Abstract
Female choice can drive the evolution of extravagant male traits. In invertebrates, the influence of prior social experience on female choice has only recently been considered. To better understand the evolutionary implications of experience-mediated plasticity in female choice, we investigated the effect of acoustic experience during rearing on female responsiveness to male song in the field cricket Teleogryllus oceanicus. Acoustic experience has unique biological relevance in this species: a morphological mutation has rendered over 90 per cent of males on the Hawaiian island of Kauai silent in fewer than 20 generations, impeding females' abilities to locate potential mates. Females reared in silent conditions mimicking Kauai were less discriminating of male calling song and more responsive to playbacks, compared with females that experienced song during rearing. Our results to our knowledge, are the first demonstration of long-term effects of acoustic experience in an arthropod, and suggest that female T. oceanicus may be able to compensate for the reduced availability of long-range male sexual signals by increasing their responsiveness to the few remaining signallers. Understanding the adaptive significance of experience-mediated plasticity in female choice provides insight into processes that facilitate rapid evolutionary change and shape sexual selection pressure in natural populations.
Keywords: acoustic experience, mate choice, phenotypic plasticity, rapid evolution, sexual selection, Teleogryllus oceanicus
1. Introduction
Genetic models of sexual selection predict that female mate choice can drive the exaggeration of secondary sexual characters in males (Lande 1981; Parker 1983; Andersson 1994). However, a number of non-genetic factors affecting female choice have captured the attention of researchers owing to their potentially large effects on the strength and direction of sexual selection. These include mate search costs, female condition, predation risk, novel male effects and female search strategy (Janetos 1980; Bakker & Milinski 1991; Hedrick & Dill 1993; Widemo & Sæther 1999; Cotton et al. 2006). Prior social experience has also been shown to affect the outcome of female mate choice in vertebrates (Bakker & Milinski 1991; Rosenqvist & Houde 1997; Galef & White 2000; Slagsvold et al. 2002; Magurran & Ramnarine 2004), but this idea has only recently gained traction in invertebrate research (Wagner et al. 2001; Hebets 2003; Dukas 2005; Fincke et al. 2007; Hebets & Vink 2007).
Plasticity in female mate choice is expected to influence the rate of male trait evolution (Chaine & Lyon 2008), and the aim of our study was to better understand how social experience mediates such plasticity, thereby influencing evolutionary change in wild populations. We considered two unresolved questions. First, what properties of social experience affect female behaviour in the context of mate choice? Until now, it has been difficult to distinguish between tactile, olfactory, visual or auditory influences during pre-mating experience in invertebrates (Koudele et al. 1987; Hebets 2003; Fincke et al. 2007). Second, what are the evolutionary consequences if social experience does affect mate choice? To our knowledge, there are few clear examples of how experience-mediated plasticity in mate choice affects the evolutionary dynamics of a rapidly evolving natural system.
We capitalized on a study system in which prior social experience might have unusually significant evolutionary consequences. Male Polynesian field crickets, Teleogryllus oceanicus, normally produce a calling song by rubbing their forewings together. The call attracts females for mating. However, a morphological mutation has evolved in response to an acoustically orienting predator, the fly Ormia ochracea, and recently swept through a T. oceanicus population on the Hawaiian island of Kauai. In fewer than 5 years (approx. 16–20 generations), over 90 per cent of male crickets on Kauai now exhibit a flatwing morphology that renders them mute (Zuk et al. 2006; Tinghitella 2008). Lacking the wing structures normally used to produce sound, these males appear to adopt a satellite strategy by intercepting females responding to the few remaining callers (Cade 1980; Zuk et al. 2006). The mutation probably persists owing to the advantage silent flatwing males enjoy against the parasitoid fly. The increased abundance of flatwing males has rapidly and dramatically altered the acoustic environment that individuals on Kauai experience; silence or near-silence now predominates on Kauai, as the vast majority of males cannot produce song.
In this study, we investigated how females were able to accommodate the abrupt loss of the male sexual signal to which they normally respond. Male calling song is the only known signal available to females to locate sexually receptive males from a distance; gryllids do not produce long-range pheromones (Tregenza & Wedell 1997) and individuals are typically spaced at least 1 m apart in the field. The population continues to thrive, however. We hypothesized that the acoustic environment in which females develop mediates plasticity in female mate choice, and that this plasticity helped to accommodate the loss of male song. In the laboratory, we manipulated females' acoustic experience during rearing to test whether females reared in silent conditions mimicking Kauai would be more responsive to male song, better enabling them to locate the few singing males that remain or flatwing satellite males in close proximity.
2. Material and methods
(a) Study organisms
A T. oceanicus laboratory colony was established using eggs from approximately 25 females collected on Kauai in 2006. We allowed four generations to elapse prior to the start of the experiment to avoid maternal effects. All crickets were reared in silence in 15 l containers in a 25°C incubator until sex differences became apparent during their fourth instar, whereupon we transferred them to individual 118 ml plastic containers. Crickets were provided with Fluker's cricket food, Purina rabbit food and water twice weekly.
(b) Acoustic environments
We separated the fourth instar females into one of two acoustic environments: ‘No Song’ or ‘Song’. Each environment consisted of an identical 25°C Precision 818 incubator on the same photo-reversed 12 L : 12 D cycles.
In the No Song incubator, the individually housed females remained in silence until we tested them. In the Song incubator, females were individually housed in an identical fashion but were exposed to male calling song broadcast from Sony SRS-M30 speakers during the dark phase of the light : dark cycle, when T. oceanicus is active.
Teleogryllus oceanicus song contains a trill-like long chirp followed by a series of paired pulses; the proportion of long chirp in a song is an important parameter evaluated by females during mate choice (Simmons et al. 2001; Simmons 2004; Bailey 2008). In the Song incubator, we broadcast six song models containing 0, 20, 40, 60, 80 and 100 per cent long chirp, each from a separate speaker. We broadcast all six song models simultaneously to approximate a wild setting, in which females are exposed to variation in male calling song. Females' positions in the Song incubator were randomly rotated every day to ensure that they were exposed to all song models during rearing. Females in the No Song environment were similarly manipulated on a daily basis to minimize handling differences between the treatments.
The sound pressure level in the Song incubator was adjusted to 80–85 dB at the lid of the containers. A pilot study showed that the plastic lids have an acoustic impedance of 10 dB but do not affect the peak carrier frequency or temporal components of T. oceanicus song transmitted through them (see the electronic supplementary data). Thus, song intensities experienced by female crickets in the Song environment ranged from 70 to 75 dB, which are typical of a male cricket singing from a distance of 50 cm (Simmons et al. 2001).
We took measures to decrease the likelihood that micro-environmental properties of the incubators differentially affected female behaviour. Incubators were cleaned with 10 per cent bleach solution before the experiment to eliminate residual pathogens. The incubators produced the same level of background white noise (53 dB). Each female was individually housed, and therefore separated from the environment within the incubator, with the exception of acoustic cues and the small amount of air that passed through the holes in the container lids. Finally, a previous study assessed mate choice behaviour in the same population, using females reared in either of the incubators (n=10 for each incubator; Bailey 2008). None of the components of mate choice (discrimination, responsiveness and preference) differed between the two incubators (all p>0.11).
(c) Time frame
Female T. oceanicus become sexually responsive 4–5 days after eclosion (N. Bailey 2008, personal observation). To gauge female responsiveness during the most biologically realistic time frame possible and avoid age-related variation in responsiveness (Prosser et al. 1997; Gray 1999), we tested all adult females at 6 days post-eclosion. Testing all females at an early stage also diminished the possibility of confounding habituation effects: females in the Song environment might show reduced response effort in subsequent tests, if they had grown accustomed to being unable to contact singing males. We minimized any habituation effects by performing phonotaxis trials just after the onset of sexual receptivity (Sakaluk 1982). Finally, the effects of recent exposure to song, ‘prior male’ effects, have been documented in other cricket species, and can influence subsequent mating decisions at least 20 min after exposure (Wagner et al. 2001). We therefore removed females from the Song environment and kept them acoustically isolated for a single light cycle; all crickets experienced a minimum of 16 hours of undisturbed silence prior to testing.
(d) Phonotaxis trials
We assessed female responsiveness by conducting a single phonotaxis trial at 25°C under red light with each female. We tested 150 females from the No Song treatment and 150 females from the Song treatment, and within each treatment, we tested 25 females with each song model. The 0 and 100 per cent song models exceed the normal range of variation in the wild, but were included to test the entire range of possible male trait values. Females were tested only once. The cross-sectional design mitigated the known effects of immediate prior acoustic experience on female phonotaxis behaviour that would have confounded our measures of responsiveness if we repeatedly tested each individual (Wagner et al. 2001).
A single female was placed 293 cm from the end of a rectangular chamber lined with acoustic foam (305×28×29 cm), and a plastic container 11 cm in diameter was carefully inverted over her. She was allowed to rest in silence for 2 min, after which we simultaneously lifted the container and began playback of one of the six song models from a speaker at the opposite end of the chamber. The playback intensity was 60 dB at the point of release, which approximated the intensity of a cricket singing at the distance of the speaker. Disturbance to females was negligible during all portions of the trial, which lasted 5 min.
We measured the following four characteristics of female responsiveness: (i) latency to initiate any walking movement after playback began, (ii) whether or not a female moved towards and touched the speaker at the opposite end of the chamber during the 5 min (positive versus negative response), (iii) response time if the response was positive and (iv) distance from the playback speaker at the end of the trial if the response was negative. Females that settled closer to a singing male may be more likely to eventually respond to him in wild situation, where they are not limited to a 5 min response period, and may also encounter flatwing satellites that have responded to the singing male.
(e) Analysis
We tested the effects of acoustic experience and song model on the proportion of females responding positively to playbacks using a three-way G-test of independence (Sokal & Rohlf 1969). General linear models (GLMs) were used to test the effects of acoustic environment on movement latency in all crickets (n=276), response latency in those that positively responded (n=187) and distance moved in those that did not (n=113). Size did not covary with any of the measures of responsiveness (all p≥0.365), so we did not include it as a covariate in the analyses. All distance and latency data were natural log transformed to ensure normality. Analyses were performed in Minitab v. 12.21 and Jmp v. 6.0.
3. Results
(a) Acoustic experience decreased responsiveness
Females from Kauai reared in a silent environment mimicking the current situation in the field were more responsive to male song than females from the same population reared in an environment in which they were exposed to biologically realistic levels of male calling song (tables 1 and 2, figures 1–3).
Table 1.
Three-way G-test of independence exploring how acoustic rearing environment (Song versus No Song) and playback song model affected the proportion of females exhibiting positive phonotactic responses. (Significant p-values are indicated in italics.)
| d.f. | G | p | |
|---|---|---|---|
| overall G | 16 | 50.06 | <0.001 |
| acoustic environment | 1 | 33.63 | <0.001 |
| song model | 5 | 7.06 | 0.008 |
| acoustic environment×song model | 5 | 8.88 | 0.114 |
Table 2.
GLMs exploring how acoustic rearing environment (Song versus No Song), playback song model and the interaction between the two affected female crickets' response latency, time of first movement and distance to the speaker. (Significant p-values are indicated in italics.)
| d.f. | F | p | |
|---|---|---|---|
| response latency | |||
| acoustic environment | 1 | 10.15 | 0.002 |
| song model | 5 | 0.85 | 0.514 |
| acoustic environment×song model | 5 | 2.95 | 0.027 |
| error | 175 | ||
| time of first movement | |||
| acoustic environment | 1 | 4.83 | 0.029 |
| song model | 5 | 2.15 | 0.060 |
| acoustic environment×song model | 5 | 1.50 | 0.190 |
| error | 264 | ||
| distance to speaker | |||
| acoustic environment | 1 | 3.71 | 0.057 |
| song model | 5 | 1.08 | 0.378 |
| acoustic environment×song model | 5 | 0.31 | 0.908 |
| error | 101 |
Figure 1.
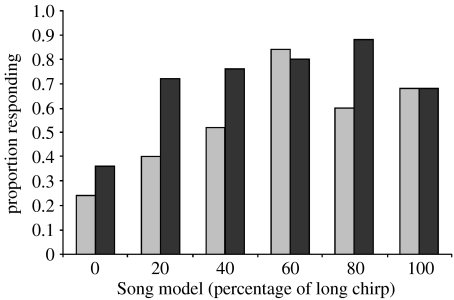
Likelihood that females positively responded to calling song playbacks. Females exposed to song (Song) during rearing are represented by grey bars, and those reared in silence (No Song) by black bars. Each female was tested with one of six song models varying in the proportion of long chirp (0–100% long chirp); a positive response was scored if the female moved to the end of the testing arena and touched the playback speaker before the end of the 5 min trial.
Figure 2.
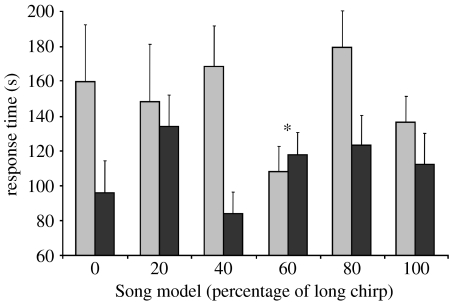
Female response latencies. Females exposed to song (Song) during rearing are represented by grey bars, and those reared in silence (No Song) by black bars. Response latency directly reflects the amount of time a female takes to reach an acoustic stimulus starting from the time it is perceived; females with shorter response latencies are interpreted to be more responsive. The asterisk highlights the 60 per cent long-chirp song model that has been shown to be the most preferred song model in population-wide preference tests (Bailey 2008). Bars indicate 1 s.e.
Figure 3.
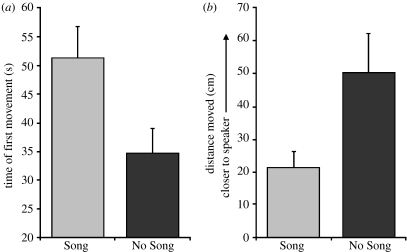
(a) Latency of the first movement for all crickets tested and (b) distance moved from the starting point for all females that did not respond positively during the trial. Bars indicate 1 s.e.
All aspects of female responsiveness were affected in a parallel manner by acoustic experience: silent rearing conditions increased both the likelihood and speed of females moving to a source of male calling song. Overall, females were less likely to respond positively to a playback if they had been exposed to song during rearing (table 1, figure 1). Of the females that did respond, those exposed to song during rearing took longer to reach the speaker during playbacks (table 2, figure 2). Crickets also took longer to initiate walking behaviour, if they had experienced song during rearing, and of the females that did not respond positively during playback tests, those reared in silence showed a marginally non-significant trend towards moving closer to the speaker by the end of their 5 min trial, compared to those exposed to song during rearing (table 2, figure 3).
(b) Acoustic experience increased discrimination in responding females
Females exposed to song during rearing were more discriminating of male song. A significant interaction between song model and acoustic environment indicated that the effect of acoustic environment on response latency was not consistent for all song models (GLM: F5,175=2.95, p=0.027). Overall, females previously experiencing song showed reduced response latencies compared to those reared in silence, but females from both treatments responded rapidly when presented with the one song model that is known from previous work to be the most preferred by Kauai females: the 60 per cent long chirp model (Bailey 2008; figure 2). A post hoc comparison revealed that the 60 per cent long chirp model significantly contributed to the interaction between acoustic environment and song model (t5,175=2.56, p=0.011). Thus, females responded rapidly to the most preferred song, regardless of their acoustic experience. The proportion of females from the two acoustic environment treatments that positively responded to playbacks did not differ depending upon the song model tested (G-test, G5=8.88, p=0.114).
4. Discussion
Acoustic experience in the absence of any other social cues was sufficient to alter female mate choice in T. oceanicus. Females that heard male calling song during rearing were less responsive to song during playback tests, but exercised greater discrimination. Several authors have advanced the idea that experience with potential mates may actually be required for the choosy sex to develop preferences. For instance, in a mixed population of brush-legged and non-ornamented wolf spiders (genus Schizocosa), juvenile females exposed to either type of male later displayed strong preferences for brush-legged males, whereas unexposed females showed no preference (Hebets & Vink 2007). Similarly, male damselflies (Enallagma civile) showed learned sensory biases towards female morphs to which they had been previously exposed, whereas those reared in solitude showed no preference (Fincke et al. 2007).
Here we used six different song models to gauge whether or not the effect of acoustic environment was consistent across all values of the male trait, potentially indicating interactions between acoustic rearing environment and mating preferences exerted by females. The models varied in a single parameter that has been demonstrated to be important in T. oceanicus mating decisions (Simmons et al. 2001). Females from both treatments responded rapidly when presented with the one song model that is known from previous work to be the most preferred by females from this population (Bailey 2008; figure 2). Thus, females experiencing song during rearing were slower to respond to male song, unless it was the most preferred song. By contrast, females reared in silence did not appear to discriminate as strongly against non-preferred songs. This corroborates Hebets & Vink's (2007) observation, but instead of acoustic experience driving the development of preference, it seems more plausible that the lack of experience drives indiscriminate mating responses in T. oceanicus. Females may have pre-existing preferences, but only exercise them when they perceive that enough males are available to choose from, by altering their threshold of mate acceptance or by sampling more males, depending upon their search strategy.
The adaptive significance of behavioural plasticity has often been considered in the context of coping with a new or abruptly altered environment (Losos et al. 2000; Price et al. 2003; Ghalambor et al. 2007). Selection is expected to favour individuals that can accommodate variation in environmental constraints and selection pressures through behavioural flexibility or learning (Wcislo 1989; West-Eberhard 2003). On Kauai, the population of field crickets is unique because their environment changed as a direct result of a phenotypic modification of the crickets themselves. Evidence from our laboratory study strongly supports the idea that phenotypic plasticity in mate choice, mediated solely by the acoustic environment, allowed female crickets to compensate for the reduced availability of singing males on Kauai by responding more readily, more quickly, and with less discrimination to the few calling males that remain in the population. As silent flatwing males appear to act as satellites to the few remaining callers (Zuk et al. 2006), females responding to the same signal are more likely to encounter and mate with a silent flatwing or the calling male himself.
The future trajectory of the Kauai population remains to be seen. Theoretical and empirical studies have shown that female responsiveness can affect the strength of sexual selection on male traits (Lande 1981; Butlin 1993; Ritchie et al. 2005), and the relative strength of sexual selection on male song exerted by female choice may be relaxed in the Kauai population owing to indiscriminately responding females. In addition, declines in one component of female choice, such as responsiveness, may diminish the effects of other components such as preference or discrimination (Gilburn & Day 1999; Bailey 2008). If negative frequency-dependent selection on the male morph leads to fluctuations in the proportion of flatwings in the population, then the acoustic environment females perceived in the field will similarly fluctuate. Since acoustic experience during rearing appears to shape female choice, sexual selection on male song may be periodically relaxed, in turn maintaining greater genetic variation for male song than would be expected in a normal population. A recent study of lark buntings supports this idea. Chaine & Lyon (2008) found that temporal flexibility in female preferences was adaptive, and dampened sexual selection on male traits. The potential for predictable changes in sexual selection pressure on male calling song makes continued monitoring of the T. oceanicus population on Kauai a priority for future studies.
It is somewhat surprising that acoustic rearing environment in and of itself was sufficient to modify female behaviour in T. oceanicus. Mating history and olfactory cues have been shown to affect female responses (Cade 1979; Koudele et al. 1987; Bateman 2001; Bateman et al. 2004), but to our knowledge, our results are the first demonstration that acoustic stimuli produce long-term effects on mate choice in an insect. Acoustic experience affected female responsiveness well after the completion of a full light cycle (over 16 hours), suggesting that acoustic effects persist beyond the previously recognized ‘prior male’ effects in other invertebrates (Wagner et al. 2001). Complex learning and memory capabilities are thoroughly documented in many insect groups, especially in the context of other behaviours such as foraging (Dukas 2004, 2005; Worden & Papaj 2005), and our results open the door to areas of future research investigating the behavioural basis of rapid evolution and the maintenance of genetic variation in traits under strong sexual selection.
Acknowledgements
This work was funded by NSF grants to M.Z. and support from the UC Riverside Academic Senate. We are grateful to M. Ritchie for helpful comments on earlier drafts of the manuscript. A. Nguyen, R. Nanda, P. Rueda and C. Switzer were of great assistance in rearing crickets and performing phonotaxis trials. We thank R. Tinghitella for helping to collect crickets in the field and for her statistical acumen.
Supplementary Material
Results of a pilot study showing acoustic properties of songs recorded outside and inside the containers used to house female crickets
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bailey N.W. Love will tear you apart: different components of female choice exert contrasting selection pressures on male field crickets. Behav. Ecol. 2008;19:960–966. doi:10.1093/beheco/arn054 [Google Scholar]
- Bakker T.C.M, Milinski M. Sequential female choice and the previous male effect in sticklebacks. Behav. Ecol. Sociobiol. 1991;29:205–210. doi:10.1007/BF00166402 [Google Scholar]
- Bateman P.W. Changes in phonotactic behavior of a bushcricket with mating history. J. Insect. Behav. 2001;14:333–343. doi:10.1023/A:1011167128430 [Google Scholar]
- Bateman P.W, Ferguson J.W.H, Ferreira M. The influence of physical and acoustic experience on sequential mate preference in the cricket Gryllus bimaculatus. Is song important? J. Insect. Behav. 2004;17:843–855. doi:10.1023/B:JOIR.0000048993.21896.23 [Google Scholar]
- Butlin R.K. The variability of mating signals and preferences in the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae) J. Insect. Behav. 1993;6:125–140. doi:10.1007/BF01051499 [Google Scholar]
- Cade W.H. Effect of male-deprivation on female phonotaxis in field crickets (Orthoptera: Gryllidae; Gryllus) Can. Entomol. 1979;111:741–744. [Google Scholar]
- Cade W.H. Alternative male reproductive behaviors. Fla. Entomol. 1980;63:30–45. doi:10.2307/3494654 [Google Scholar]
- Chaine A.S, Lyon B.E. Adaptive plasticity in female choice dampens sexual selection on male ornaments in the lark bunting. Science. 2008;319:459–462. doi: 10.1126/science.1149167. doi:10.1126/science.1149167 [DOI] [PubMed] [Google Scholar]
- Cotton S, Small J, Pomiankowski A. Sexual selection and condition-dependent mate preferences. Curr. Biol. 2006;16:R755–R765. doi: 10.1016/j.cub.2006.08.022. doi:10.1016/j.cub.2006.08.022 [DOI] [PubMed] [Google Scholar]
- Dukas R. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Syst. 2004;35:347–374. doi:10.1146/annurev.ecolsys.35.112202.130152 [Google Scholar]
- Dukas R. Learning affects mate choice in female fruit flies. Behav. Ecol. 2005;16:800–804. doi:10.1093/beheco/ari057 [Google Scholar]
- Fincke O.M, Fargevieille A, Schultz T.D. Lack of innate preference for morph and species identity in mate-searching Enallagma damselflies. Behav. Ecol. Sociobiol. 2007;61:1121–1131. doi:10.1007/s00265-006-0345-3 [Google Scholar]
- Galef B.G, White D.J. Evidence of social effects on mate choice in vertebrates. Behav. Process. 2000;51:1–3. doi: 10.1016/s0376-6357(00)00126-1. doi:10.1016/S0376-6357(00)00126-1 [DOI] [PubMed] [Google Scholar]
- Ghalambor C.K, McKay J.K, Carroll S.P, Reznick D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007;21:394–407. doi:10.1111/j.1365-2435.2007.01283.x [Google Scholar]
- Gilburn A.S, Day T.H. Female mating behaviour, sexual selection and chromosome I inversion karyotype in the seaweed fly, Coelopa frigida. Heredity. 1999;82:276–281. doi: 10.1038/sj.hdy.6884830. doi:10.1038/sj.hdy.6884830 [DOI] [PubMed] [Google Scholar]
- Gray D.A. Intrinsic factors affecting female choice in house crickets: time cost, female age, nutritional condition, body size, and size-relative reproductive investment. J. Insect. Behav. 1999;12:691–700. doi:10.1023/A:1020983821436 [Google Scholar]
- Hebets E.A. Subadult experience influences adult mate choice in an arthropod: exposed female wolf spiders prefer males of a familiar phenotype. Proc. Natl Acad. Sci. USA. 2003;100:13 390–13 395. doi: 10.1073/pnas.2333262100. doi:10.1073/pnas.2333262100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebets E.A, Vink C.J. Experience leads to preference: experienced females prefer brush-legged males in a population of syntopic wolf spiders. Behav. Ecol. 2007;18:1010–1020. doi:10.1093/beheco/arm070 [Google Scholar]
- Hedrick A.V, Dill L.M. Mate choice by females is influenced by predation risk. Anim. Behav. 1993;46:193–196. doi:10.1006/anbe.1993.1176 [Google Scholar]
- Janetos A.C. Strategies of female mate choice: a theoretical analysis. Behav. Ecol. Sociobiol. 1980;7:107–112. doi:10.1007/BF00299515 [Google Scholar]
- Koudele K, Stout J.F, Reichert D. Factors which influence female crickets' (Acheta domesticus) phonotactic and sexual responsiveness to males. Physiol. Entomol. 1987;12:67–80. doi:10.1111/j.1365-3032.1987.tb00725.x [Google Scholar]
- Lande R. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. doi:10.1073/pnas.78.6.3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos J.B, Creer D.A, Glossip D, Goellner R, Hampton A, Roberts G, Haskell N, Taylor P, Ettling J. Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution. 2000;54:301–305. doi: 10.1111/j.0014-3820.2000.tb00032.x. doi:10.1554/0014-3820(2000)054[0301:EIOPPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Magurran A.E, Ramnarine I.W. Learned mate recognition and reproductive isolation in guppies. Anim. Behav. 2004;67:1077–1082. doi:10.1016/j.anbehav.2003.10.010 [Google Scholar]
- Parker G.A. Mate quality and mating decisions. In: Bateson P, editor. Mate choice. Cambridge University Press; Cambridge, UK: 1983. pp. 141–166. [Google Scholar]
- Price T.D, Qvarnstrom A, Irwin D.E. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. B. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. doi:10.1098/rspb.2003.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser M.R, Murray A.M, Cade W.H. The influence of female age on phonotaxis during single and multiple song presentations in the field cricket, Gryllus integer (Orthoptera: Gryllidae) J. Insect. Behav. 1997;10:437–450. doi:10.1007/BF02765609 [Google Scholar]
- Ritchie M.G, Saarikettu M, Hoikkala A. Variation, but no covariance, in female preference functions and male song in a natural population of Drosophila montana. Anim. Behav. 2005;70:849–854. doi:10.1016/j.anbehav.2005.01.018 [Google Scholar]
- Rosenqvist G, Houde A. Prior exposure to male phenotypes influences mate choice in the guppy, Poecilia reticulate. Behav. Ecol. 1997;8:194–198. doi:10.1093/beheco/8.2.194 [Google Scholar]
- Sakaluk S.K. Onset of phonotaxis and age at first mating in female house crickets, Acheta domesticus (Orthoptera: Gryllidae) J. NY Entomol. Soc. 1982;90:136–141. [Google Scholar]
- Slagsvold T, Hansen B.T, Johannessen L.E, Lifjeld J.T. Mate choice and imprinting in birds studied by cross-fostering in the wild. Proc. R. Soc. B. 2002;269:1449–1455. doi: 10.1098/rspb.2002.2045. doi:10.1098/rspb.2002.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L.W. Genotypic variation in calling song and female preferences of the field cricket Teleogryllus oceanicus. Anim. Behav. 2004;68:313–322. doi:10.1016/j.anbehav.2003.12.004 [Google Scholar]
- Simmons L.W, Zuk M, Rotenberry J.T. Geographic variation in female preference functions and male songs of the field cricket Teleogryllus oceanicus. Evolution. 2001;55:1386–1394. doi: 10.1111/j.0014-3820.2001.tb00660.x. doi:10.1554/0014-3820(2001)055[1386:GVIFPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. W. H. Freeman and Company; San Francisco, CA: 1969. Biometry. [Google Scholar]
- Tinghitella R.M. Rapid evolutionary change in a sexual signal: genetic control of the mutation ‘flatwing’ that renders male field crickets (Teleogryllus oceanicus) mute. Heredity. 2008;100:261–267. doi: 10.1038/sj.hdy.6801069. doi:10.1038/sj.hdy.6801069 [DOI] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Definitive evidence for cuticular pheromones in a cricket. Anim. Behav. 1997;54:979–984. doi: 10.1006/anbe.1997.0500. doi:10.1006/anbe.1997.0500 [DOI] [PubMed] [Google Scholar]
- Wagner W.E, Smeds M.R, Wiegmann D.D. Experience affects female responses to male song in the variable field cricket Gryllus lineaticeps (Orthoptera, Gryllidae) Ethology. 2001;107:769–776. doi:10.1046/j.1439-0310.2001.00700.x [Google Scholar]
- Wcislo W.T. Behavioral environments and evolutionary change. Annu. Rev. Ecol. Syst. 1989;20:137–169. doi:10.1146/annurev.es.20.110189.001033 [Google Scholar]
- West-Eberhard M.J. Oxford University Press; Oxford, UK: 2003. Developmental plasticity and evolution. [Google Scholar]
- Widemo F, Sæther S.A. Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends Ecol. Evol. 1999;14:26–31. doi: 10.1016/s0169-5347(98)01531-6. doi:10.1016/S0169-5347(98)01531-6 [DOI] [PubMed] [Google Scholar]
- Worden B.D, Papaj D.R. Flower choice copying in bumble-bees. Biol. Lett. 2005;1:504–507. doi: 10.1098/rsbl.2005.0368. doi:10.1098/rsbl.2005.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M, Rotenberry J.T, Tinghitella R.M. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2006;2:521–524. doi: 10.1098/rsbl.2006.0539. doi:10.1098/rsbl.2006.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of a pilot study showing acoustic properties of songs recorded outside and inside the containers used to house female crickets