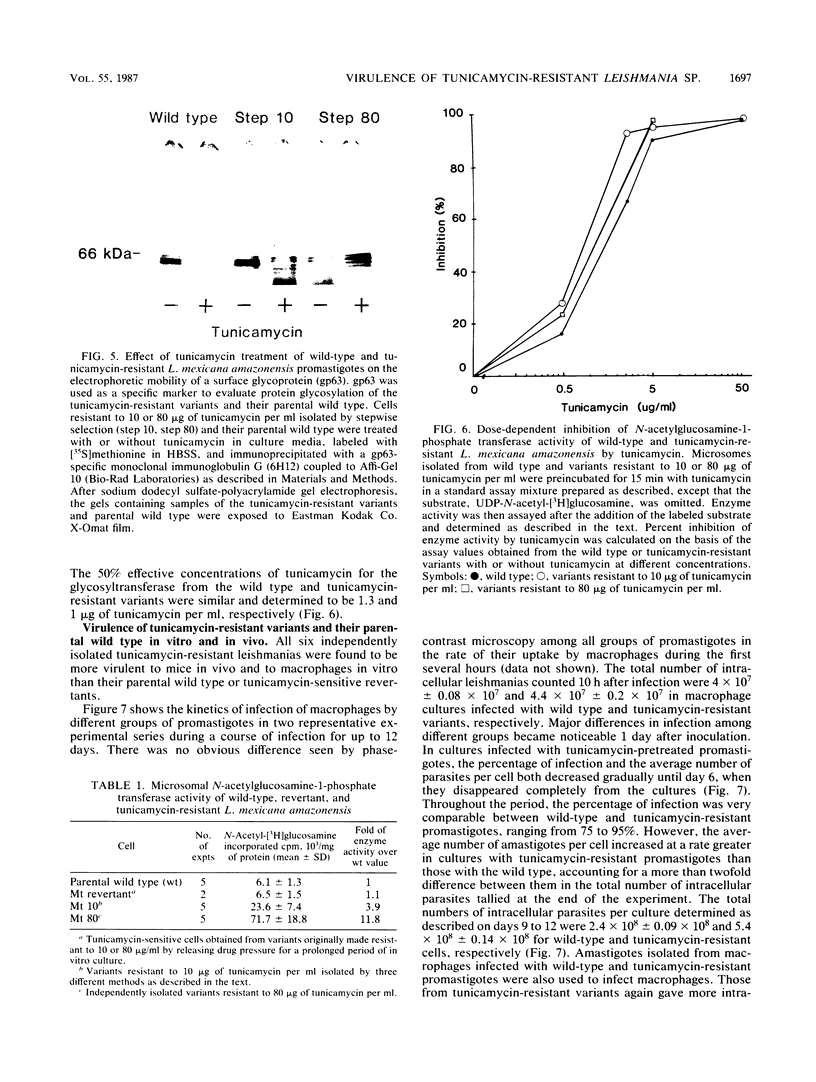
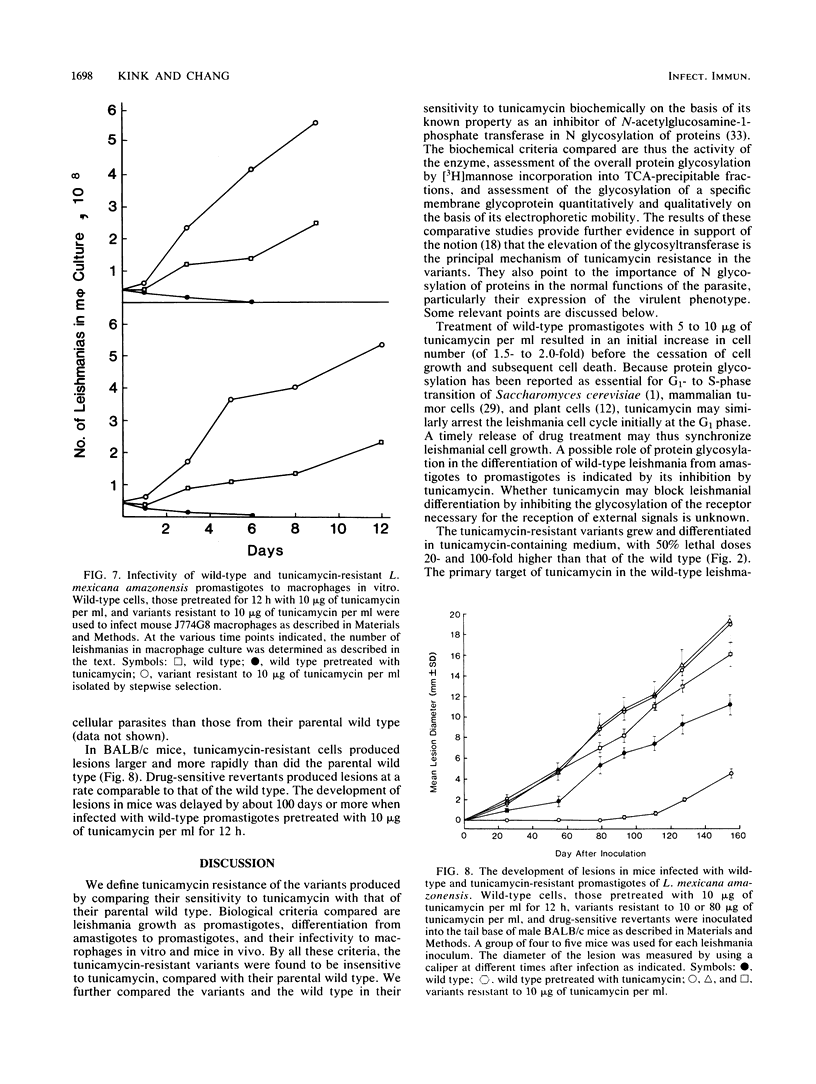
A parasitic protozoan, Leishmania mexicana amazonensis, was previously made resistant to tunicamycin (J.A. Kink and K.-P. Chang, Proc. Natl. Acad. Sci. USA 84:1253-1257, 1987). In the present study, six different tunicamycin-resistant variants were biologically and biochemically compared with their parental wild type to further delineate the mechanism of tunicamycin resistance and that of their virulence observed. In contrast to their parental wild type, all tunicamycin-resistant variants were found to grow and differentiate in tunicamycin-containing medium. The 50% lethal doses of tunicamycin for variants resistant to 10 or 80 micrograms of tunicamycin per ml were 20- and 100-fold higher, respectively, than that of the wild type. Specific activity of the microsomal N-acetylglucosamine-1-phosphate transferase was 4- to 12-fold higher in the tunicamycin-resistant cells than in their parental wild type and tunicamycin-sensitive revertants. The level of the enzyme activity is proportional to the degree of drug resistance. Inhibition kinetics studies showed that the enzyme from all groups was equally sensitive to the drug, with a 50% effective concentration of 1 to 1.3 micrograms of tunicamycin per ml. Thus, tunicamycin resistance of the variants is caused primarily by an increased level of their enzyme without alteration of its structure. Protein glycosylation determined by the incorporation of 2-D-[3H]mannose was about twofold higher in the tunicamycin-resistant variants than in their parental wild type. The increased glycosyltransferase activity in the latter apparently renders their protein glycosylation insensitive to the inhibition by tunicamycin. A major membrane glycoprotein of 63 kilodaltons (gp63) on the leishmania surface was found to be about threefold higher in the tunicamycin-resistant variants than in the wild type, as determined by immunoprecipitation with a monoclonal antibody specific for this antigen. Tunicamycin treatment of the wild type and tunicamycin-resistant variants caused changes in the electrophoretic mobility of this molecule, indicating a higher degree of its glycosylation in the latter cells. The tunicamycin-resistant variants parasitized macrophages in vitro more effectively than did the wild type, accounting for their virulence seen in mice. Thus, a high level of the glycosyltransferase enables the tunicamycin-resistant cells not only to overcome the inhibitory effect of tunicamycin on protein glycosylation but also to express their virulence, possibly by regulating N glycosylation of leishmanial proteins critical for leishmanias to establish intracellular parasitism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold E., Tanner W. An obligatory role of protein glycosylation in the life cycle of yeast cells. FEBS Lett. 1982 Nov 1;148(1):49–53. doi: 10.1016/0014-5793(82)81240-4. [DOI] [PubMed] [Google Scholar]
- Barnes G., Hansen W. J., Holcomb C. L., Rine J. Asparagine-linked glycosylation in Saccharomyces cerevisiae: genetic analysis of an early step. Mol Cell Biol. 1984 Nov;4(11):2381–2388. doi: 10.1128/mcb.4.11.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B. Lectin-resistant cell surface variants of eukaryotic cells. Int Rev Cytol. 1982;75:101–165. doi: 10.1016/s0074-7696(08)61003-7. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Chang K. P. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc Natl Acad Sci U S A. 1986 Jan;83(1):100–104. doi: 10.1073/pnas.83.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. S., Inserra T. J., Kink J. A., Fong D., Chang K. P. Expression and size heterogeneity of a 63 kilodalton membrane glycoprotein during growth and transformation of Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1986 Feb;18(2):197–210. doi: 10.1016/0166-6851(86)90038-1. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int Rev Cytol Suppl. 1983;14:267–305. [PubMed] [Google Scholar]
- Chang K. P., Fong D. Cell biology of host-parasite membrane interactions in leishmaniasis. Ciba Found Symp. 1983;99:113–137. doi: 10.1002/9780470720806.ch7. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Steiger R. F., Dave C., Cheng Y. C. Effects of methylglyoxal bis(ganylhydrazone) on trypanosomatid flagellates: inhibition of growth and nucleoside incorporation in Trypanosoma brucei. J Protozool. 1978 Feb;25(1):145–149. doi: 10.1111/j.1550-7408.1978.tb03887.x. [DOI] [PubMed] [Google Scholar]
- Dagger F., Ayesta C., Hernandez A. G. The effect of tunicamycin on Leishmania braziliensis cell growth, cell morphology and ultrastructure. Biol Cell. 1984;50(2):173–189. doi: 10.1111/j.1768-322x.1984.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharides. CRC Crit Rev Biochem. 1984;16(1):21–49. doi: 10.3109/10409238409102805. [DOI] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Tubulin biosynthesis in the developmental cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassy M. C., Ferrone S. Ia antigen expression is increased on tunicamycin-resistant human B-lymphoid cells. Clin Immunol Immunopathol. 1984 Jul;32(1):90–100. doi: 10.1016/0090-1229(84)90046-1. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Hernández A. G. Leishmanial excreted factors and their possible biological role. Ciba Found Symp. 1983;99:138–156. [PubMed] [Google Scholar]
- Kink J. A., Chang K. P. Tunicamycin-resistant Leishmania mexicana amazonensis: expression of virulence associated with an increased activity of N-acetylglucosaminyltransferase and amplification of its presumptive gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1253–1257. doi: 10.1073/pnas.84.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweider M., Lemesre J. L., Darcy F., Kusnierz J. P., Capron A., Santoro F. Infectivity of Leishmania braziliensis promastigotes is dependent on the increasing expression of a 65,000-dalton surface antigen. J Immunol. 1987 Jan 1;138(1):299–305. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nolan T. J., Farrell J. P. Inhibition of in vivo and in vitro infectivity of Leishmania donovani by tunicamycin. Mol Biochem Parasitol. 1985 Aug;16(2):127–135. doi: 10.1016/0166-6851(85)90081-7. [DOI] [PubMed] [Google Scholar]
- Nolan T. J., Herman R. Effects of long-term in vitro cultivation on Leishmania donovani promastigotes. J Protozool. 1985 Feb;32(1):70–75. doi: 10.1111/j.1550-7408.1985.tb03015.x. [DOI] [PubMed] [Google Scholar]
- Rine J., Hansen W., Hardeman E., Davis R. W. Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6750–6754. doi: 10.1073/pnas.80.22.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovis L., Baekkeskov S. Sub-cellular fractionation of Trypanosoma brucei. Isolation and characterization of plasma membranes. Parasitology. 1980 Jun;80(3):507–524. doi: 10.1017/s0031182000000974. [DOI] [PubMed] [Google Scholar]
- Rovis L., Dube S. Identification and characterisation of two N-acetylglucosaminyltransferases associated with Trypanosoma Brucei microsomes. Mol Biochem Parasitol. 1982 Mar;5(3):173–187. doi: 10.1016/0166-6851(82)90019-6. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Hieny S., Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985 Jul;135(1):564–569. [PubMed] [Google Scholar]
- Saraiva E. M., Andrade A. F., Pereira M. E. Cell surface carbohydrate of Leishmania mexicana amazonensis: differences between infective and non-infective forms. Eur J Cell Biol. 1986 Apr;40(2):219–225. [PubMed] [Google Scholar]
- Savage K. E., Baur P. S. Effect of tunicamycin, an inhibitor of protein glycosylation, on division of tumour cells in vitro. J Cell Sci. 1983 Nov;64:295–306. doi: 10.1242/jcs.64.1.295. [DOI] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv Carbohydr Chem Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- Stanley P. Glycosylation mutants of animal cells. Annu Rev Genet. 1984;18:525–552. doi: 10.1146/annurev.ge.18.120184.002521. [DOI] [PubMed] [Google Scholar]
- Steiger R. F., Steiger E. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J Protozool. 1977 Aug;24(3):437–441. doi: 10.1111/j.1550-7408.1977.tb04771.x. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Van Dyke K. Concurrent inhibition by tunicamycin of glycosylation and parasitemia in malarial parasites (Plasmodium falciparum) cultured in human erythrocytes. Pharmacology. 1981;23(3):165–170. doi: 10.1159/000137545. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Raizada M. K., Schutzbach J. S. Formation of alpha-1,2-mannosyl-mannose by an enzyme preparation from rabbit liver. J Biol Chem. 1977 Oct 25;252(20):7235–7242. [PubMed] [Google Scholar]