Abstract
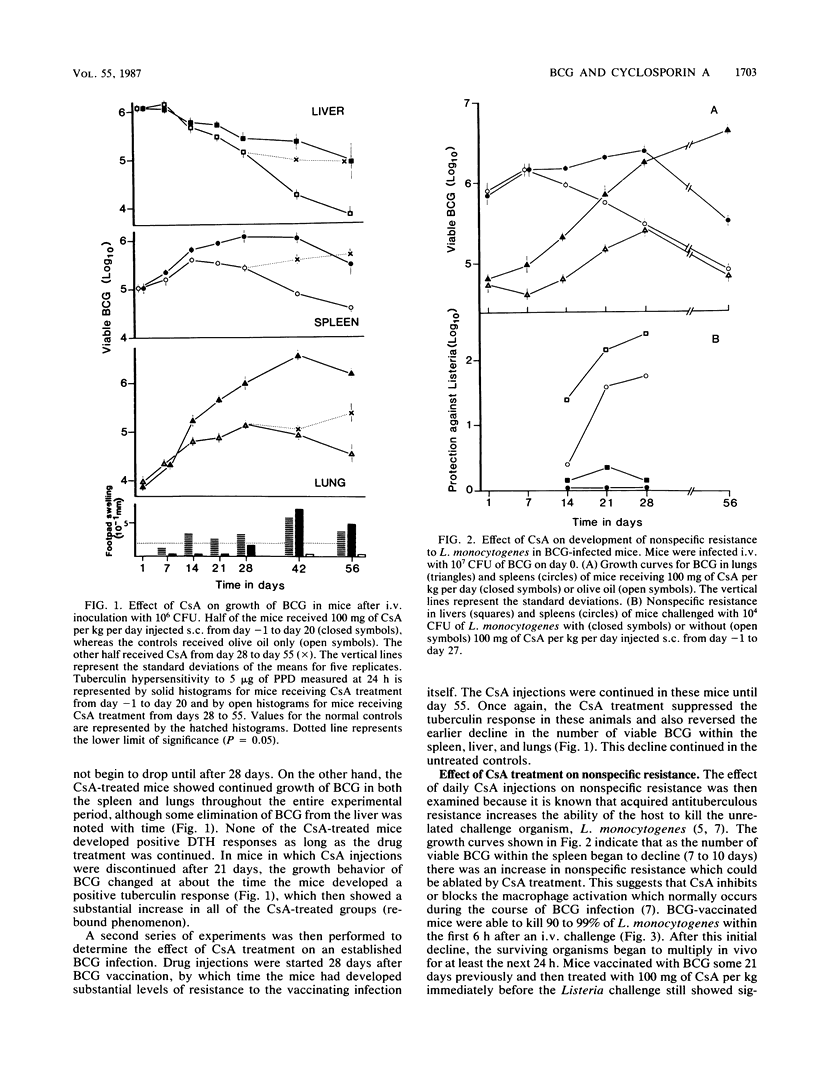
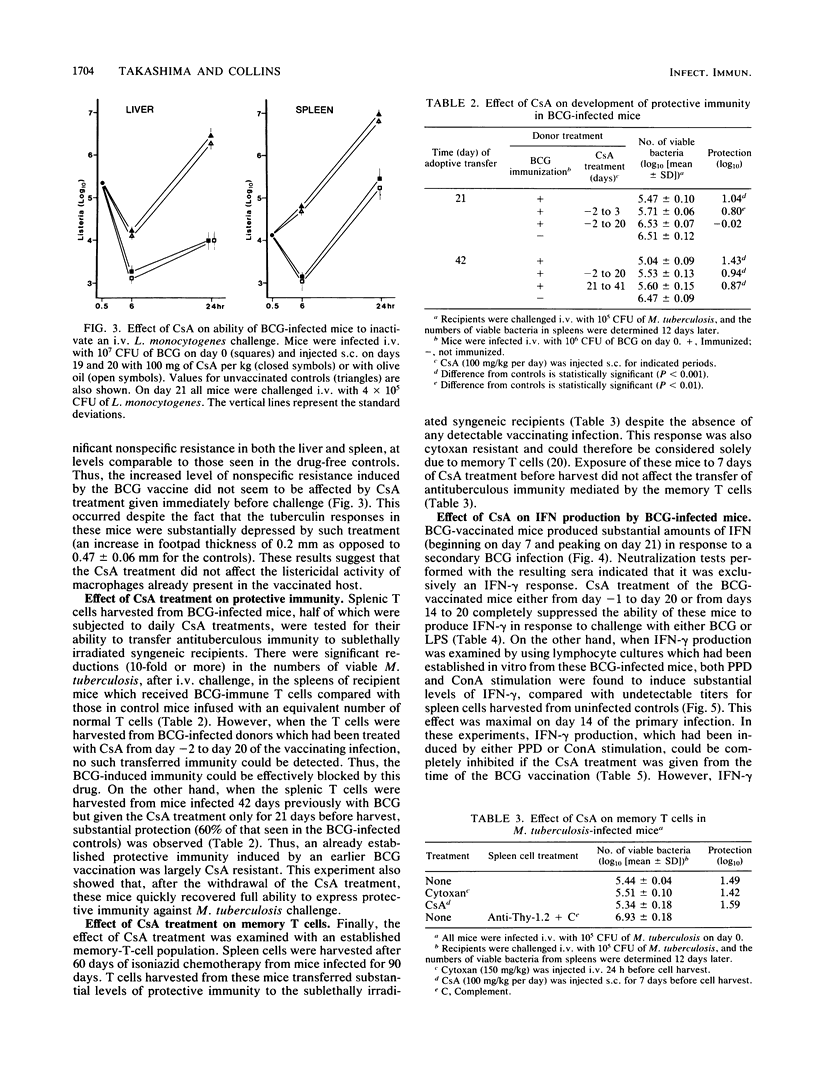
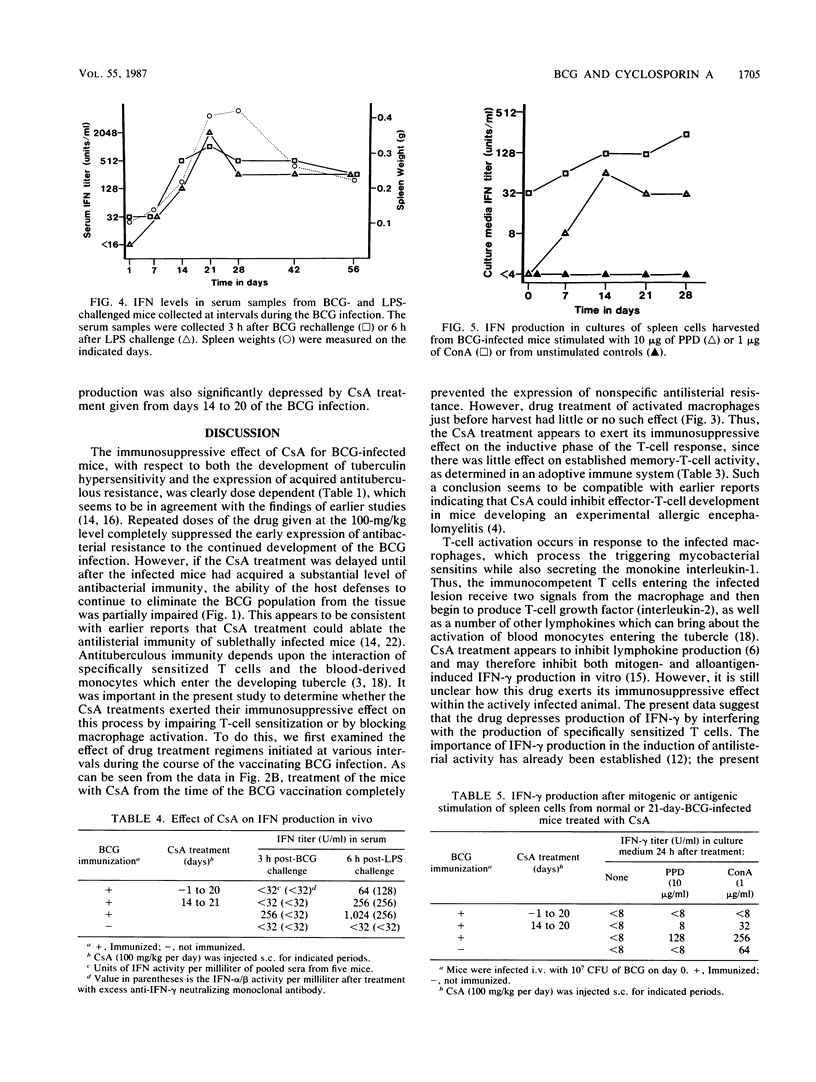
The effect of increasing doses of cyclosporin A (CsA) given to mice infected intravenously with Mycobacterium bovis BCG was investigated. Development of both tuberculin hypersensitivity and acquired antituberculous resistance was suppressed in a dose-responsive manner. Daily dosages at 100 mg/kg of body weight prevented any reduction in the BCG counts within the lungs, liver, or spleen. This effect was associated with lowered nonspecific resistance to a Listeria monocytogenes challenge and a decline in specific protective immunity adoptively transferred to naive recipients. CsA treatment had no effect on antilisterial activity by activated macrophages or on the antituberculous immunity expressed by specific memory T cells. CsA treatment inhibited the ability of BCG-vaccinated mice to produce gamma interferon (IFN-gamma) after a secondary stimulation with live BCG or with lipopolysaccharide. Spleen cells from BCG-infected mice which were exposed to daily treatment with CsA showed reduced IFN-gamma production in response to purified protein derivative or concanavalin A stimulation, suggesting that the immunosuppressive effect of CsA on BCG-infected mice was expressed by inhibiting the development of effector T cells responsible for the production of IFN-gamma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti S., Boraschi D., Luini W., Tagliabue A. Effects of in vivo treatments with cyclosporin-A on mouse cell-mediated immune responses. Int J Immunopharmacol. 1981;3(4):357–364. doi: 10.1016/0192-0561(81)90031-x. [DOI] [PubMed] [Google Scholar]
- Berger R., Meingassner J. G., Knapp W. In vitro effects of cyclosporin A on human B-cell responses. Scand J Immunol. 1983 Mar;17(3):241–249. doi: 10.1111/j.1365-3083.1983.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton C., Allsopp G., Cuzner M. L. The effect of cyclosporin A on the adoptive transfer of experimental allergic encephalomyelitis in the Lewis rat. Clin Exp Immunol. 1982 Jan;47(1):127–132. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M. The relative immunogenicity of virulent and attenuated strains of tubercle bacilli. Am Rev Respir Dis. 1973 Jun;107(6):1030–1040. doi: 10.1164/arrd.1973.107.6.1030. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Wayne L. G., v Montalbine The effect of cultural conditions on the distribution of Mycobacterium tuberculosis in the spleens and lungs of specific pathogen-free mice. Am Rev Respir Dis. 1974 Aug;110(2):147–156. doi: 10.1164/arrd.1974.110.2.147. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Curtis J., Turk J. L. The effect of hydrocortisone and cyclosporin A on bacillus Calmette-Guérin epitheloid cell granulomas. Cell Immunol. 1985 Jun;93(1):189–198. doi: 10.1016/0008-8749(85)90399-5. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Augmented induction of interferons during Listeria monocytogenes infection. J Infect Dis. 1986 May;153(5):960–969. doi: 10.1093/infdis/153.5.960. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L., Patel P. J. Enhanced production of murine interferon gamma by T cells generated in response to bacterial infection. J Exp Med. 1982 Jul 1;156(1):112–127. doi: 10.1084/jem.156.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huegin A. W., Cerny A., Hengartner H., Zinkernagel R. M. Suppression by cyclosporin A of murine T-cell-mediated immunity against viruses in vivo and in vitro. Cell Immunol. 1985 Feb;90(2):464–473. doi: 10.1016/0008-8749(85)90211-4. [DOI] [PubMed] [Google Scholar]
- Hügin A. W., Cerny A., Wrann M., Hengartner H., Zinkernagel R. M. Effect of cyclosporin A on immunity to Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):12–17. doi: 10.1128/iai.52.1.12-17.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman V. K., Klimpel G. R. Cyclosporin A inhibits the production of gamma interferon (IFN gamma), but does not inhibit production of virus-induced IFN alpha/beta. Cell Immunol. 1983 May;78(1):122–129. doi: 10.1016/0008-8749(83)90265-4. [DOI] [PubMed] [Google Scholar]
- Kunkl A., Klaus G. G. Selective effects of cyclosporin A on functional B cell subsets in the mouse. J Immunol. 1980 Dec;125(6):2526–2531. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Crossprotection against nontuberculous mycobacterial infections by Mycobacterium tuberculosis memory immune T lymphocytes. J Exp Med. 1986 Jan 1;163(1):203–208. doi: 10.1084/jem.163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Cook L. A., Vilcek J. Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science. 1983 Jul 1;221(4605):63–65. doi: 10.1126/science.6407112. [DOI] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Davis C. E. Models of T cell deficiency in listeriosis: the effects of cortisone and cyclosporin A on normal and nude BALB/c mice. J Immunol. 1983 Jul;131(1):450–453. [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]



