Abstract
Most research on the effects of exposure to stressful stimuli during embryonic development has focused on post-embryonic behaviour that appears to be abnormal or maladaptive. Here, we tested whether exposure to some stressful stimuli (predatory cues) can lead to post-embryonic behaviour that is adaptive. When eggs of ringed salamanders (Ambystoma annulatum) were exposed to chemical cues from predators, post-hatching larvae showed reduced activity and greater shelter-seeking behaviour; larvae that had been exposed to control cues did not show these behaviours. In addition, wood frog (Rana sylvatica)tadpoles learned to respond to chemical cues from unfamiliar predators with danger based on embryonic conditioning. Therefore, if embryonic experience is a good predictor of future risk, learning associated with exposure to negative stimuli during development may be adaptive.
Keywords: embryos, learning, predation, Ambystoma annulatum, Rana sylvatica
1. Introduction
Learning by embryos primarily has been associated with maternal/auditory recognition (Hepper 1996) or attraction to food (Hepper & Waldman 1992; Sneddon et al. 1998). By contrast, exposure to negative or stressful stimuli during embryological development is generally considered to result in behaviour categorized as abnormal or deficient, including anxiety and depression (Vallée et al. 1997; Dugovic et al. 1999; Lemaire et al. 2000). The possible evolutionary function of embryonic learning associated with negative stimuli has not received much attention.
One well-known mechanism of learning is classical conditioning, where an individual is presented simultaneously with a familiar and unfamiliar stimulus; the individual later responds to the unfamiliar stimulus in the same way as it responded to the familiar stimulus. Embryos of some primates are able to associate auditory stimuli (tones or music) with vibration or maternal relaxation (Hepper 1997; Kawai et al. 2004). However, it is not known whether such prenatal learning is adaptive or simply is a by-product of maturation of the central nervous system (Hepper 1996).
For aquatic vertebrates, predator-related learning plays an integral role in the success of antipredator behaviour. Predator-related learning generally takes the following two forms: (i) exposure to increased predation risk influences subsequent behaviour (Lima & Bednekoff 1999) and (ii) associative learning, whereby individuals learn to associate novel stimuli, such as unfamiliar predators or specific habitats, with danger (Brown 2003). For most aquatic vertebrates, risk of predation, and thus benefits of learned recognition of predation risk, is highest for small (i.e. young) individuals (Stangel 1988). Therefore, selection should favour individuals that can learn early in development. We tested the hypothesis that embryonic amphibians use information about predation risk to alter post-hatching behaviour in ways that reduce exposure to predation. Specifically, we examined post-hatching responses of (i) ringed salamanders (Ambystoma annulatum) that were exposed to syntopic predators as embryos and (ii) wood frogs (Rana sylvatica) that were exposed to an associative learning protocol that paired stimuli from an unfamiliar (allotropic) predator and a known fright stimulus (wood frog tadpole alarm chemical).
2. Material and methods
(a) Does embryonic exposure to predators influence post-hatching activity and habitat choice?
We collected eggs (approx. 20 clutches and 500 eggs at Harrison (1969) developmental stage 1) from three ponds in Stone County, MO, USA on 11 November 2005. At the same time, we collected free-swimming larvae of A. annulatum (n=15; 19–29 mm total length) to provide the predatory stimuli (larger larvae are cannibals of small larvae (Nyman et al. 1993)) and Rana catesbeiana (bullfrog) tadpoles (n=15; 21–36 mm total length) to provide a non-predatory control stimulus. Salamander larvae were fed either zooplankton or blackworms (Lumbriculus variegatus) and tadpoles were fed algae pellets.
Each clutch was divided into three groups, with five to eight eggs in each group. Each group was randomly assigned to one of three chemical treatments: water from tanks containing either (i) predatory (cannibalistic) A. annulatum larvae, (ii) non-predatory tadpoles or (iii) no animals (blank). Substrates (plants versus rocks) also were randomly assigned, but had no effect on the results; therefore, this variable will not be discussed further. Stimulus animals were kept individually in plastic boxes (9×9×8 cm) with 500 ml of water. Each group of eggs was kept in a plastic box (30×15×11 cm) for 3 days, during which time 50 ml of stimulus water was added twice daily. After the 3-day exposure period, each group of eggs (now at Harrison stages 35–36) was placed in containers (9×9×8 cm) with clean water (500 ml) until hatching (4–13 days later; Harrison stages 39–42). There were no statistically significant effects of treatment on the size (F=1.77, d.f.=2, p=0.17) or stage (F=0.67, d.f.=2, p=0.51) of larvae at hatching, although such an effect has been reported for another species (Sih & Moore 1993). For each experiment, two larvae were tested from each group and no larva was tested more than once.
In the first experiment, we hypothesized that larvae exposed to the high-risk treatment as embryos would show lower post-hatching activity than those in the low-risk treatments; low activity is an antipredatory response for this species (Mathis et al. 2003). We quantified activity of free-swimming larvae as soon as larval yolk sacs were depleted (12.0–17.5 mm total length; post-hatching stages 4–8 (Watson & Russell 2000)). Test chambers were modified 12 ml test tubes that were oriented horizontally with the open end blocked and lines marked every 12 mm. A 4 mm diameter hole was drilled in the top left end of the tube for introduction of the test larva. Immediately after introduction (which constituted a mild disturbance), we quantified activity as number of lines crossed in 5 min. Statistical analysis was an ANOVA followed by Tukey's post hoc comparisons.
In a second experiment, we hypothesized that larvae exposed to the high-risk treatment as embryos would have a stronger tendency to occupy habitats containing plants than larvae in the low-risk treatment. In their natural habitat, larval A. annulatum often are found in vegetation around the edges of the pond (A. Mathis & N. Windel 2005, personal observations) probably because it provides refuge from predators (e.g. Brodman & Jaskula 2002). Aquatic vegetation is the most abundant shelter available in the ponds. To distinguish between a specific choice for plants versus a choice for any available shelter, we included an alternative shelter choice of rocks; rocks are rare in the ponds where the eggs were collected. We divided a plastic container (30×15×11 cm) into three equal sections: one randomly chosen end had rocks; the opposite end had vegetation (Polygonum sp.); and a neutral area (no added substrate) was in the centre. Larvae (12–17 mm; post-hatching stages 5–7) were introduced into an open-ended vertical tube in the centre of the neutral zone. After 10 min, the tube was removed and the position of the larvae was noted after 1 and 5 hours. Most larvae were still in the neutral zone after 1 hour, and so data for latency to move to cover and initial time in cover were not analysed statistically. For 1 and 5 hour choice data, we compared post-hatching habitat choice (rock versus vegetation) to a random distribution using the Fisher's exact probability test for each treatment.
(b) Can embryos learn to recognize unfamiliar predators using classical conditioning?
Two weeks prior to collection of eggs, we filled a 1900 l tub with well water to serve as a holding tank for wood frog eggs and left it outdoors. The tub was seeded with aquatic plants, zooplankton and phytoplankton from a local pond using a fine mesh dip net, ensuring that the holding and testing water contained a full array of algae and plankton. The predators, fire-belly newts (Cynops pyrrhogaster), obtained from a commercial supplier, were kept in a 7 l tub containing mosquito larvae (Culex restuans) and fairy shrimp (Streptocephalus spp.).
All eggs and tadpoles used in this experiment were collected from a single pond in northeastern Alberta in April 2006. Egg laying occurred 16–27 April. Four egg clutches laid early in the season were transferred into a pool containing pond water and aquatic plants, and were left to develop until hatching. These tadpoles were used for preparation of the alarm stimulus. The alarm stimulus was prepared immediately prior to being used, by crushing 20 tadpoles (approx. 8–12 mm in length) in 50 ml of well water using a mortar and pestle and then dividing the stimulus solution into five aliquots of 10 ml each.
For the embryo-training portion of the study, the conditioned stimulus (water from tanks with allopatric predators, fire-belly newts) was obtained by putting four newts into 375 ml of conditioned well water for 24 h; the water was changed daily. For the test of post-hatching behaviour, the newt odour was obtained by putting nine newts into a tub containing 3 l of conditioned well water for 24 h.
To serve as test embryos, we collected five clutches of wood frog eggs and divided each clutch into three groups of approximately 50 eggs. The 15 groups were then individually transferred into 3.5 l buckets containing 3 l of conditioned well water. The 50 eggs consisted of a single mass with the egg jelly intact. The buckets were then placed on trays, partially filled with pond water, floating on the pond to equalize the temperature of the egg buckets with that of the pond. Random temperature checks revealed only a 0.1°C difference between the 15 buckets. Embryos were at Gosner (1960) developmental stages 12–15 (the neural fold stage), and training began immediately.
For each clutch, the three groups of eggs were randomly assigned to a training treatment with (i) 10 ml of injured tadpoles cues paired with 20 ml of newt odour, (ii) 10 ml of conditioned well water paired with 20 ml of newt odour or (iii) 30 ml of conditioned well water. Eggs were treated every day for 6 days at 16.00 hours; the stimuli were slowly injected on the side of the buckets to minimize disturbance to the eggs. At 19.00, a 100 per cent water change was performed, with the experimenter wearing latex gloves to avoid contamination of the water. Daily treatments continued for 6 days until the embryos within the eggs appeared fully formed (approx. Gosner stage 22/24) but had not hatched; all eggs hatched within 24 h. We verified that the embryos had not hatched as they were curled up inside their egg shell; tadpoles straighten out immediately upon hatching. Tadpoles were provided with rabbit food and the water was partially changed every second day.
The number of individuals tested per treatment was unbalanced due to differential mortality of the clutches. Mortality included individuals in all clutches, one entire clutch and two subgroups of 50 eggs from other clutches. Mortality was primarily due to infection by pathogenic water moulds (Saprolegnia spp.) that were common in amphibian clutches at our field site. Hence, we tested tadpoles from three different clutches in the first treatment (crushed tadpoles paired with newt odour—sample size of 17, 14 and 13), four clutches in the second treatment (water paired with newt odour—sample size of 17, 16, 16 and 7) and three clutches in the last treatment (water only—sample size of 18, 11 and 16). There were many surviving tadpoles in some buckets that were not tested.
Two weeks after hatching (stage 25), tadpoles from all three treatments were tested for a response to newt odour. Individual tadpoles were transferred into plastic cups containing 0.5 l of conditioned well water and left to acclimatize for 45 min. The trials consisted of a 4 min prestimulus period, a 30 s injection period and a 4 min post-stimulus period. Behaviour (activity) of the tadpoles was recorded, and a decrease in activity was considered a fright response (Petranka & Hayes 1998). To measure activity, we drew a line across the centre of the cup and counted the number of line crosses during the two observation periods. We considered that a tadpole crossed a line when its entire body was on the other side of the line. During the injection period, the 5 ml of the newt stimulus solution was emptied slowly on the side of the cup to minimize disturbance. The trials were performed outdoors, and all trials were performed on the same day to avoid day/temperature effects.
We calculated the change in line crosses from the prestimulus baseline. We compared treatments using a one-way nested ANOVA, in which the responses of tadpoles were nested within clutch and the clutches were nested within treatments; data were normal and homoscedastic. Post hoc Bonferroni comparisons were performed after a significant treatment effect to compare among treatments.
3. Results and discussion
(a) Does embryonic exposure to predators influence post-hatching activity and habitat choice?
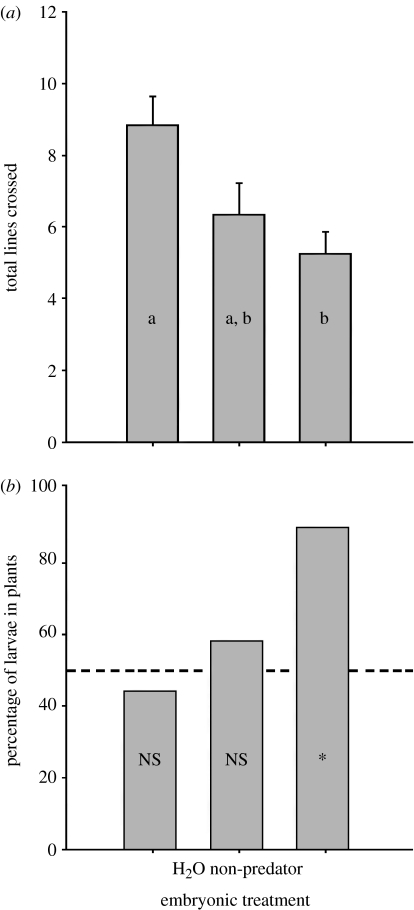
In the first experiment, there was a significant effect of treatment on post-hatching activity (F=5.53; d.f.=2; n: predator=76, non-predator=65, blank=74; p=0.005). Larvae that had been exposed to chemical cues from predators as embryos were significantly less active than those exposed to the blank treatment (Tukey's test: p=0.004). There was no significant difference between the tadpole and blank (p>0.05) or tadpole and predator treatment groups (p>0.50; figure 1a).
Figure 1.
Post-hatching behaviour of Ambystoma annulatum larvae that had been exposed as embryos to chemical stimuli from predators (cannibalistic conspecifics), non-predators (tadpoles of R. catesbeiana) or to a blank control. (a) Activity measured as total number of lines crossed in 5 min (mean±1 s.e.). Statistical analysis was an ANOVA followed by Tukey's post hoc comparisons; different letters indicate significant differences. (b) Habitat choice measured as proportion of larvae found in the plants after 5 hours. Statistical analysis was the Fisher's exact probability test; *p=0.011; n.s., p>0.15.
In the second experiment, larvae had been exposed to predator cues as embryos occupied the vegetation habitat significantly more often than the rock habitat after 5 hours (n=20; p=0.022; figure 1b). For larvae exposed to blank (n=31; p=0.664) and non-predator cues (n=32; p=0.50), there was no significant difference between the proportion of individuals choosing the rock side and those choosing the vegetation side (figure 1b).
Experience of embryos with predatory stimuli influenced post-hatching behaviour in ways that were consistent with antipredator behaviour. Following embryonic exposure to syntopic predators (potentially cannibalistic older larvae), A. annulatum larvae showed reduced activity and a preference for vegetated habitats. Reduced activity is a known response to predatory stimuli for this species in both laboratory and field experiments (Mathis et al. 2003), and this behaviour typically results in increased survival of amphibian larvae (Skelly 1994). A similar result was seen in an experiment with common frogs (Rana temporaria; Saglio & Mandrillon 2006). Attraction to vegetation by larvae exposed to predation risk as embryos is likely an antipredator strategy (e.g. Orizaola & Braña 2003); vegetation is the primary refuge available in natural pond habitats for this population. Control larvae were not differentially attracted to plants, indicating a possible cost to plant use, such as decreased prey availability or increased risk of predation by some predators (dytiscid beetles: Holomuzki 1985). Differential use of vegetation for larvae in the predator treatment did not reflect a general preference for structure over non-structure because the vegetation was not used significantly more often than rocks in the non-predator treatments. Moreover, use of the vegetated habitat did not depend upon being exposed to vegetation during the embryonic period (see §2). Although the proximate mechanisms for these changes in behaviour are unknown, embryonic learning may be hormonally mediated (Davis et al. 2005; Uller & Olsson 2006) or related to changes in neurological (amygdalar) biology (Salm et al. 2004).
(b) Can embryos learn to recognize unfamiliar predators?
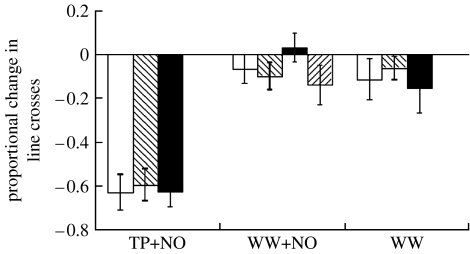
There was a significant effect of treatment (F2,135=45.57, p<0.001) on post-hatching activity, but no effect of clutch (F3,135=0.23, p=0.88) and no significant interaction between the treatment and clutch (F6,135=0.53, p=0.71; figure 2). Post hoc Bonferroni comparisons showed that tadpoles initially exposed to crushed tadpoles paired with newt odour during embryonic development responded differently to newt odour than the ones initially exposed to either water paired with newt odour (difference=−0.56, p<0.001) or water only (difference=−0.50, p<0.001). Tadpoles initially exposed to water paired with newt odour did not respond differently to the newt odour than the tadpoles initially exposed to water only (difference=0.06, p=1.000).
Figure 2.
Activity measured as proportional change in line crosses (mean±1 s.e.) for wood frog tadpoles that had been exposed to tadpole alarm cues paired with newt odour (TP+NO), well water paired with newt odour (WW+NO) or well water only (WW) in the egg. Different shading patterns represent the responses of tadpoles from different clutches.
Amphibians are subject to a wide range of predators that include aquatic insects, fishes, other amphibians, snakes, wading birds and some mammals (Petranka 1998). The suite of predators present in a given pond at any one time may be unpredictable. Our data on R. sylvatica indicate that embryos can learn to recognize unfamiliar predators by associating the odour of the predator with a chemical alarm cue that is released from damaged conspecific tadpoles. Tadpoles that were exposed to predator-recognition training as embryos exhibited decreased activity when exposed to the novel predator as tadpoles whereas tadpoles that were not trained with the alarm cue did not. Such results could be explained by classical conditioning or sensitization to the introduction to new odours. However, we feel that classical conditioning is a more parsimonious explanation given that there was a single conditioning trial followed by a two-week delay between conditioning and testing; sensitization lasting longer than a few hours generally requires multiple conditioning events (Pinsker et al. 1973). The responses of tadpoles trained as embryos are similar to amphibians and other aquatic vertebrates that are exposed to predator-recognition training post-hatching (Woody & Mathis 1998; Brown 2003). Such learning is adaptive because trained individuals typically experience higher survival than individuals that are not trained (Mirza & Chivers 2001).
Predatory cues generally indicate threat levels at a given moment in time. Prey give immediate responses to these cues and then their behaviour gradually returns to pre-threat levels (Waldman 1982). When past or present threat levels are good predicators of future threat, then individuals that alter future behaviour based on past levels of threat should experience higher fitness. The ‘ghost of predation future’ (Kats & Dill 1998) may be an adaptive explanation for anxiety-like behaviour that is present post-hatching (or after birth) for individuals who received stressful stimuli as embryos.
Acknowledgments
Permission to collect ringed salamander eggs was provided by the Missouri Department of Conservation and salamander experimental protocols were approved by the Missouri State IACUC (no. 98H). Wood frog eggs were collected under Alberta Research Permit no. 06-067 and Collection Permit no. 23410, and wood frog experimental protocols were approved under the University of Saskatchewan Animal Care protocol no. 20060014.
We thank Missouri State University Department of Biology and the Natural Sciences and Engineering Research Council of Canada for funding.
References
- Brodman R, Jaskula J. Activity and microhabitat use during interactions among five species of pond-breeding salamander larvae. Herpetologica. 2002;58:346–354. doi:10.1655/0018-0831(2002)058[0346:AAMUDI]2.0.CO;2 [Google Scholar]
- Brown G.E. Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish. 2003;4:227–234. doi:10.1046/j.1467-2979.2003.00132.x [Google Scholar]
- Davis P.D, Glynn L.M, Schetter C.D, Hobel C, Chicz-Demet A, Sandman C.A. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Devel. Neurosci. 2005;27:299–305. doi: 10.1159/000086709. doi:10.1159/000086709 [DOI] [PubMed] [Google Scholar]
- Dugovic C, Maccari S, Weibel L, Turek F.W, Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. J. Neurosci. 1999;19:8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosner K.L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Harrison R.G. Yale University Press; New Haven, CT: 1969. Organization and development of the embryo. [Google Scholar]
- Hepper P.G. Fetal memory: does it exist? What does it do? Acta Pædiatr. Suppl. 1996;416:16–20. doi: 10.1111/j.1651-2227.1996.tb14272.x. doi:10.1111/j.1651-2227.1996.tb14272.x [DOI] [PubMed] [Google Scholar]
- Hepper P.G. Memory in utero? Develop. Med. Child Neurol. 1997;39:343–346. doi: 10.1111/j.1469-8749.1997.tb07442.x. [DOI] [PubMed] [Google Scholar]
- Hepper P.G, Waldman B. Embryonic olfactory learning in frogs. Q. J. Exp. Psychol. B. 1992;44:179–197. doi: 10.1080/02724999208250611. [DOI] [PubMed] [Google Scholar]
- Holomuzki J.R. Life history aspects of the predaceous diving beetle, Dytiscus dauricus (Gebler), in Arizona. Southwest. Nat. 1985;30:485–490. doi:10.2307/3671043 [Google Scholar]
- Kats L.B, Dill L.M. The scent of death: chemosensory assessment of predation risk by prey animals. Écoscience. 1998;5:361–394. [Google Scholar]
- Kawai N, Morokuma S, Tomonaga M, Horimoto N, Tanada M. Associative learning and memory in a chimpanzee fetus: learning and long-lasting memory before birth. Dev. Psychobiol. 2004;44:116–122. doi: 10.1002/dev.10160. doi:10.1002/dev.10160 [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous D.N. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl Acad. Sci. USA. 2000;97:11 032–11 037. doi: 10.1073/pnas.97.20.11032. doi:10.1073/pnas.97.20.11032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S.L, Bednekoff P.A. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 1999;153:649–659. doi: 10.1086/303202. doi:10.1086/303202 [DOI] [PubMed] [Google Scholar]
- Mathis A, Murray K.L, Hickman C.R. Do experience and body size play a role in responses of larval ringed salamanders, Ambystoma annulatum, to predator kairomones? Laboratory and field assays. Ethology. 2003;109:159–170. doi:10.1046/j.1439-0310.2003.00849.x [Google Scholar]
- Mirza, R. S. & Chivers, D. P. 2001 Do chemical alarm signals enhance survival in aquatic vertebrates? An analysis of the current research paradigm. In Chemical signals in vertebrates, vol. 9 (eds A. Marchlewska-Koj, J. J. Lepri & D. Müller-Schwarze), pp. 277–284. New York, NY: Plenum.
- Nyman S, Wilkinson R.F, Hutcherson J.E. Cannibalism and size relations in a cohort of larval ringed salamanders (Ambystoma annulatum) J. Herpetol. 1993;27:78–84. doi:10.2307/1564909 [Google Scholar]
- Orizaola G, Braña F. Response of predator-naive newt larvae to food and predator presence. Can. J. Zool. 2003;81:1845–1850. doi:10.1139/z03-160 [Google Scholar]
- Petranka J.W. Smithsonian Institution Press; Washington, DC: 1998. Salamanders of the United States and Canada. [Google Scholar]
- Petranka J, Hayes L. Chemically mediated avoidance of a predatory odonate (Anax junius) by American toad (Bufo americanus) and wood frog (Rana sylvatica) tadpoles. Behav. Ecol. Sociobiol. 1998;42:263–271. doi:10.1007/s002650050438 [Google Scholar]
- Pinsker H.M, Hening W.A, Carew T.J, Kandel E.R. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. doi:10.1126/science.182.4116.1039 [DOI] [PubMed] [Google Scholar]
- Saglio P, Mandrillon A.-L. Embryonic experience to predation risk affects tadpoles of the common frog (Rana temporaria) Archiv. Hydrobiol. 2006;166:505–523. doi:10.1127/0003-9136/2006/0166-0505 [Google Scholar]
- Salm A.K, Pavelko M, Krouse E.M, Webster W, Kraszpulski M, Birkle D.L. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Develop. Brain Res. 2004;148:159–167. doi: 10.1016/j.devbrainres.2003.11.005. doi:10.1016/j.devbrainres.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Sih A, Moore R.D. Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am. Nat. 1993;142:947–960. doi: 10.1086/285583. doi:10.1086/285583 [DOI] [PubMed] [Google Scholar]
- Skelly D.K. Activity level and the susceptibility of anuran larvae to predation. Anim. Behav. 1994;47:464–468. doi:10.1006/anbe.1994.1063 [Google Scholar]
- Sneddon H, Hadden R, Hepper P.G. Chemosensory learning in the chicken embryo. Physiol. Behav. 1998;64:133–139. doi: 10.1016/s0031-9384(98)00037-7. doi:10.1016/S0031-9384(98)00037-7 [DOI] [PubMed] [Google Scholar]
- Stangel P.W. Premetamorphic survival of the salamander Ambystoma maculatum in eastern Massachusetts. J. Herpetol. 1988;22:345–347. doi:10.2307/1564160 [Google Scholar]
- Uller T, Olsson M. Direct exposure to corticosterone during embryonic development influences behavior in an ovoviviparous lizard. Ethology. 2006;112:390–397. doi:10.1111/j.1439-0310.2006.01164.x [Google Scholar]
- Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J. Neurosci. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman B. Quantitative and developmental analyses of the alarm reaction in the zebra danio Brachydanio rerio. Copeia. 1982;1982:1–9. doi:10.2307/1444261 [Google Scholar]
- Watson S, Russell A.P. A posthatching developmental staging table for the long-toed salamander, Ambystoma macrodactylum krausei. Amphib.-Reptil. 2000;21:143–154. doi:10.1163/156853800507336 [Google Scholar]
- Woody D.R, Mathis A. Acquired recognition of chemical stimuli from an unfamiliar predator: associative learning by adult newts, Notophthalmus viridescens. Copeia. 1998;1998:1027–1031. doi:10.2307/1447352 [Google Scholar]