Abstract
Increasing evidence of links between climate change, anthropogenic stress and coral disease underscores the importance of understanding the mechanisms by which reef-building corals resist infection and recover from injury. Cellular inflammation and melanin-producing signalling pathway are two mechanisms employed by invertebrates to remove foreign organisms such as pathogens, but they have not been recorded previously in scleractinian corals. This study demonstrates the presence of the phenoloxidase (PO) activating melanin pathway in two species of coral, Acropora millepora and a massive species of Porites, which both develop local pigmentation in response to interactions with a variety of organisms. l-DOPA (3-(3,4-dihydroxyphenyl)-l-alanine) substrate-based enzyme activation assays demonstrated PO activity in healthy tissues of both species and upregulation in pigmented tissues of A. millepora. Histological staining conclusively identified the presence of melanin in Porites tissues. These results demonstrate that the PO pathway is active in both coral species. Moreover, the upregulation of PO activity in areas of non-normal pigmentation in A. millepora and increased melanin production in pigmented Porites tissues suggest the presence of a generalized defence response to localized stress. Interspecific differences in the usage of pathways involved in innate immunity may underlie the comparative success of massive Porites sp. as long-lived stress tolerators.
Keywords: scleractinian corals, melanin, innate immunity, phenoloxidase, amoebocyte
1. Introduction
Recent sustained increases in reports of coral disease (Peters 1997; Harvell et al. 1999, 2007) have suggested that factors regulating diseases of reef corals have changed in the last few decades, possibly through changes in the abundance and virulence of causative agents and/or changes in host-defensive responses involved in disease resistance. As impacts from global climate change and local anthropogenic influences escalate, causative agents and their virulence are likely to increase (Harvell et al. 1999; Ritchie 2006). Concomitantly, corals are likely to become more susceptible if their disease-resistance mechanisms are compromised. Scleractinian corals are the dominant calcifying organisms on coral reefs (Fagerstrom 1987) and are therefore vital for the fabrication and maintenance of the reef ecosystem. In the light of their key role, investigations of disease-resistance mechanisms in corals and the impacts of changing environmental conditions, such as ocean warming, on these mechanisms are urgently required.
Non-normally pigmented tissues in scleractinian corals have been investigated as syndromes or diseases in multiple scleractinian species (Aeby 2003; Bongiorni & Rinkevich 2005; Ravindran & Raghukumar 2006a,b). Discoloured tissues are typically pink, purple or blue and are observed in a variety of circumstances associated with stressful interactions (Kawaguti 1944; Rinkevich & Sakai 2001; Aeby 2003; Ravindran & Raghukumar 2006a,b). Because such observations are characteristically associated with agents that compromise coral tissue integrity, it has been suggested that these signs of non-normal pigmentation are associated with a generalized response of the coral to a physical or pathogenic challenge (Willis et al. 2004; Bongiorni & Rinkevich 2005; Ravindran & Raghukumar 2006b). The intriguing possibility therefore arises that such non-normal tissue pigmentation is associated with the activation of a general immune response.
Immunity refers to internal protection against infectious diseases (Stedman 2000) and involves a chain of response mechanisms induced in recognition of an invading foreign body. Because invertebrates lack adaptive immunity, they are dependent on an innate immune system to provide effective non-specific defences (Rinkevich 1999; Cooper 2002; Cerenius & Soderhall 2004), such as the inflammation response (Rowley 1996). Sparks (1972) defined the inflammation response as an attempt to destroy, dilute or isolate the destructive agent and the affected host cells. As such, one of the most characteristic mechanisms of inflammation is the rapid infiltration of immune-type cells and initiation of phagocytosis. Phagocytic cells act by engulfing and destroying invading pathogens (Rowley 1996), or by accumulating and encapsulating large foreign organisms, thereby providing a defensive barrier and structural integrity to weakened areas (Fonatine 1971; Rowley 1996). Cells with phagocytic potential are ubiquitous across the animal kingdom (Cooper 2002) and have been characterized in some anthozoans (Patterson & Landolt 1979; Olano & Bigger 2000; Mullen et al. 2004; Ellner et al. 2007; Mydlarz et al. 2008). In addition, phagocytic cells have been found to produce oxygen radicals, thought to be involved in intracellular killing of phagocytosed material, in both anthozoans (Hutton & Smith 1996) and molluscs (Adema et al. 1991). Although putative amoebocytes have been proposed (Domart-Coulon et al. 2006; Vargas-Ángel et al. 2007), to date phagocytic cells have not been characterized for scleractinians and the specific mechanisms of innate immunity in hard corals are poorly known (reviewed in Mullen et al. 2004 and Mydlarz et al. 2006).
In addition to the aggregation of phagocytic cells, the creation of a defensive barrier can be achieved with melanin deposition (Sparks 1972; Bayne 1990; Rowley 1996; Nappi & Christensen 2005). Melanin is the final stage of the phenoloxidase (PO)-activating cascade, which is widely recognized as a key component of invertebrate immunity (Coles & Pipe 1994; Asokan et al. 1997; Soderhall & Cerenius 1998; Cerenius & Soderhall 2004; Munoz et al. 2006; Hellio et al. 2007; Butt & Raftos 2008) and, in some cases, is associated directly with specialized phagocytes (Porchet-Henneré & Vernat 1992; Butt & Raftos 2008). The activation of the melanin synthesis pathway in invertebrates initially involves proteolysis of zymogen prophenoloxidase to give the active form of PO (Cerenius & Soderhall 2004; Nappi & Christensen 2005). Demonstration of a positive correlation between quantities of the active isomer PO and disease resistance in bivalves (Butt & Raftos 2008) and crustaceans (Persson et al. 1987) highlights the key role this enzyme plays in immune responses.
Despite an increasing focus on coral disease research to understand and reduce the rate of reef degradation worldwide, little attention has been paid to disease-resistance mechanisms in scleractinian corals. This study investigated the presence of key invertebrate inflammatory response mechanisms in areas of non-normally pigmented tissue in two species of scleractinian coral that have contrasting susceptibility to disease: Acropora millepora, from the family Acroporidae, which is one of the most susceptible families (Willis et al. 2004), and a massive species of Porites, which has comparatively low susceptibility to disease on the Great Barrier Reef (GBR; Willis et al. 2004). Comparisons of PO activity, cell infiltration and the location of melanin deposits between healthy and non-normally pigmented tissues (referred to as simply pigmented hereafter; figure 1) provide novel insights into potential mechanisms of innate immunity in two scleractinian coral species.
Figure 1.
Macroscopic signs of pigmentation response in (a) A. millepora showing blue pigmentation encircling a wound and normal coloration of the surrounding healthy branches, and (b) massive Porites sp. showing pink pigmentation encircling lesions and normal coloration of the surrounding healthy tissue. (Photos credited to Giles Winstanley.)
2. Material and methods
(a) Sample collection
Pigmented and healthy samples were collected from 28 colonies of A. millepora and 20 colonies of a massive species of Porites for comparisons of PO activity and histological microstructure from fringing reefs surrounding Orpheus Island in the central section of the GBR. Sampling pigmented and healthy tissues from each colony controlled for potential genetic factors underlying differences in pigmentation. Healthy samples had coloration that was characteristic of the species, whereas pigmented samples displayed a distinct blue coloration in A. millepora and pink coloration in Porites sp. (figure 1). Pigmented tissue sampled was generally associated with areas of wound healing. For protein and enzyme assays, samples were immediately snap frozen in liquid nitrogen and stored at −20°C. Samples for histology were fixed in 4 per cent formaldehyde–sea water solution.
(b) Sample processing
For protein and enzyme assays, coral tissues were removed from the skeletons of all healthy and pigmented samples of A. millepora (n=28 per category) and Porites sp. (n=20 samples per category) using an airbrush (Panaache H2) and stored in 100 mM phosphate buffer with 5 mM 2-mercaptoethanol (Sigma-Aldrich M7522). Tissue adjacent to points of fragmentation caused by the sampling process was avoided. Tissue slurries were homogenized using a tissue homogenizer (Janke and Kunkel, IKA-Labortechnik) for 1 min, and freeze-dried to concentrate extracts and further disrupt cells. Samples were rehydrated with 2 ml of molecular grade water (Sigma W4502) immediately before use.
(c) Biochemical analyses
PO activity was quantified by measuring the darkening of the colourless substrate l-DOPA to coloured dopachrome as a result of enzymatic oxidation (Munoz et al. 2006; Hellio et al. 2007). For this assay, three 20 μl aliquots of each sample extract were placed in wells of a 96-well microtitre plate and left at room temperature for 20 min. Then 100 μl of molecular grade water (Sigma W4502) and 50 μl of 20 mM l-DOPA (3-(3, 4-dihydroxyphenyl)-l-alanine; Fluka 37830) in 100 mM phosphate buffer (pH 5.0) were added to each sample. The l-DOPA and extracts were tested for independent effects using controls. To quantify the oxidized product of PO activity, absorbance for each sample was read at 490 nm at time 0 (immediately after the addition of l-DOPA) and after 30 min using a Multiskan Ascent plate reader (Labsystems, Finland). Data represent the linear phase of the reaction over 30 min and were normalized to mg protein for each sample. Protein concentration was determined using the Peterson's Lowry total protein standard assay (Peterson 1977, Sigma-Aldrich TP0300) with bovine serum albumin as a standard and performed according to the protocols provided by the manufacturer. Absorbance at 595 nm was measured for each sample using a Multiskan Ascent plate reader (Labsystems, Finland). PO activity between healthy and pigmented samples was compared using t-tests.
(d) Histological tissue comparisons
Samples of healthy and pigmented tissues were collected from each of five A. millepora and five massive Porites colonies from Pioneer Bay, Orpheus Island. The samples were fixed in 4 per cent formaldehyde–sea water solution, decalcified progressively in 3–10% formic acid and stored in 70% ethanol. Histological samples were processed overnight in an automated tissue processor and embedded in paraffin wax. Wax blocks were sectioned at 5 μm and sections were stained with either Mayer's haematoxylin and Young's eosin–erythrosin stain (H&E) or Fontana–Masson melanin stain. In the melanin-specific stain, melanin granules reduce silver nitrate to metallic silver, which results in a histochemical reaction that precipitates black material wherever melanin is located (Porchet-Henneré & Vernat 1992).
Photographs were taken of the epidermal layers in the free body wall region in longitudinal sections of coral polyps for each A. millepora sample and in transverse sections of polyps for each Porites sample using an Olympus DP12 dedicated camera head mounted on an Olympus microscope. Counts of symbiotic algae (zooxanthellae) within 100 μm2 of gastrodermis and counts of granular cells, characterized by aggregations of pigment granules, within 100 μm2 of both epithelial tissue layers were recorded within fifteen randomly selected areas of both healthy and pigmented tissue sections. Tissue areas were calculated using imaging software (Image J). Mean numbers of zooxanthellae were compared statistically between healthy and pigmented samples of each genus and mean numbers of granular cells were compared between healthy and pigmented samples of Porites. The non-parametric Mann–Whitney U test was used in both cases as assumptions of normality and homogeneity of variances were not met.
3. Results
(a) Characterization of pigmented A. millepora tissue
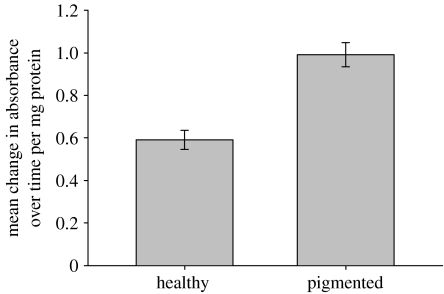
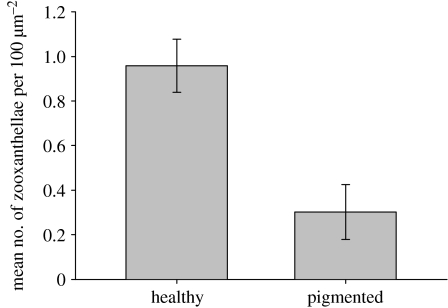
PO activity was significantly greater in blue pigmented tissues of A. millepora than in healthy tissues (figure 2). This was indicated by a greater mean change in absorbance over time per mg protein (t=2.212, p=0.031). Conversely, the mean number of zooxanthellae per 100 μm2 of gastrodermis (figure 3) was threefold greater in healthy tissues than in pigmented tissues of A. millepora (U=7.0; p<0.01).
Figure 2.
Comparison of mean (±s.e.) change in PO activity, as measured by the oxidation of l-DOPA over time, between healthy and pigmented samples of A. millepora (n=28; t=2.212, p=0.031).
Figure 3.
Comparison of mean (±s.e.) number of zooxanthellae per 100 μm2 of gastrodermis between healthy and pigmented samples of A. millepora (n=15; U=7.0; p<0.01).
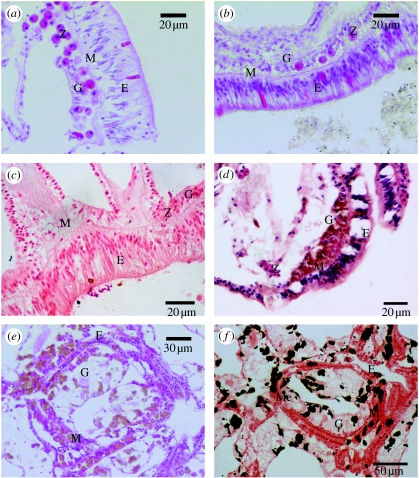
In healthy tissues of A. millepora, the two epithelial cell layers, the epidermis and gastrodermis, plus the connective layer, the mesogloea, were all present and well structured. Each contained intact diagnostic cells and structures, i.e. zooxanthellae in the gastrodermis and nematocysts in the free body wall epidermis (figure 4a). All three layers were also discernible in the majority of pigmented tissue samples; however, the mesogloea appeared reduced in thickness and tissue layers appeared highly granular (figure 4b). Moreover, zooxanthellae densities were reduced and there were aggregations of unidentified oval cells in the epidermis. No melanin deposits were located in the A. millepora tissue using the Fontana–Masson stain (figure 4c).
Figure 4.
Histological sections showing (a) healthy free body wall epithelial layers of A. millepora, with abundant zooxanthellae in the gastrodermis (H&E); (b) pigmented free body wall epithelial layers of A. millepora, with granular cell layers and depleted numbers of zooxanthellae in the gastrodermis (H&E); (c) pigmented free body wall epithelial layers of A. millepora showing no melanin deposits (Fontana–Masson stain); (d) Porites free body wall epithelial layers showing pigment cells in the gastrodermis of a healthy sample; (e) Porites free body wall epithelial layers showing pigment cells in both the epidermis and the gastrodermis of a pigmented sample; (f) Porites free body wall epithelial layers showing black stained melanin deposits (Fontana–Masson stain) in the same pigmented sample as in (e). E, epithelium; G, gastrodermis; M, mesogloea; Z, zooxanthellae; Me, melanin.
(b) Characterization of pigmented Porites sp. tissue
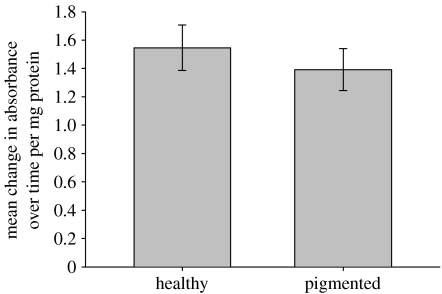
PO activity did not differ significantly between the healthy and pigmented tissue samples of Porites (figure 5), as demonstrated by a lack of significant difference in absorbance over time (t=0.479, p=0.634). Histological examination of Porites sp. tissues revealed the presence of granular cells in both healthy and pigmented tissues (figure 4d,e). Both the epithelial layers and the connective layer were comparatively uniform in thickness and typical in appearance in healthy tissue sections (figure 4d). By contrast, in pigmented sections (figure 4e), the mesoglea was reduced in thickness, the epidermal cell layer was irregular and disrupted, and it contained an increased number of granular cells. Granules stained black in the Fontana–Masson stain (figure 4f), confirming that the pigment in the granular cells observed was melanin.
Figure 5.
Comparison of mean (±s.e.) change in PO activity, as measured by the oxidation of l-DOPA over time, between healthy and pigmented samples of Porites sp. (n=20; t=0.479, p=0.634).
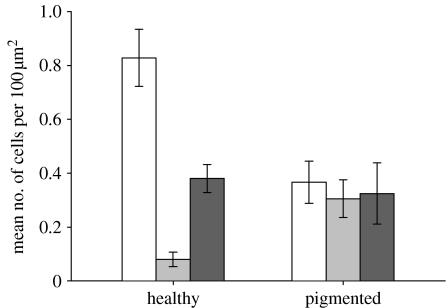
Comparisons of histological sections revealed that the mean number of zooxanthellae per 100 μm2 of gastrodermis was more than twofold greater in healthy (0.8 cells 100 μm−2 gastrodermis) than in pigmented (0.35 cells 100 μm−2 gastrodermis) samples (U=669.0; p<0.01; figure 6). The mean number of granular cells per 100 μm2 of tissue was more than fourfold greater in the epidermal layer of pigmented samples than in the epidermal layer of healthy samples (U=52.5, p=0.012), which corresponded to four times more melanin in pigmented tissues. By contrast, comparisons of gastrodermal tissue layers between healthy and pigmented samples of Porites sp. revealed no significant difference in the mean number of granular cells per 100 μm2 of tissue (figure 6), with the highest counts ranging between approximately 0.34 and 0.37 cells per 100 μm2 (U=73, p=0.101). Overall, the lowest number of granular cells (less than 0.1 cells per 100 μm2) was detected in the epidermal layer of healthy samples, and the highest in the gastrodermal layer of healthy samples (0.38 cells per 100 μm2). However, there was approximately 1.5 times the number of granular cells in the combined gastrodermal and epidermal layers of pigmented samples than in the combined epithelial layers of healthy samples (figure 6).
Figure 6.
Porites healthy versus pigmented tissue comparisons for mean (±s.e.) densities of: zooxanthellae (n=15, U=669.0, p<0.01; white bars), epidermal granular cells (n=15, U=52.5, p=0.012; grey bars) and gastrodermal granular cells (n=15, U=73, p=0.101; black bars).
4. Discussion and conclusions
Phenoloxidase activity and the presence of melanin deposits, which are both commonly associated with the mechanisms of innate immunity in invertebrates, were confirmed for the first time in a scleractinian coral in this study. Moreover, upregulation of PO activity in pigmented tissues of A. millepora and increased melanin deposits in pigmented tissues of Porites provide clear evidence that pathways associated with innate immunity are upregulated in corals with compromised health. The PO pathway is important in inflammatory defence due to the production of cytotoxic intermediates (Nappi & Christensen 2005; Mydlarz & Harvell 2007) and can result in the formation of a physical barrier for encapsulation (Rowley 1996; Petes et al. 2003). Although causes of non-normal pigmentation in tissues sampled in this study were unknown, associations with biochemical activities related to innate immunity suggest that pigmentation may be part of a generalized inflammatory-like response in these two species of corals.
(a) Characterization of pigmented A. millepora tissue
Symbiotic zooxanthellae are foreign organisms within coral tissues and may be expelled locally as part of innate immune mechanisms activated in response to stress. Low densities of zooxanthellae within the gastrodermal layer of pigmented tissues of A. millepora confirmed that their health was compromised. Increased PO activity, which has been shown to be inducible in immunological studies of a variety of invertebrates as part of their resistance mechanisms (Cerenius & Soderhall 2004; Munoz et al. 2006; Hellio et al. 2007; Butt & Raftos 2008), provided further evidence that pigmented tissues were immunologically challenged. Histologically, however, there was no evidence of cell infiltration indicative of an inflammation response, such as those that have been associated with phagocytic cellular responses in other anthozoans (Olano & Bigger 2000). Lack of cellular infiltration in immunologically challenged tissues of A. millepora provides support for speculations that cellular inflammation responses are not as well developed in scleractinians as in gorgonians (Mullen et al. 2004; Vargas-Ángel et al. 2007; Mydlarz et al. 2008). The lack of melanin deposits detected by the melanin-specific Fontana–Masson stain in A. millepora was unexpected if the pigmentation was part of a generalized inflammation response, but there are several potential explanations, all of which warrant further investigation. First, the presence of PO does not necessarily lead to the deposition of melanin (Butt & Raftos 2008). Second, although PO is typically sequestered to reduce the impacts of its reactivity and subsequent cytotoxicity to tissues, cell-free PO has been found in the haemolymph of multiple bivalves, although generally in lower concentrations than cellular PO (Deaton et al. 1998; Jordan & Deaton 2005; Butt & Raftos 2008), and could provide an explanation for the granular appearance of tissue layers of pigmented A. millepora. If A. millepora uses cell-free PO, then the presence of PO within the extracellular matrix of all tissue layers of A. millepora would negate the requirement for infiltration of cells as a cellular inflammation response (Olano & Bigger 2000).
(b) Characterization of pigmented Porites sp. tissue
Similar levels of PO activity in both the pigmented and healthy tissues of Porites sp. indicate a relatively high level of residual activity that could result in constant production and deposition of melanin in both tissue types. Histological investigations supported the comparatively constant PO activity found biochemically in Porites tissues, as melanin deposits were found in both the healthy and pigmented samples of Porites sp. However, the 1.5-fold greater number of granular cells in pigmented tissues is consistent with these tissues being immunologically challenged. Since only activated PO was measured in this study, it is possible that the inactive form, prophenoloxidase, might be present in differing quantities and might provide a better indicator of melanin deposition in these two tissue types.
Differences in the distribution of granular cells in epidermal versus gastrodermal layers of Porites sp. between healthy and pigmented tissues are also consistent with their role in immunological defence. The fourfold greater density of granular cells in the epidermal layer of pigmented tissues compared with healthy tissues highlights their potential role in defence as the epidermal layer is the most susceptible layer to an externally invading organism. In pigmented tissues, the high and approximately equal numbers of granular cells that were found in epidermal and gastrodermal layers suggest either an upregulation or migration of granular cells into the epidermal layer of compromised tissue, as would occur with amoebocytes as part of an inflammation response (Olano & Bigger 2000). We hypothesize that melanin-containing granular cells in Porites sp. are involved in encapsulation, similar to chromophore cells identified as putative amoebocytes during an inflammatory-like response in Porites compressa (Domart-Coulon et al. 2006). Our results support the role of melanization and PO as part of a general inflammatory-like response, identify melanin as the biochemical content of the granular cells and highlight the potential for amoebocytes to be present within the tissue layers of scleractinian corals.
The significantly lower density of zooxanthellae in pigmented tissues than in healthy tissues is consistent with the findings of Ravindran & Raghukumar (2006a,b), and indicates that tissue health was compromised in the pigmented samples of this study. Conversely, the presence of melanin-containing granular cells within the gastrodermis of healthy tissue may explain the relative tolerance of Porites sp. to thermal stress that results in bleaching in more sensitive coral genera (Brown 1997; Marshall & Baird 2000).
(c) Interspecific comparison
Although both A. millepora and Porites sp. demonstrate the presence and activation of PO-associated pathways in tissues showing signs of compromised health (i.e. pigmentation combined with significantly reduced zooxanthellae densities), there are clear differences in the usage of the biochemical cascade between the two species. Differences in the characterization of pigmented tissues between the two coral species suggest differing optimization of the melanin pathway. A. millepora had more than twofold lower constitutive levels of PO than Porites sp. and no observable melanin deposits or melanin-containing putative amoebocytes (Domart-Coulon et al. 2006). This is consistent with the suggestions by Mullen et al. (2004) and Vargas-Ángel et al. (2007) that the use of cellular defences is variable among anthozoans. This not only suggests a general lower resistance, but also it can be hypothesized that A. millepora exploits the cytotoxic and non-cellular mechanisms of the PO cascade, whereas Porites sp. uses a higher concentrated cellular PO and takes advantage of both the cytotoxic and barrier-forming potential of the PO cascade. The differences in the use of the PO cascade and cellular infiltration may relate to the differences in constitutive disease and/or bleaching resistance, as in other marine invertebrates including oysters (Ford et al. 1993; Butt & Raftos 2008), clams (Allam et al. 2001) and crustaceans (Perssons et al. 1987). Therefore, the development of different immune mechanisms could be related to the differences in life history of these two corals.
The two genera, Porites and Acropora, have very disparate life-history strategies and, undoubtedly, partition energy differently among activities associated with growth, reproduction and maintenance. The continuous production of PO and melanin in Porites sp. requires a constant investment of energy, leading to a reduction of resources available for growth and reproduction. Nevertheless, these PO levels would provide a continual level of resistance to infection, supporting observations of lower disease susceptibility for this genus on the GBR (Willis et al. 2004). By contrast, the more rapidly growing acroporids (Oliver 1985), which are one of the most susceptible families to both bleaching (Brown & Suharsono 1990; Brown 1997; Marshall & Baird 2000) and disease (Willis et al. 2004; Page & Willis 2006), have a lower level of residual PO and no melanin deposits. Such differences in immunity are particularly pertinent, given the current rate of global climate change and potential implications of ocean warming for the health of reef corals (Hoegh-Guldberg 1999; Hughes et al. 2003). In summary, this study demonstrates the presence of the melanization cascade as part of an inflammatory-like immune response of scleractinian corals and highlights the presence of melanin-containing putative amoebocytes. It also contributes to current understanding of how differential development of innate immunity might contribute to ecological and life-history differences among coral species.
Acknowledgments
Funding was provided by the Australian Research Council through the ARC Centre of Excellence for Coral Reef Studies and the Coral Disease Working Group in the GEF Coral Reef Targeted Research program.
References
- Aeby G.S. Corals in the genus Porites are susceptible to infection by a larval trematode. Coral Reefs. 2003;22:216. doi:10.1007/s00338-003-0310-9 [Google Scholar]
- Adema C.M, Van der Knaap W.P.W, Sminia T. Molluscan hemocytemediated cytotoxicity: the role of reactive oxygen intermediates. Rev. Aquat. Sci. 1991;4:201–223. [Google Scholar]
- Allam B, Ashton-Alcox K.A, Ford S.E. Haemocyte parameters associated with resistance to brown ring disease in Ruditapes spp. clams. Dev. Comp. Immunol. 2001;25:365–375. doi: 10.1016/s0145-305x(00)00072-0. doi:10.1016/S0145-305X(00)00072-0 [DOI] [PubMed] [Google Scholar]
- Asokan R, Arumugam M, Mullainadhan P. Activation of prophenoloxidase in the plasma and haemocytes of the marine mussel, Perna viridis Linnaeus. Dev. Comp. Immunol. 1997;21:1–12. doi: 10.1016/s0145-305x(97)00004-9. doi:10.1016/S0145-305X(97)00004-9 [DOI] [PubMed] [Google Scholar]
- Bayne C.J. Phagocytosis and non-self recognition in invertebrates. BioScience. 1990;40:723–731. doi:10.2307/1311504 [Google Scholar]
- Bongiorni L, Rinkevich B. The pink-blue spot syndrome in Acropora eurystoma (Eilat Red Sea): a possible marker of stress? J. Zool. 2005;108:247–256. doi: 10.1016/j.zool.2005.05.002. doi:10.1016/j.zool.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Brown B.E. Coral bleaching: causes and consequences. Coral Reefs. 1997;16:129–138. doi:10.1007/s003380050249 [Google Scholar]
- Brown B.E, Suharsono Damage and recovery of coral reefs affected by El Niño related seawater warming in the Thousand Islands, Indonesia. Coral Reefs. 1990;8:163–170. doi:10.1007/BF00265007 [Google Scholar]
- Butt D, Raftos D. Phenoloxidase-associated cellular defence in the Sydney rock oyster, Saccostrea glomerata, provides resistance against QX disease infections. Devel. Comp. Immunol. 2008;32:299–306. doi: 10.1016/j.dci.2007.06.006. doi:10.1016/j.dci.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Cerenius L, Soderhall K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. doi:10.1111/j.0105-2896.2004.00116.x [DOI] [PubMed] [Google Scholar]
- Coles J.A, Pipe R.K. Phenoloxidase activity in the haemolymph and haemocytes of the marine mussel Mytilus edulis. Fish Shellfish Immunol. 1994;4:337–352. doi:10.1006/fsim.1994.1030 [Google Scholar]
- Cooper E.L. Comparative immunology. Curr. Pharma Des. 2002;8:99–110. doi:10.2174/1381612023396546 [Google Scholar]
- Deaton L.E, Jordan P.J, Dankert J.R. Phenoloxidase activity in the hemolymph of bivalve mollusks. J. Shellfish Res. 1998;18:223–226. [Google Scholar]
- Domart-Coulon I.J, et al. Comprehensive characterisation of skeletal growth anomalies of the finger coral Porites compressa. Coral Reefs. 2006;25:531–543. doi:10.1007/s00338-006-0133-6 [Google Scholar]
- Ellner S.E, Jones L.E, Mydlarz L.D, Harvell C.D. Within-host disease ecology in the sea fan Gorgonia ventalina: modeling the spatial immunodynamics of a coral-pathogen interaction. Am. Nat. 2007;170:E143–E161. doi: 10.1086/522841. doi:10.1086/522841 [DOI] [PubMed] [Google Scholar]
- Fagerstrom J.A. Wiley; New York, NY: 1987. The evolution of reef communities. [Google Scholar]
- Fontaine C.T. Exoskeleton intrusions-a wound repair process in Penaid shrimp. J. Invertebr. Pathol. 1971;18:301–303. doi: 10.1016/0022-2011(71)90165-0. doi:10.1016/0022-2011(71)90165-0 [DOI] [PubMed] [Google Scholar]
- Ford S.E, Kanaley S.A, Littlewood D.T.J. Cellular responses of oysters infected with Haplosporidium nelsoni: changes in circulating and tissue-infiltrating haemocytes. J. Invert. Pathol. 1993;61:49–57. doi: 10.1006/jipa.1993.1009. doi:10.1006/jipa.1993.1009 [DOI] [PubMed] [Google Scholar]
- Harvell C.D, et al. Emerging marine diseases-climate links and anthropogenic factors. Science. 1999;286:1505–1510. doi: 10.1126/science.285.5433.1505. doi:10.1126/science.285.5433.1505 [DOI] [PubMed] [Google Scholar]
- Harvell C.D, Jordan E, Merkel S, Raymundo L, Rosenberg E, Smith G, Weil E, Willis B.L. Coral disease: environmental change and the balance between coral and microbes. Oceanography. 2007;20:58–81. [Google Scholar]
- Hellio C, Bado-Nilles A, Gagnaire B, Renault T, Thomas-Guyon H. Demonstration of a true phenoloxidase activity and activation of a ProPO cascade in Pacific oyster, Crassostrea gigas (Thunberg) in vitro. Fish Shellfish Immunol. 2007;22:433–440. doi: 10.1016/j.fsi.2006.06.014. doi:10.1016/j.fsi.2006.06.014 [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O. Coral bleaching, climate change and the future of the world's coral reefs. Mar. Freshw. Res. 1999;50:839–866. doi:10.1071/MF99078 [Google Scholar]
- Hughes T.P, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. doi:10.1126/science.1085046 [DOI] [PubMed] [Google Scholar]
- Hutton D, Smith V.J. Antibacterial properties of isolated amoebocyte from the sea anemone Actinia equina. Biol. Bull. 1996;191:441–451. doi: 10.2307/1543017. doi:10.2307/1543017 [DOI] [PubMed] [Google Scholar]
- Jordan P.J, Deaton L.E. Characterization of phenoloxidase from Crassostrea virginica hemocytes and the effect of Perkinsus marinus on phenoloxidase activity in the hemolymph of Crassostrea virginica and Geukensia demissa. J. Shellfish Res. 2005;24:477–482. [Google Scholar]
- Kawaguti S. On the physiology of reef corals VI. Study on the pigments. Palao. Trop. Biol. Station Stud. 1944;2:617–673. [Google Scholar]
- Marshall P.A, Baird A.H. Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs. 2000;19:155–163. doi:10.1007/s003380000086 [Google Scholar]
- Mullen K, Peters E.C, Harvell C.D. Coral resistance to disease. In: Rosenberg E, Loya Y, editors. Coral health and disease. Springer; New York, NY: 2004. pp. 377–399. [Google Scholar]
- Munoz P, Meseguer J, Esteban M.A. Phenoloxidase activity in three commercial bivalve species. Changes due to natural infestation with Perkinsus atlanticus. Fish Shellfish Immunol. 2006;20:12–19. doi: 10.1016/j.fsi.2005.02.002. doi:10.1016/j.fsi.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Mydlarz L.D, Harvell C.D. Peroxidase activity and inducibility in the sea fan coral exposed to a fungal pathogen. Comp. Biochem. Physiol. A. 2007;146:54–62. doi: 10.1016/j.cbpa.2006.09.005. doi:10.1016/j.cbpa.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Mydlarz L.D, Jones L.E, Harvell C.D. Innate immunity, environmental drivers, and disease ecology of marine and freshwater marine invertebrates. Annu. Rev. Ecol. Evol. Syst. 2006;37:251–288. doi:10.1146/annurev.ecolsys.37.091305.110103 [Google Scholar]
- Mydlarz L.D, Holthouse S.F, Peters E.C, Harvell C.D. Cellular responses in sea fan corals: granular amoebocytes react to pathogen and climate stressors. PLoS ONE. 2008;3:1–9. doi: 10.1371/journal.pone.0001811. doi:10.1371/journal.pone.0001811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi A.J, Christensen B.M. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005;35:443–459. doi: 10.1016/j.ibmb.2005.01.014. doi:10.1016/j.ibmb.2005.01.014 [DOI] [PubMed] [Google Scholar]
- Olano C.T, Bigger C.H. Phagocytic Activities of the Gorgonian Coral Swiftia exserta. J. Invert Path. 2000;76:176–184. doi: 10.1006/jipa.2000.4974. doi:10.1006/jipa.2000.4974 [DOI] [PubMed] [Google Scholar]
- Oliver J. Recurrent seasonal bleaching and mortality of corals on the Great Barrier Reef. Proc. 5th Int. Coral Reef Symp. 1985;4:201–206. [Google Scholar]
- Page C, Willis B.L. Distribution, host range and large-scale spatial variability in black band disease prevalence on the Great Barrier Reef, Australia. Dis. Aquat. Org. 2006;69:41–51. doi: 10.3354/dao069041. doi:10.3354/dao069041 [DOI] [PubMed] [Google Scholar]
- Patterson M.J, Landolt M.L. Cellular reaction to injury in the anthozoan Anthopleura elegantissima. J. Invertebr. Pathol. 1979;33:189–196. doi:10.1016/0022-2011(79)90152-6 [Google Scholar]
- Persson M, Cerenius L, Soderhall K. The influence of haemocyte number on the resistance of the freshwater crayfish, Pacifastacus leniusculus Dana, to the parasitic fungus Aphanomyces astaci. J. Fish Diseases. 1987;10:471–477. doi:10.1111/j.1365-2761.1987.tb01098.x [Google Scholar]
- Peters E.C. Diseases of coral-reef organisms. In: Birkeland C, editor. Life and death of coral reefs. Chapman and Hall; New York, NY: 1997. pp. 114–139. [Google Scholar]
- Peterson G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. doi:10.1016/0003-2697(77)90043-4 [DOI] [PubMed] [Google Scholar]
- Petes L.E, Harvell C.D, Peters E.C, Webb M.A.H, Mullen K.M. Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar. Ecol. Prog. Ser. 2003;264:167–171. doi:10.3354/meps264167 [Google Scholar]
- Porchet-Henneré E, Vernat G. Cellular immunity in an annelid (Nereis diversicolor, Polychaeta): Production of melanin by a subpopulation of granulocytes. Cell Tissue Res. 1992;269:167–174. doi: 10.1007/BF00384737. doi:10.1007/BF00384737 [DOI] [PubMed] [Google Scholar]
- Ravindran J, Raghukumar C. Histological observations on the scleractinian coral Porites lutea affected by pink-line syndrome. Curr. Sci. 2006a;90:720–724. [Google Scholar]
- Ravindran J, Raghukumer C. Pink-line syndrome, a physiological crisis in the scleractinian coral Porites lutea. Mar. Biol. 2006b;149:347–356. doi:10.1007/s00227-005-0192-1 [Google Scholar]
- Rinkevich B. Invertebrates versus vertebrates innate immunity: in the light of evolution. Scand. J Immunol. 1999;50:456–460. doi: 10.1046/j.1365-3083.1999.00626.x. doi:10.1046/j.1365-3083.1999.00626.x [DOI] [PubMed] [Google Scholar]
- Rinkevich B, Sakai K. Interspecific interactions among species of the coral genus Porites from Okinawa. Jpn. J. Zool. 2001;104:91–97. doi: 10.1078/0944-2006-00014. [DOI] [PubMed] [Google Scholar]
- Ritchie K.B. Regulation of marine microbes by coral mucus and mucus associated bacteria. Mar. Ecol. Prog. Ser. 2006;322:1–14. doi:10.3354/meps322001 [Google Scholar]
- Rowley A.F. The evolution of inflammatory mediators. Mediat. Inflamm. 1996;5:3–13. doi: 10.1155/S0962935196000014. doi:10.1155/S0962935196000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks A.K. Academic Press; London, UK: 1972. Invertebrate pathology, non communicable diseases. [Google Scholar]
- Stedman T.L. 27th edn. Lippincott Williams & Wilkins; Baltimore, MD: 2000. Stedman's medical dictionary. [Google Scholar]
- Soderhall K, Cerenius L. Role of prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998;10:23–28. doi: 10.1016/s0952-7915(98)80026-5. doi:10.1016/S0952-7915(98)80026-5 [DOI] [PubMed] [Google Scholar]
- Vargas-Ángel B, Peters E.C, Kramarsky-Winter E, Gilliam D.S, Dodge R.E. Cellular reactions to sedimentation and temperature stress in the Caribbean coral Montastraea cavernosa. J. Invert. Path. 2007;95:140–145. doi: 10.1016/j.jip.2007.01.003. doi:10.1016/j.jip.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Willis B.L, Page C.A, Dinsdale E.A. Coral disease on the Great Barrier Reef. In: Rosenberg E, Loya Y, editors. Coral health and disease. Springer; New York, NY: 2004. pp. 69–104. [Google Scholar]