Abstract
Many traits in animals reduce the rate of attack from visually hunting predators, including camouflage, warning signals and mimicry. In addition, some animal markings may reduce the likelihood that an attack ends in successful capture. These might include dazzle markings, high-contrast patterns that make the estimation of speed and trajectory difficult. However, until now, no study has experimentally tested whether some markings may achieve such an effect. We developed a computer ‘game’ where human ‘predators’ have to capture computer-generated prey moving across a background. In two experiments, we find that although uniform camouflaged targets were among the hardest to capture, so were a range of high-contrast conspicuous patterns, such as bands and zigzags. Prey were also more difficult to capture against more heterogeneous than uniform backgrounds, and at faster speeds of movement. As such, we find the first experimental evidence that conspicuous patterns, similar to those found in a wide range of real animals, make the capture of moving prey more challenging. Various anti-predator markings may work prey during motion, and some animals may combine such dazzle patterns with other functions, such as camouflage, thermoregulation, sexual and warning signals.
Keywords: protective coloration, motion, conspicuousness, vision, predation, dazzle
1. Introduction
Animal markings result from multiple selection pressures (Stevens 2007), generally including the reduction of predation risk (Ruxton et al. 2004). Visual signals encode a variety of information in motion, form and texture (Rosenthal 2007). As such, some markings may prevent predators from accurately judging the speed and trajectory of their prey, making effective tracking and thus capture difficult (Stevens 2007). Indeed, it is likely that many markings on animals have been influenced by selection to avoid capture when moving, especially because animals are often easiest to detect when in motion (Hailman 1977).
Thayer (1909) predicted that certain arrangements of markings on animals would make estimates of speed and trajectory difficult, an idea used by the military, with dazzle markings once common on ships during war times (Behrens 1999). In nature, ‘motion dazzle’ markings are essentially high-contrast anti-predator patterns, which may prevent predators from accurately judging the speed and trajectory of a moving prey item. It is often argued that the markings on some animals may create visual illusions when the animals are moving, which interfere with motion detection mechanisms in vertebrate predators (Jackson et al. 1976; Brodie 1992; Shine & Madsen 1994).
In humans, there is good evidence that a range of target and background features can affect the perception of motion, including stimulus contrast (Thompson & Stone 1997; Anstis 2003), and background texture (Blakemore & Snowden 2000). There are various possibilities as to how dazzle markings may impair speed and direction estimates in predators, although these are currently largely speculative (see §4). A range of stripes, bands and zigzag patterns are widespread in nature, occurring most often in reptiles (Jackson et al. 1976), mammals (Ruxton 2002), fishes (Marshall 2000) and some insects. It is possible that these may have a motion dazzle function. However, little research has focused on anti-predator markings and prey motion, with no study experimentally testing whether some markings do make prey capture more difficult than others. The only evidence for such an idea stems from correlation analyses between the life history of certain snake species and their coloration type (e.g. Jackson et al. 1976; Brodie 1992), plus some anecdotal accounts. For example, Jackson et al. (1976) noted that they had observed in the snake Chionactis occipitalis the appearance of the bands on the snakes moving in the opposite direction of the true movement. Overall, there is no experimental evidence that markings common in animals have a dazzle effect, and research on anti-predator coloration has focused almost exclusively on static prey. Essentially, almost nothing is known about how animal coloration may be affected by selection to avoid the accurate tracking of speed and direction by predators.
We investigated whether some markings make it more difficult for human ‘predators’ to capture moving prey, and whether any effects were influenced by the complexity of the background and the speed of the prey. Using humans to assess the markings of real animals is often inappropriate due to between-species differences in visual perception (Stevens 2007). However, humans can be highly effective in deriving general principles about animal coloration when using set-ups and stimuli designed specifically to be presented to people. This has been effective, for example, in research into the evolution of aposematism and locomotory behaviour (e.g. Sherratt et al. 2004). Even more relevant to our study, humans charged with ‘capturing’ snake-like computer images have been used to argue that prey speed scaled to body length is a better measure of vulnerability to predation than absolute speed (Van Damme & Van Dooren 1999). Furthermore, vertebrates share many common features of spatial vision, and so the general principles should be widely applicable. Our experimental system avoided the ethical concerns surrounding staged predation experiments and allowed us to compare the effects of patterning in prey that were otherwise identical. Experiments using real prey would almost certainly introduce confounding factors through variation between morphs in, for example, behaviour or value to predators.
2. Material and methods
(a) General methods
We created a computer ‘game’ in the software Scratch (2007), whereby a single achromatic prey item at a time moved across a grey-scale display (28 cm wide by 21 cm tall) at a constant speed. Prey would unpredictably change direction (how often depended on how long each target was displayed without capture and the number of times it reached the display edge) between 1° and 3° clockwise as they moved, and would bounce back off the edges of the screen in a trajectory according to that which they hit the display edge, with the addition of a 2° anticlockwise turn. Although these changes in movement direction seem small, they made it more difficult to predict exactly where the prey would move, making prey capture a challenge. Participants caught a prey item by clicking on them with the mouse (with the cursor marked by a red cross on the screen), following which the prey would disappear and, after a delay of 0.5 s, reappear on the screen in a random position.
In both experiments, prey were 3 cm long and 1.2 cm tall (2.86×1.15° of visual angle subtended on the viewer's eye) targets. Prey were presented one at a time, appearing from a random position. Within a single trial, individuals had to capture as many prey items of a given type within 1 min. This was repeated for each combination of prey and background/speed (labelled ‘treatment’). Treatment order was balanced for both experiments, such that each treatment appeared an equal number of times in each order.
We created the treatments in Photoshop Elements v. 5 (Adobe Systems, Inc.), as low-compression (10%), high-resolution (600 dpi) JPEG files. Backgrounds were digital images of static natural substrates, taken with a Fuji Finepix S7000 camera, converted to low-compression JPEGs, using only the MW sensors image, such that they were grey scale (the MW sensor of the camera used has a spectral sensitivity similar to that of a human MW cone; M. Stevens 2006, unpublished data). The average grey value of the displayed image on the monitor was adjusted in Photoshop, such that it fell midway between the luminance of the white and black markings of the prey on a ratio scale (see below). For both experiments, we used a sample of backgrounds of each type to ensure that any effects were due to background type and not a specific image.
We removed the effects of colour to simplify the experiment and allow calibrations for luminance. Both experiments were conducted on the same (38 cm wide by 30 cm tall) flat-screen monitor (Hanns.G HU196D) that was calibrated for human luminance perception with a Minolta LS-110 (Osaka, Japan) luminance meter. This was achieved by displaying an image with sets of squares with grey values ranging from 0 to 255 (on an 8 bit scale), and by measuring the luminance (cd m−2) of each patch. We ensured that each section of the screen contained the full range of grey values displayed to account for any differences in display intensity (excluding the outer margins of the screen in the experiments also minimized this possibility). We then plotted grey value against luminance to determine the value of the background that would represent an intermediate grey between the white and black markings on the prey. Viewing distances (60 cm) and ambient light conditions (standard fluorescent laboratory lights) were approximately constant.
In both experiments, participants (72 in experiment 1 and 50 in experiment 2) were chosen to be naive to the experimental aims and unlikely to have an idea of what the experiment was designed to test. We did not give any more information than was necessary to play the game. Otherwise, we haphazardly selected individuals, male and female, from a range of professions, between 18 and 55 years of age. No subject was used more than once over both experiments. Prior to the experimental trials, each person had a 1 min training period, where they tried to catch a black prey against a white background. Neither this prey nor background was used in the main experiment.
Results were analysed with a general linear model (GLM), with the factors prey type, order of presentation and either background (experiment 1) or speed (experiment 2), with human subject as a random factor. We undertook planned orthogonal post hoc comparisons (Ruxton & Beauchamp 2008), by rerunning the GLM with the factor ‘prey’ replaced with each comparison in turn. This is more powerful than undertaking a series of unplanned comparisons, and best reflects our hypotheses (Ruxton & Beauchamp 2008).
(b) Experiment 1
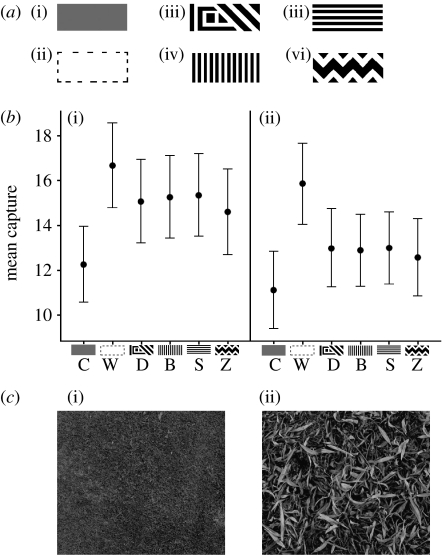
Experiment 1 comprised six prey types: a camouflaged grey matching the average background luminance (C); a conspicuous unmarked white treatment (W); a treatment with simple dazzle markings (D); two target types with stripes running perpendicular (bands, B) or parallel to the direction of prey movement (stripes, S); and a prey item with zigzag markings (Z; figure 1a). Although the camouflaged target matched the average background luminance, owing to spatial variation in the backgrounds, it was still detectable. These moved at approximately 18 cm (17.06° of visual angle) per second across the background, made from images of grass and leafy substrates, such that each subject had a total of 12, 1 min, experimental trials. The two background types provided natural style backgrounds of either relatively uniform (short grass) or heterogeneous (leafy) composition (figure 1c). We undertook the following planned comparisons: (i) white versus the aggregate of all other prey types, (ii) grey versus the aggregate of all patterned prey types, (iii) dazzle versus the aggregate of the striped and zigzagged prey, (iv) zigzagged versus the striped and banded prey, and (v) banded versus the striped prey. We wanted to know whether conspicuous patterned prey would be easier or more difficult to catch than uniform camouflaged and conspicuous targets, and whether there was any difference between the different prey markings. We predicted that if some markings make estimates of speed and trajectory difficult, then some or all of the patterned prey would be caught less than the conspicuous control. We also tested for the influence of background type, predicting that a more heterogeneous background (leaves) would make prey capture more difficult than a more uniform (grass) background, because speed perception in humans is affected by background texture (Blakemore & Snowden 2000).
Figure 1.
(a) Prey types used in experiment 1: (i) camouflaged grey (C), (ii) conspicuous white (W), (iii) dazzle (D), (iv) bands (B), (v) stripes (S), and (vi) zigzag (Z). (b) Mean number of prey caught by the participants in 1 min, plus standard error, against (i) leafy (heterogeneous) and (ii) grassy (uniform) backgrounds. (c) Samples of the (i) grass and (ii) leaf backgrounds used in the experiment.
(c) Experiment 2
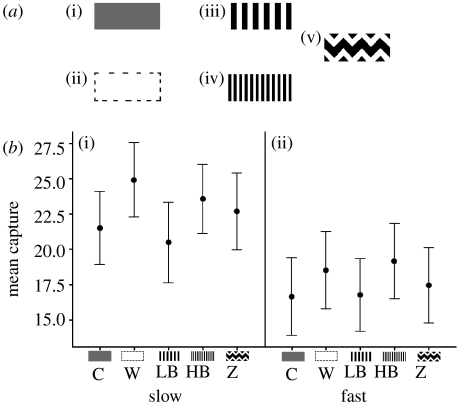
Experiment 2 investigated the effect of several pattern types at two different speeds: fast (20 cm s−1; 18.93° of visual angle s−1) and slow (15 cm s−1; 14.25° of visual angle s−1). We had camouflaged grey (C), conspicuous white (W), and zigzag (Z) marked targets, and in addition there were two treatments, one with thick low-spatial-frequency bands (LB) and the other with thin high-spatial-frequency bands (HB; figure 2a). All prey were presented against the leafy type backgrounds used in experiment 1. We undertook the following planned comparisons: (i) white versus the aggregate of all other prey types, (ii) grey versus the aggregate of all patterned prey types, (iii) zigzagged versus the banded prey, and (iv) thin- versus thick-banded prey. Here, we wished to know whether some prey markings could influence the ease of prey capture, but also whether this would be affected by prey speed. We predicted that prey would be harder to capture at faster speeds.
Figure 2.
(a) Prey types used in experiment 2: (i) camouflaged grey (C), (ii) conspicuous white (W), (ii) low SF bands (LB), (iv) high SF bands (HB), and (v) zigzag (Z) . (b) Mean number of prey caught by the participants in 1 min, plus standard error, at (i) slow and (ii) fast speeds.
3. Results
(a) Experiment 1
Prey were harder to catch on the leafy than the grass background (F1,863=64.37, 863, p<0.001), and capture rates differed between prey types (F5,863=27.43, 863, p<0.001; figure 1b). The conspicuous white target was caught more than the other treatments (F1=76.49, p<0.001), the camouflaged grey target was caught less than the aggregate of the patterned prey types (F1=55.80, p<0.001), there was no difference between the dazzle and other patterned prey types (F1=0.12, p=0.726), or between the banded and striped targets (F1=0.80, p=0.779), although there was a non-significant trend for the zigzag marked targets to be harder to catch than the aggregate of the striped and banded prey (F1=3.42, d.f.=1, p=0.065). There were significant effects of order (F11,863=9.80, p<0.001) and participant (F71,863=48.43, p<0.001).
(b) Experiment 2
Prey were harder to catch at fast than at slow speeds (F1,499=205.40, p<0.001) and capture rates differed between prey types (F4,499=12.54, p<0.001; figure 2b). The conspicuous white target was caught more than the other treatments (F1=19.06, p<0.001), the camouflaged grey was caught less than the aggregate of the patterned prey types (F1=4.32, p=0.038), there was no difference between the zigzag marked prey and the other patterned prey (F1=0.02, p=0.877), but broadbanded targets were harder to capture than thin-banded targets (F1=29.35, p<0.001). There were significant effects of order (F9,499=3.09, p=0.001) and participant (F49,499=50.25, p<0.001).
4. Discussion
The results from our two experiments show that some conspicuous patterns are highly effective at hindering capture. As such, it is likely that some markings on real animals have evolved under selection pressure to make the estimation of speed and direction difficult for predators, particularly in species that are either highly active and/or under selection pressure to be conspicuous (e.g. for sexual signalling or thermoregulation). In addition, the decreased capture rates of some prey types, in particular broad bands and zigzags, compared with others (e.g. thin stripes) suggest that some specific pattern forms are particularly effective in preventing the estimation of speed and direction by predators.
Our current experiments are not capable of revealing exactly why conspicuous patterns made capture more difficult. In experiment 2, targets were harder to catch at fast than slow speeds, but there was no interaction with prey type. This indicates that the decreased ability of participants to capture the broadbanded and zigzagged prey was not due to a flicker fusion camouflage effect (sensu Pough 1976). We also note that at no time in the experiments did the markings on the prey begin to blur into a uniform appearance, even for the thinly banded targets, again indicating that the results are not down to speed induced camouflage. Therefore, we assert that the markings did indeed prevent accurate estimates of speed and direction, and are due to a dazzle effect. While there are various possibilities, the underlying mechanisms are unclear. A range of stimuli can give rise to illusions, such as motion-after effects and illusory motion reversal (Snowden et al. 2006), but these are unlikely to have occurred under our test conditions and with the stimuli used. However, it is well known that certain geometric patterns and arrangements of colour (for instance, incorporated into ‘op art’) can produce the illusion of flicker or motion from a stationary display, possibly due to small involuntary eye movements (saccades) producing image ‘shifts’ that are picked up by low-level motion detectors (Zanker & Walker 2004). Other ‘peripheral drift’ illusions can also create a strong impression of movement (Kitaoka & Ashida 2003). Such illusions involve static repeating elements, which produce responses in direction-sensitive neurons based on differences in element luminance contrast (Conway et al. 2005). While speculative, it is possible that certain combinations of luminance in real animal markings generate similar illusions. Finally, also relevant is the ‘aperture problem’, where a line or edge is observed moving behind a fixed aperture. Here, because the motion component parallel to the line itself cannot be inferred, only perpendicular movement is detectable and the true movement vector of the line is ambiguous; movement often seems to occur at right angles to the line (Bruce et al. 2003). The problem concerns motion-detecting receptive fields, which detect movement over a small area, and thus are sensitive only to movement perpendicular to their own receptive field (Bruce et al. 2003). As such, the estimates of many neurons need to be combined into a global estimate of motion. If this is not possible, then the veridical motion of a stimulus may be hard to judge, especially if, as in our targets, the stimulus consists of a range of bars and lines moving in oblique directions. As such, the aperture problem may be key to understanding misdirected local motion signals in striped patterns (cf. Zanker 2004).
Understanding the various illusions described above in terms of human vision is a difficult task, and so linking such ideas to the coloration, visual systems and behaviour of non-human animals is challenging. There are various anecdotal accounts of illusions generated by animals (mainly snakes) including individuals appearing stationary when in fact they are moving, or simply an inability to judge speed and direction in general (Brodie 1992; Shine & Madsen 1994). The perception of motion in peripheral drift and related illusions is enhanced by high levels of repetition and regularity because this excites a larger number of motion detectors. Certainly, a range of animals have repeating, high luminance-contrast patterns, not dissimilar to those used in such illusions. In addition, markings consisting of angled lines and patterns may also induce an aperture-type problem, inhibiting the detection of true movement.
In experiment 1, prey were more difficult to capture against the more heterogeneous leafy background than against the uniform grass. However, the order of the treatment capture rates was unaffected, indicating that the relative value of different pattern types may not be changed by the background on which the prey are found. In non-human animals, detection of moving signals is challenging when environmental motion (e.g. swaying plants/leaves) is also present, and this may influence the form of motion-based signals (Peters 2008). In our set-up, the prey were always presented on top of the background, whereas in many natural situations, the view of an animal may frequently be fully or partially occluded as it moves through the habitat, potentially further decreasing the ability of the predator to capture it. Such occlusion of the prey outline may also lead to effects associated with the aperture problem.
In experiment 2, we found that the thick-banded targets were harder to capture than the thin-banded targets. At the fast speed, it is possible that this result was, at least in part, due to interference of the prey pattern flicker rate by the screen flicker. The flicker of the thin-banded targets was 80 Hz (based on calculating the time taken for one complete cycle of white and black stripes), whereas the monitor refresh rate was 60 Hz. The flicker of the thick-banded prey patterns at the fast speeds was 40 Hz. At the slow speed, however, the flicker of the thin and thick prey stripes was 50 and 30 Hz, respectively, illustrating that there may still be a genuine advantage of having thick rather than thin stripes. This is an area that would be fruitful to investigate further, especially on a display with a high flicker rate. In addition, a greater range of prey speeds should be tested, including movement slower than the relatively fast speeds used here.
In both experiments, the camouflaged uniform grey prey were the most difficult to capture. As such, it seems that matching the overall coloration or luminance of the background is a good strategy to avoid predation not just when motionless, but also when moving as well. However, there are costs to crypsis (Ruxton et al. 2004), such as being limited to one or a few background types, and so camouflage is not always an optimal solution. Furthermore, animals may possess conspicuous markings for mate choice, thermoregulation or as warning signals, and so our present findings indicate that specific arrangements of conspicuous markings may make it more difficult for a predator to catch its prey. It follows that a range of markings on animals may therefore be combining multiple functions. For example, it is possible that some combinations of stripe colours, such as in some fishes, have a distance-dependent effect, being involved in signalling in close proximity but camouflage from afar (Marshall 2000). Alternatively, various snakes signal their toxicity through colourful stripes (e.g. coral snakes; Brodie 1993). Such multiple functions may even be directed towards different predator groups with different visual perceptions (Jackson et al. 1976).
Although we found no interaction between the effects of prey appearance and speed of movement in our experiments, it may be that dazzle effects such as those demonstrated here can be enhanced by particular forms of locomotion. That is, dazzle coloration may interact with protean movements (Humphries & Driver 1967), and so evaluation of the trajectories of fleeing prey should consider potential sensory implications. Alternatively, dazzle effects may afford protection to prey moving for non-defensive reasons, which are suddenly attacked by a previously undetected predator. Thus, dazzle schemes might be prevalent among highly mobile prey with predators that rely on an accurate strike for effective prey capture. Dazzle coloration also occurs later in the sequence of a predator–prey encounter than camouflage and warning coloration. Prey with a very effective aposematic signal, for example, may therefore have little need of dazzle, since predators would be deterred from making attacks by the warning signal, leaving the dazzle scheme rarely employed. As such, we may expect prey species to combine aposematism and dazzle markings if they face a suite of predators, only some of which are deterred by the warning signal.
Overall, these experiments provide the first support for the idea that some biologically relevant markings, similar to those found on real animals, are highly effective in preventing capture, seemingly because they make estimates of speed and trajectory difficult. Markings that function in this way are also likely to impact on the camouflage of the bearer when at rest; this reduction in background matching may be compensated for by a disruptive or distractive effect if the signal has multiple functions. Additionally, the use of dazzle strategies must also be linked to behaviour (cf. Jackson et al. 1976; Brodie 1992). Finally, features that confuse viewers as to the speed and direction of the bearer may also be selected in predators as well as prey, since many animals with striped patterns are also predators (e.g. snakes, various insects). Pursuit predators may gain an advantage if prey are less able to effectively deploy evasive manoeuvres through a failure to accurately track the chasing predator. Our future understanding of animal coloration will be extended by considering the multivariate nature of many markings, and is an area where interdisciplinary research would be of great advantage (Rosenthal 2007). In particular, future work should aim to understand how multiple selection pressures have given rise to the specific markings seen in animals, and how these relate to behaviour.
Acknowledgments
We thank two reviewers for their thoughtful and perceptive suggestions, David Tolhurst for use of his luminance meter and Tom Troscianko for his advice. M.S., D.H.Y. and G.D.R. designed the experiments, D.H.Y. and M.S. ran the experiments, G.D.R. and M.S. performed the statistics and M.S. and G.D.R. wrote the paper. M.S. was supported by a Research Fellowship from Girton College, Cambridge, and G.D.R. was supported by NERC grants NE/D010772/1, NE/D010500/1 and NE/E016626/1.
References
- Anstis S. Moving objects appear to slow down at low contrasts. Neural Netw. 2003;16:933–938. doi: 10.1016/S0893-6080(03)00111-4. doi:10.1016/S0893-6080(03)00111-4 [DOI] [PubMed] [Google Scholar]
- Behrens R.R. The role of artists in ship camouflage during world war I. Leonardo. 1999;32:53–59. doi:10.1162/002409499553000 [Google Scholar]
- Blakemore M.R, Snowden R.J. Textured backgrounds alter perceived speed. Vision Res. 2000;40:629–638. doi: 10.1016/s0042-6989(99)00214-x. doi:10.1016/S0042-6989(99)00214-X [DOI] [PubMed] [Google Scholar]
- Brodie E.D.I. Correlational selection for color pattern and antipredator behavior in the garter snake Thamnophis ordinoides. Evolution. 1992;46:1284–1298. doi: 10.1111/j.1558-5646.1992.tb01124.x. doi:10.2307/2409937 [DOI] [PubMed] [Google Scholar]
- Brodie E.D.I. Differential avoidance of coral snake banded patterns by free-ranging avian predators in Costa Rica. Evolution. 1993;47:227–235. doi: 10.1111/j.1558-5646.1993.tb01212.x. doi:10.2307/2410131 [DOI] [PubMed] [Google Scholar]
- Bruce V, Green P.R, Georgeson M.A. 4th edn. Psychology Press; Hove, UK: 2003. Visual perception. [Google Scholar]
- Conway B.R, Kitaoka A, Yazdanbakhsh A, Pack C.C, Livingstone M.S. Neural basis for a powerful static motion illusion. J. Neurosci. 2005;25:5651–5656. doi: 10.1523/JNEUROSCI.1084-05.2005. doi:10.1523/JNEUROSCI.1084-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailman J.P. Indiana University Press; London, UK: 1977. Optical signals: animal communication and light. [Google Scholar]
- Humphries D.A, Driver P.M. Erratic display as a device against predators. Science. 1967;156:1767–1768. doi: 10.1126/science.156.3783.1767. doi:10.1126/science.156.3783.1767 [DOI] [PubMed] [Google Scholar]
- Jackson J.F, Ingram W, Campbell H.W. The dorsal pigmentation pattern of snakes as an antipredator strategy: a multivariate approach. Am. Nat. 1976;110:1029–1053. doi:10.1086/283125 [Google Scholar]
- Kitaoka A, Ashida H. Phenomenal characteristics of the peripheral drift illusion. Vision. 2003;15:261–262. [Google Scholar]
- Marshall N.J. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. B. 2000;355:1243–1248. doi: 10.1098/rstb.2000.0676. doi:10.1098/rstb.2000.0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R.A. Environmental motion delays the detection of movement-based signals. Biol. Lett. 2008;4:2–5. doi: 10.1098/rsbl.2007.0422. doi:10.1098/rsbl.2007.0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pough F.H. Multiple cryptic effects of crossbanded and ringed patterns of snakes. Copeia. 1976;1976:834–836. doi:10.2307/1443481 [Google Scholar]
- Rosenthal G.G. Spatiotemporal dimensions of visual signals in animal communication. Annu. Rev. Ecol. Evol. Syst. 2007;38:155–178. doi:10.1146/annurev.ecolsys.38.091206.095745 [Google Scholar]
- Ruxton G.D. The possible fitness benefits of striped coat coloration for zebra. Mamm. Rev. 2002;32:237–244. doi:10.1046/j.1365-2907.2002.00108.x [Google Scholar]
- Ruxton G.D, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav. Ecol. 2008;19:690–693. doi:10.1093/beheco/arn020 [Google Scholar]
- Ruxton G.D, Sherratt T.N, Speed M.P. Oxford University Press; Oxford, UK: 2004. Avoiding attack. [Google Scholar]
- Scratch 2007 Lifelong kindergarten group, M.M. L. See http://scratch.mit.edu
- Sherratt T.N, Rashed A, Beatty C.D. The evolution of locomotory behavior in profitable and unprofitable simulated prey. Oecologia. 2004;138:143–150. doi: 10.1007/s00442-003-1411-4. doi:10.1007/s00442-003-1411-4 [DOI] [PubMed] [Google Scholar]
- Shine R, Madsen T. Sexual dichromatism in snakes of the genus Viperia: a review and a new evolutionary hypothesis. J. Herpetol. 1994;28:114–117. doi:10.2307/1564692 [Google Scholar]
- Snowden R.J, Thompson P, Troscianko T. Oxford University Press; Oxford, UK: 2006. Basic vision: an introduction to visual perception. [Google Scholar]
- Stevens M. Predator perception and the interrelation between protective coloration. Proc. R. Soc. B. 2007;274:1457–1464. doi: 10.1098/rspb.2007.0220. doi:10.1098/rspb.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer G.H. Macmillan; New York, NY: 1909. Concealing-coloration in the animal kingdom: an exposition of the laws of disguise through color and pattern: being a summary of Abbott H. Thayer's discoveries. [Google Scholar]
- Thompson P, Stone L.S. Contrast affects flicker and speed perception differently. Vision Res. 1997;37:1255–1260. doi: 10.1016/s0042-6989(96)00302-1. doi:10.1016/S0042-6989(96)00302-1 [DOI] [PubMed] [Google Scholar]
- Van Damme R, Van Dooren T.J.M. Absolute versus per unit body length speed of prey as an estimator of vulnerability to predation. Anim. Behav. 1999;57:347–352. doi: 10.1006/anbe.1998.0980. doi:10.1006/anbe.1998.0980 [DOI] [PubMed] [Google Scholar]
- Zanker J.M. Looking at op art from a computational viewpoint. Spat. Vis. 2004;17:75–94. doi: 10.1163/156856804322778279. doi:10.1163/156856804322778279 [DOI] [PubMed] [Google Scholar]
- Zanker J.M, Walker R. A new look at op art: towards a simple explanation of illusory motion. Naturwissenschaften. 2004;91:149–156. doi: 10.1007/s00114-004-0511-2. doi:10.1007/s00114-004-0511-2 [DOI] [PubMed] [Google Scholar]