Abstract
Complex biotic interactions shape ecological communities of plants, herbivores and their natural enemies. In studies of multi-trophic interactions, the presence of small, invisible micro-organisms associated with plants and those of a fourth above-ground trophic level have often been neglected. Incorporating these neglected factors improves our understanding of the processes within a multi-trophic network. Here, we ask whether the presence of a fungal endosymbiont, which alters plant quality by producing herbivore-toxic substances, trickles up the food chain and affects the performance and host-selection behaviour of aphid secondary parasitoids. We simultaneously offered hosts from endophyte-free and endophyte-infected environments to secondary parasitoids. Older and more experienced parasitoid females discriminated against hosts from the endophyte-infected environment. Developing in lower quality hosts from the endophyte-infected environment reduced the lifespan of secondary parasitoids. This indicates that aphid secondary parasitoids can perceive the disadvantage for their developing offspring in parasitoids from the endophyte environment and can learn to discriminate against them. In the field, this discrimination ability may shift the success of primary parasitoids to endophyte-infected plants, which co-occur with endophyte-free plants. Ultimately, the control of aphids depends on complex interactions between primary and secondary parasitoids and their relative sensitivity to endophytic fungi.
Keywords: Asaphes vulgaris, bottom-up cascade, endophytic fungi, mummy parasitoids, Neotyphodium lolii, oviposition strategy
1. Introduction
The mediating effect of plants on the interaction between herbivores and their natural enemies plays a crucial role in structuring natural communities (Ohgushi et al. 2007). However, natural communities often consist of more than three trophic levels: plants; herbivores; and natural enemies (Pimm & Lawton 1977). For example, in insect communities, primary parasitoids are commonly attacked by a range of secondary parasitoids (Sullivan 1987). The activity of secondary parasitoids can limit the success of primary parasitoids and may disrupt the effectiveness of essential biological control (Rosenheim 1998). In aphid communities, secondary parasitoids are ubiquitous, diverse and may have a strong impact on interactions among plants, herbivores and primary parasitoids (Müller et al. 1999; Sullivan & Völkl 1999). Nevertheless, studies on multi-trophic interactions in insect communities often neglect the presence of this fourth trophic level (Brodeur 2000; but see Harvey et al. 2003; Soler et al. 2005).
Aside from ignoring consumers at the top of food webs, the presence of micro-organisms associating with plants at the bottom of food webs has also been frequently overlooked (Polis & Strong 1996). For example, endophytic fungi (=endophytes) are endosymbionts of a large variety of plant species (Arnold & Lutzoni 2007). Endophytes of the genus Neotyphodium are associated with temperate grasses and produce herbivore-toxic alkaloids (Clay 1990). These alkaloids can change food plant quality for herbivores and the effects can cascade up the food chain and propagate to higher trophic levels (Omacini et al. 2001; Müller & Krauss 2005; de Sassi et al. 2006; Härri 2007). The potential of endophytic fungi to affect secondary parasitoids, however, has never been investigated experimentally. Secondary parasitoids are intimately linked to primary parasitoids with their larvae feeding directly on the pre-pupae and pupae of the primary parasitoid (Quicke 1997). In addition, adult females of many species also feed directly on the hosts (host feeding; Jervis & Kidd 1986; Jervis et al. 2008). It is therefore likely that the presence of endophytes affects the behaviour and the performance of secondary parasitoids.
Spatial heterogeneity in plant quality can affect multi-trophic interactions (van Nouhuys & Hanski 2002). Spatial mosaics in endophyte infection in European grasslands (Saikkonen et al. 2000; Zabalgogeazcoa et al. 2003) leave the secondary parasitoids with the choice of hosts from either endophyte-free or endophyte-infected grasses. Such a choice confronts the parasitoids with a sequence of decisions when encountering a host: should the host be accepted for oviposition, ignored or used as protein-rich food? Host choice by parasitoids has been studied experimentally and theoretically, with the majority of the experimental studies focusing on host choice by primary parasitoids, whereas theoretical models often ignore important differences between primary and secondary parasitoids (Godfray 1994). If eggs were cheap and not limited, and the time required for host attack minimal, parasitoids would be predicted to attack all hosts they encounter. However, as many secondary parasitoids are either time and/or egg limited, oviposition becomes more costly and a precise host choice is important (Heimpel et al. 1996). Most aphid secondary parasitoids are synovigenic, which means that they continue to mature eggs during the adult stage (Quicke 1997). For many synovigenic species, the process of egg maturation requires proteins that are obtained by host feeding (Harvey 2008) and slows down with progressing age (Godfray 1994). Synovigenic parasitoids can therefore become egg limited either temporarily when deprived of hosts or after encountering a large number of hosts within a short time period or permanently with increasing age (Rosenheim et al. 2000). With progressive, age-dependent egg limitation, females should preferentially oviposit into the highest quality hosts (Iwasa et al. 1984) and use lower quality hosts for host feeding (Kidd & Jervis 1991). The ability to distinguish between hosts of different qualities may depend on the parasitoid's experience (Vet et al. 1990). Older and more experienced females are predicted to make more precise host choice decisions than younger ones. If host choice by secondary parasitoids indeed occurs, it may define the impact of the endophyte on secondary parasitoids and the structure of the associated aphid–parasitoid community.
Here, we investigate the cascading effect of primary parasitoid hosts of the endophyte-infected and endophyte-free environments on secondary parasitoid choices. We assumed that primary parasitoids that develop in aphids from endophyte-infected grasses are of reduced quality and predicted that this reduced host quality should influence the host choice behaviour and offspring performance of aphid secondary parasitoids, by (i) increasing larval development time, (ii) reducing adult lifespan, (iii) resulting in a male-biased sex ratio as theory predicts that fertilized eggs (=females) are laid preferentially into high-quality hosts whereas unfertilized eggs (=males) are laid into low-quality hosts (Charnov et al. 1981) and (iv) reducing the weight of adults that had developed in such low-quality hosts. We further tested whether females are able to distinguish between mummies from endophyte-free (E−) and endophyte-infected (E+) environments, depending on age and experience, predicting that (v) older females should be more selective, (vi) host experience would lead to increased choice of E− mummies for oviposition and (vii) the lower quality hosts (E+ mummies) would preferentially be used for host feeding.
2. Material and methods
(a) Model system
The system used to address our hypotheses consisted of the endophytic fungi Neotyphodium lolii Glen, Bacon, Hanlin, a specialist on the grass Lolium perenne L. The aphid species Metopolophium festucae Theobald served as hosts for the primary parasitoids Aphidius ervi Haliday whose mummies served as hosts for the secondary parasitoids Asaphes vulgaris Wlk. The aphid species M. festucae is relatively insensitive to the presence of N. lolii, whereas the fecundity of A. ervi is reduced when developing within M. festucae feeding on L. perenne infected by N. lolii (Härri 2007).
Seeds of L. perenne were provided by Brian Tapper, AgResearch, New Zealand. The seeds were either infected with N. lolii (wild-type; E+) or endophyte free (E−). All of the seeds belonged to the agricultural cultivar Grassland Samson. Endophyte infection was lost by selectively choosing plants with unsuccessful endophyte transmission (B. Tapper 2004, personal communication). After the experiment, the endophyte infection status of the plants was verified with the Phytoscreen Neotyphodium immunoblot assays (Agrinostics Ltd., Watkinsville, GA, USA). Infection level of E− plants was 3 per cent, whereas 80 per cent of the E+ plants were infected.
The stock culture of M. festucae was started in summer 2005 with a few individuals collected close to the University of Zurich, Switzerland. Since then, the culture was kept on L. perenne Arion (commercially available endophyte-free fodder grass). The stock culture of A. ervi was started with 250 individuals from Andermatt Biocontrol AG, Switzerland, in Spring 2006. Aphidius ervi was maintained on M. festucae feeding on L. perenne Arion. The stock culture of A. vulgaris was started with a few individuals obtained from the collected aphid mummies close to the University of Zurich, Switzerland, in Summer 2006. The stock culture was maintained on A. ervi.
Asaphes vulgaris (Pteromalidae, Chalcidoidea) is an ectoparasitic, solitary mummy parasitoid. Females oviposit on the surface of the pupae of the primary parasitoid and the hatched larva feeds on the primary parasitoid pre-pupa or pupa within the mummified aphid (Christiansen-Weniger 1992). When ovipositing or host feeding, females inject venom that kills the primary parasitoid pupae immediately (=idiobionts). Proteins gained from host feeding are allocated to the maturation of eggs. In the absence of hosts, the females can reallocate nutrients from the eggs into the somatic maintenance by resorption (=oosorption; Godfray 1994). The process of oosorption can lead to egg limitation in synovigenic species (Rosenheim et al. 2000).
(b) Experimental set-up
To obtain 1-day-old mummies from the E− and E+ environments, aphids from the stock culture were placed on either E− or E+ pots. After 10 days, A. ervi females were added, and a few days later, the freshly formed mummies were collected over four consecutive days. The explanatory factors were the presence of endophytes (‘endophyte’), age of A. vulgaris females (‘age’) and A. vulgaris female experience (‘experience’). In the endophyte choice treatment, 14 E− and 14 E+ mummies were offered simultaneously to one female A. vulgaris that had no previous experience with the E+ or E− environments. The choice experiments were conducted in Petri dishes and one Petri dish represented one replicate. The 28 mummies represented ad libitum number of hosts, as in all trials some primary parasitoids were emerging from the mummies. The mummies were glued to the Petri dish with a honey–water solution in a checkerboard pattern to ensure that individuals had the same probability of encountering E− and E+ mummies. To each Petri dish, one female and one male A. vulgaris were added. The mating partners had the same age. The factor age was manipulated using mating pairs of different ages. From the nine mating pairs used in the experiment, the youngest was 5 days old and the oldest 14 days old with 1 day difference between each of the pairs (age class 8 was missing). Each of the mating pairs was kept without aphid hosts after emergence until they entered the experimental arena where they were left together with the 14 E− and 14 E+ mummies for 24 hours. The factor experience was obtained by offering each mating pair fresh mummies (14 E− and 14 E+) in a new Petri dish over the four consecutive days.
Three days after female and male A. vulgaris were removed from the Petri dishes, the mummies were put individually into gelatine capsules. The gelatine capsules were checked daily for the emergence of A. vulgaris. The time until emergence (=development time) was recorded. Emerged A. vulgaris were sexed and put singly into a plastic vial (5×2 cm) closed with a foam plug. A fresh piece of apple was provided as a sugar source every second day, which reduced the overall lifespan and workload compared with daily feedings (S. A. Härri 2007, personal observation). The relative differences in lifespan between individuals developing with E− and E+ hosts are most likely not affected by the feeding regime. Time until death (=adult lifespan) was recorded in days. After death, each individual was weighed. The remaining mummies were dissected to detect host feeding or the death of the secondary parasitoid larvae. All stock cultures and experiments were conducted under controlled climatic conditions at 22°C with an 16 L : 8 D light regime.
(c) Statistical analyses
All statistical analyses were performed with R (v. 2.7.0 for Mac OS X). Values are represented as mean±s.e. The choice between E− and E+ mummies was analysed with a generalized mixed-effects model (glmmPQL—function) with ‘experience’, ‘day’, ‘endophyte’ and all their interactions as fixed effects and ‘female identity’ as random effect using the quasi-Poisson error structure to account for overdispersion of the count data (Venables & Ripley 2002). To account for the repeated measurements over the four consecutive days, experience was nested within female identity for the random factor.
The life-history traits, development time, lifespan and weight, of the emerged A. vulgaris offspring were analysed with linear mixed-effects model (lme—function) with ‘age’, ‘experience’, ‘sex’, ‘endophyte’ and including all interactions with the exception of sex for which only the interaction with endophyte was included. Female identity was included as a random factor with experience nested within female identity to account for the repeated measurements. Differences in the sex of the emerged A. vulgaris were analysed using a generalized mixed-effects model (glmmPQL—function) including the same fixed and random effects as described above, but excluding sex and its interaction with endophyte. For the four life-history traits, p-values were adjusted using Bonferroni corrections.
For all analyses, experience and day were treated as continuous factors and the first-order autocorrelation structure (corAR1) was applied to account for the dependency of the repeated measures (Pinheiro & Bates 2000). For all linear mixed-effects models, the maximum-likelihood method was used for model fit and the general positive-definite symmetric variance–covariance structure for the random effects (Pinheiro & Bates 2000). Owing to the non-orthogonality of the analyses, non-significant interaction terms were removed and only the main effects were tested. If the interactions were significant, only the highest order interaction would be interpreted.
3. Results
Test statistics and p-values for all measured traits are summarized in table 1 and table 2.
Table 1.
The influence of age, experience, endophyte and the interactions of interest on oviposition and host feeding, for the adult female generation that had a choice between mummies from E+ and E− environments. (For host feeding, all interactions were not significant and therefore not included in the analysis. The significant results are presented in bold letters. For the random factor ‘female identity’, the percentage of variation occurring between females is presented.)
| oviposition | host feeding | |
|---|---|---|
| single factors | ||
| age | F1,7=0.52 | F1,7=0.09 |
| p=0.492 | p=0.767 | |
| experience | F1,25=38.14 | F1,26=0.90 |
| p<0.001 | p=0.351 | |
| endophyte | F1,32=4.64 | F1,35=0.49 |
| p=0.039 | p=0.491 | |
| interactions | ||
| age×experience | F1,25=7.06 | — |
| p=0.014 | ||
| age×endophyte | F1,32=6.16 | — |
| p=0.019 | ||
| experience×endophyte | F1,32=1.71 | — |
| p=0.200 | ||
| age×experience×endophyte | F1,32=8.50 | — |
| p=0.006 | ||
| random factor | ||
| female identity | 14.96% | 39.93% |
Table 2.
The influence of age, experience, sex and endophyte on the measured life-history parameters of A. vulgaris. (All the interactions were not significant and were therefore not included in the analyses. The p-values were adjusted for multiple testing using Bonferroni corrections. The significant and marginally significant results are presented in bold letters. For the random factor ‘female identity’, the percentage of variation occurring between females is presented.)
| development | lifespan | weight | sex ratio | |
|---|---|---|---|---|
| single factors | ||||
| age | F1,5=6.12 | F1,5=0.38 | F1,5=3.51 | F1,5=2.55 |
| p=0.225 | p=1.00 | p=0.479 | p=0.684 | |
| experience | F1,11=5.47 | F1,11=2.10 | F1,11=0.99 | F1,11=1.16 |
| p=0.157 | p=0.700 | p=1.00 | p=1.00 | |
| sex | F1,40=21.95 | F1,40=11.89 | F1,40=9.81 | — |
| p<0.001 | p=0.004 | p=0.010 | ||
| endophyte | F1,40=0.14 | F1,40=6.74 | F1,40=0.20 | F1,41=1.64 |
| p=1.00 | p=0.053 | p=1.00 | p=0.830 | |
| random factor | ||||
| female identity | <0.01% | 0.09% | <0.01% | <0.01% |
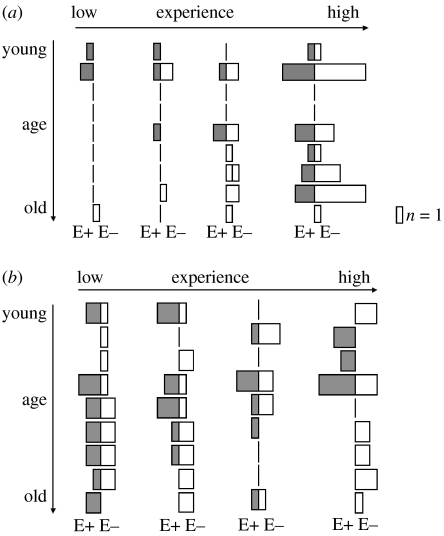
Seven of the nine A. vulgaris females reproduced successfully showing a preference for E− mummies (figure 1a). This resulted in a mean number of offspring of 2.50±0.58 from E− mummies and 1.85±0.34 from E+ mummies. The preference for E− mummies was especially pronounced for the older parasitoids and increased with experience (age×experience×endophyte; figure 1a; table 1). Contrary to our expectations, host feeding was not significantly influenced by any of the explanatory factors (figure 1b; table 1). A few mummies from which no primary or mummy parasitoid emerged and that were not used for host feeding contained dead A. ervi larvae that did not finish their development (E−: 4 mummies; E+: 8 mummies). In contrast to the primary parasitoid A. ervi, we did not observe larval and/or pupal mortality among A. vulgaris.
Figure 1.
The presence and the quantity of (a) oviposition events and (b) host-feeding events on E− (white bars) and E+ (grey bars). From left to right are the 4 days (‘experience’) and from top to down are the different ages of the females (excluding age class 8). Data show the increase in oviposition probability over the 4 days and an increase in oviposition probability with increasing age (table 1). Two females (aged 7 and 9 days at the beginning of the experiment) were not ovipositing despite showing host-feeding behaviour.
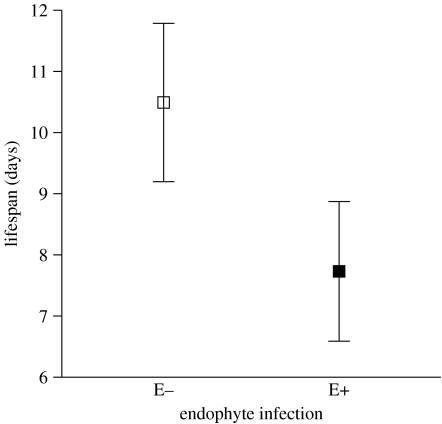
A total of 40 parasitoid offspring emerged from E− mummies and 25 from E+ mummies. The development time of A. vulgaris larvae from egg at oviposition to adult emergence was not influenced by the presence of endophytes but was shorter for males (19.98±0.09 days) than females (21.33±0.24 days; table 2). Asaphes vulgaris emerging from E+ had a significant shorter lifespan than those emerging from E− (figure 2). After the conservative Bonferroni correction, the reduction in lifespan is only marginally significant (table 2). Independent of endophyte infection, females lived significantly longer than males (females: 14.11±2.73 days, males: 9.37±0.59 days; table 2). The only factor that influenced the weight of the emerged adult wasps was their sex (females: 84.67±4.32 μg, males: 65.50±2.27 μg; table 2). Contrary to our expectation, the sex ratio of the progeny was not influenced by any of the experimental factors (table 2).
Figure 2.
The mean±s.e. lifespan for A. vulgaris offspring emerging from E− mummies (open square) and E+ mummies (filled square). The mean was calculated from the average of each Petri dish. The statistics are presented in table 2 (second column).
4. Discussion
The adult lifespan of a generalist secondary parasitoid at the top of an aphid–parasitoid food web tended to decrease when oviposition and larval development took place in hosts experiencing the endophyte environment. Female A. vulgaris improved their host choice with progressing age and oviposition experience through selection of more hosts from the endophyte-free environment compared with younger and less experienced females. Offspring performance in endophyte-free hosts was only improved in terms of lifespan and no effects on developmental time, sex ratio or weight were detected. Asaphes vulgaris parasitoids are long lived and synovigenic, maturing their eggs during adulthood. Under such conditions, a reduced lifespan will most likely result in a shorter reproductive time and thus reduced fitness. Similar fitness penalties of endophytes are known for predators and primary parasitoids (Bultman et al. 1997; de Sassi et al. 2006; Härri 2007). Ours is the first study showing experimentally that even the top trophic level in this aphid–parasitoid food web can suffer from ubiquitous endophytic fungi in agricultural grasses. It is also one of very few studies showing that variation in plant quality–-here caused by a microbial endophyte–-affects individual performance of the fourth trophic level (but see Harvey et al. 2003; Soler et al. 2005).
In the field, where endophyte infection often occurs in a heterogeneous pattern (e.g. Saikkonen et al. 2000), a fitness reduction caused by the presence of endophytes depends on the ability of secondary parasitoids to discriminate against such inferior hosts. We show that more secondary parasitoids offspring emerged from E− mummies than E+ mummies when females were given a choice. This increase in the number of offspring resulted from an increased number of oviposition events on E− mummies and not from increased larval mortality within E+ mummies as we detected no mortality among secondary parasitoid larvae. Thus, our experiment demonstrated that secondary parasitoids were able to discriminate against hosts from the endophyte-infected environment and this discrimination improved over time and with experience.
The exact cues that females used when selecting hosts from the endophyte-free environment must be linked to the aphid mummy as no plants or living aphids were present in the choice arenas. Aphid secondary parasitoids may be attracted to the presence of aphid honeydew (Buitenhuis et al. 2004), but this was not present in the choice arenas either. Host acceptance of secondary parasitoids often involves careful, time-consuming examination of a mummy by tapping it and probing the host inside with the ovipositor (Vinson 1976). As mummy size does not differ between E− and E+ (Härri 2007), the ovipositor that examines the host may perceive signals about endophyte-produced chemicals that have accumulated in the primary parasitoid. Alternatively, the mummified aphid skin could carry clues on past plant associations of the now dead aphid. Asaphes vulgaris appears to also respond to kairomones that occur in the silky cocoon of aphidiine wasp (Christiansen-Weniger 1992). Independent of the exact cue the female secondary parasitoid perceives, host preference has been shown previously for different host species (Chow & Mackauer 1999), and we provide further evidence that age and experience of A. vulgaris females play a decisive role for their reproductive success.
For primary parasitoids, it has been proposed that mothers deposit a chemical cue inside the silk surrounding the pupa, which may condition the offspring to the environment in which they developed (van Emden et al. 1996; Douloumpaka & van Emden 2003). The fact that, in our experiment, the degree of preference exhibited by female wasps for E− mummies was time dependent suggests that the mechanism is likely to be different. All A. vulgaris females concentrated on host feeding for the first couple of days, presumably to mature their eggs. The oviposition events increased with more experience, and increasing numbers of eggs were laid in E− mummies. For host-feeding behaviour, no such temporal patterns were observed and no evidence for preference was detected. Therefore, females could have learned during host feeding to distinguish high- versus low-quality hosts.
The lack of detection of host-feeding patterns may have been caused by the relatively small sample size of nine females and the short time design of the experiment. Experiments with secondary parasitoids are extremely difficult to conduct, which is probably why very little data exist for multi-trophic interactions including secondary parasitoids (Brodeur 2000; but see Harvey et al. 2003; Soler et al. 2005). We believe that our experiment provides strong evidence for the cascading effects of endophytes to the top fourth trophic level of the food web, despite the small number of females tested. Changes in secondary parasitoid number and diversity depending on endophyte infection are also supported by a field study (Omacini et al. 2001). In that study, the food chain based on endophyte-infected plants and the aphid Rhopalosiphum padi showed strong numerical responses of primary and secondary parasitoids. The co-occurring aphid M. festucae was less affected by the presence of endophytes, but its parasitoid community also showed decreased abundance and diversity. Apart from numerical responses in aphids, the pattern of reduced aphid secondary parasitoid abundance and diversity in the endophyte environment could have arisen by discrimination against hosts containing endophytes. In our laboratory experiment, the herbivore aphid M. festucae was relatively insensitive to the presence of the endophyte, while the associated primary parasitoids (Härri 2007), and as shown here a secondary parasitoid, showed reduced fitness in the presence of endophytes.
In conclusion, we showed that the fitness of aphid secondary parasitoids when developing within hosts from an endophyte-infected environment was reduced and females learned to discriminate against these hosts of lower quality. The reduction in fitness of aphid secondary parasitoids in the presence of endophytes limits their top-down control on primary parasitoids and may result in complex indirect effects for lower trophic levels in the food web.
Acknowledgments
We thank Dennis Hansen, Uli Steiner and Nancy Bunbury for their helpful discussions and comments. Jeff Harvey and three anonymous reviewers helped improving the manuscript. The project was funded by an SNSF grant to C.B.M. (631-065950).
References
- Arnold A.E, Lutzoni F. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology. 2007;88:541–549. doi: 10.1890/05-1459. doi:10.1890/05-1459 [DOI] [PubMed] [Google Scholar]
- Brodeur J. Host specificity and trophic relationships of hyperparasitoids. In: Hochberg M.E, Ives A.R, editors. Parasitoid population biology. Princeton University Press; Princeton, NJ: 2000. pp. 163–183. [Google Scholar]
- Buitenhuis R, McNeil J.N, Boivin G, Brodeur J. The role of honeydew in host searching of aphid hyperparasitoids. J. Chem. Ecol. 2004;30:273–285. doi: 10.1023/b:joec.0000017977.39957.97. doi:10.1023/b:joec.0000017977.39957.97 [DOI] [PubMed] [Google Scholar]
- Bultman T.L, Borowicz K.L, Schneble R.M, Coudron T.A, Bush L.P. Effect of a fungal endophyte on the growth and survival of two Euplectrus parasitoids. Oikos. 1997;78:170–176. doi:10.2307/3545812 [Google Scholar]
- Charnov E.L, Los- den Hartogh R.L, Jones W.T, van den Assem J. Sex ratio evolution in a variable environment. Nature. 1981;289:27–33. doi: 10.1038/289027a0. doi:10.1038/289027a0 [DOI] [PubMed] [Google Scholar]
- Chow A, Mackauer M. Host handling and specificity of the hyperparasitoid wasp, Dendrocerus carpenteri (Curtis) (Hym., Megaspilidae): importance of host age and species. J. Appl. Entomol. 1999;123:83–91. doi:10.1046/j.1439-0418.1999.00322.x [Google Scholar]
- Christiansen-Weniger, P. 1992 Wirt-Parasitoid-Beziehung zwischen Blattlausprimärparasitoiden und den Blattlaushyperparasitoiden Asaphes vulgaris Wlk. und Asaphes suspensus (Nees) (Hymenoptera: Pteromalidae). PhD thesis Christian-Albrechts-Universita¨t zu Kiel, Kiel, Germany.
- Clay K. Fungal endophytes of grasses. Annu. Rev. Ecol. Syst. 1990;21:275–297. doi:10.1146/annurev.es.21.110190.001423 [Google Scholar]
- de Sassi C, Müller C.B, Krauss J. Fungal plant endosymbionts alter life history and reproductive success of aphid predators. Proc. R. Soc. B. 2006;273:1301–1306. doi: 10.1098/rspb.2005.3442. doi:10.1098/rspb.2005.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douloumpaka S, van Emden H.F. A maternal influence on the conditioning to plant cues of Aphidius colemani Viereck, parasitizing the aphid Myzus persicae Sulzer. Physiol. Entomol. 2003;28:108–113. doi:10.1046/j.1365-3032.2003.00323.x [Google Scholar]
- Godfray H.C.J. Monographs in Behavior and Ecology. Princeton University Press; Princeton, NJ: 1994. Parasitoids. Behavioral and evolutionary ecology. [Google Scholar]
- Härri, S. A. 2007 Fungal endosymbionts of grasses, and their effects on multitrophic interactions. PhD thesis, University of Zürich, Zürich, Switzerland.
- Harvey J.A. Comparing and contrasting development and reproductive strategies in the pupal hyperparasitoids Lysibia nana and Gelis agilis (Hymenoptera: Ichneumonidae) Evol. Ecol. 2008;22:153–166. doi:10.1007/s10682-007-9164-x [Google Scholar]
- Harvey J.A, van Dam N.M, Gols R. Interactions over four trophic levels: foodplant quality affects development of a hyperparasitoid as mediated through a herbivore and its primary parasitoid. J. Anim. Ecol. 2003;72:520–531. doi:10.1046/j.1365-2656.2003.00722.x [Google Scholar]
- Heimpel G.E, Rosenheim J.A, Mangel M. Egg limitation, host quality, and dynamic behavior by a parasitoid in the field. Ecology. 1996;77:2410–2420. doi:10.2307/2265742 [Google Scholar]
- Iwasa Y, Suzuki Y, Matsuda H. Theory of oviposition strategy of parasitoids—1. Effect of mortality and limited egg number. Theor. Popul. Biol. 1984;26:205–227. doi: 10.1016/0040-5809(84)90030-3. doi:10.1016/0040-5809(84)90030-3 [DOI] [PubMed] [Google Scholar]
- Jervis M.A, Kidd N.A.C. Host-feeding strategies in hymenopteran parasitoids. Biol. Rev. Camb. Phil. Soc. 1986;61:395–434. doi:10.1111/j.1469-185X.1986.tb00660.x [Google Scholar]
- Jervis M.A, Ellers J, Harvey J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 2008;53:361–385. doi: 10.1146/annurev.ento.53.103106.093433. doi:10.1146/annurev.ento.53.103106.093433 [DOI] [PubMed] [Google Scholar]
- Kidd N.A.C, Jervis M.A. Host-feeding and oviposition strategies of parasitoids in relation to host stage. Res. Popul. Ecol. 1991;33:13–28. doi:10.1007/bf02514570 [Google Scholar]
- Müller C.B, Krauss J. Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 2005;8:450–456. doi: 10.1016/j.pbi.2005.05.007. doi:10.1016/j.pbi.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Müller C.B, Adriaanse I.C.T, Belshaw R, Godfray H.C.J. The structure of an aphid–parasitoid community. J. Anim. Ecol. 1999;68:346–370. doi:10.1046/j.1365-2656.1999.00288.x [Google Scholar]
- Ohgushi T, Craig T.P, Price P.W. Indirect interaction webs: an introduction. In: Ohgushi T, Craig T.P, Price P.W, editors. Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press; Cambridge, UK: 2007. pp. 3–15. [Google Scholar]
- Omacini M, Chaneton E.J, Ghersa C.M, Müller C.B. Symbiotic fungal endophytes control insect host–parasite interaction webs. Nature. 2001;409:78–81. doi: 10.1038/35051070. doi:10.1038/35051070 [DOI] [PubMed] [Google Scholar]
- Pimm S.L, Lawton J.H. Number of trophic levels in ecological communities. Nature. 1977;268:329–331. doi:10.1038/268329a0 [Google Scholar]
- Pinheiro J.C, Bates D.M. Statistics and computing. Springer; New York, NY: 2000. Mixed-effects models in S and S-PLUS. [Google Scholar]
- Polis G.A, Strong D.R. Food web complexity and community dynamics. Am. Nat. 1996;147:813–846. doi:10.1086/285880 [Google Scholar]
- Quicke D.L.J. Chapman & Hall; London, UK: 1997. Parasitic wasps. [Google Scholar]
- Rosenheim J.A. Higher-order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol. 1998;43:421–447. doi: 10.1146/annurev.ento.43.1.421. doi:10.1146/annurev.ento.43.1.421 [DOI] [PubMed] [Google Scholar]
- Rosenheim J.A, Heimpel G.E, Mangel M. Egg maturation, egg resorption and the costliness of transient egg limitation in insects. Proc. R. Soc. B. 2000;267:1565–1573. doi: 10.1098/rspb.2000.1179. doi:10.1098/rspb.2000.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikkonen K, Ahlholm J, Helander M, Lehtimäki S, Niemeläinen O. Endophytic fungi in wild and cultivated grasses in Finland. Ecography. 2000;23:360–366. doi:10.1034/j.1600-0587.2000.d01-1645.x [Google Scholar]
- Soler R, Bezemer T.M, van der Putten W.H, Vet L.E.M, Harvey J.A. Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J. Anim. Ecol. 2005;74:1121–1130. doi:10.1111/j.1365-2656.2005.01006.x [Google Scholar]
- Sullivan D.J. Insect hyperparasitism. Annu. Rev. Entomol. 1987;32:49–70. doi:10.1146/annurev.en.32.010187.000405 [Google Scholar]
- Sullivan D.J, Völkl W. Hyperparasitism: multitrophic ecology and behavior. Annu. Rev. Entomol. 1999;44:291–315. doi: 10.1146/annurev.ento.44.1.291. doi:10.1146/annurev.ento.44.1.291 [DOI] [PubMed] [Google Scholar]
- van Emden H.F, Sponagl B, Wagner E, Baker T, Ganguly S, Douloumpaka S. Hopkins ‘host selection principle’, another nail in its coffin. Physiol. Entomol. 1996;21:325–328. doi:10.1111/j.1365-3032.1996.tb00873.x [Google Scholar]
- van Nouhuys S, Hanski I. Multitrophic interactions in space: metacommunity dynamics in fragmented landscapes. In: Tscharntke T, Hawkins B.A, editors. Multitrophic level interactions. Cambridge University Press; Cambridge, UK: 2002. pp. 124–147. [Google Scholar]
- Venables W.N, Ripley B.D. Springer; New York, NY: 2002. Modern applied statistics with S. [Google Scholar]
- Vet L.E.M, Lewis W.J, Papaj D.R, van Lenteren J.C. A variable-response model for parasitoid foraging behavior. J. Insect Behav. 1990;3:471–490. doi:10.1007/BF01052012 [Google Scholar]
- Vinson S.B. Host selection by insect parasitoids. Annu. Rev. Entomol. 1976;21:109–133. doi:10.1146/annurev.en.21.010176.000545 [Google Scholar]
- Zabalgogeazcoa I, Vázquez de Aldana B.R, García Ciudad A, García Criado B. Fungal endophytes in grasses from semi-arid permanent grasslands of western Spain. Grass Forage Sci. 2003;58:94–97. doi:10.1046/j.1365-2494.2003.00352.x [Google Scholar]