Abstract
Biotic interactions in the plankton can be both complex and dynamic. Competition among phytoplankton is often chemically mediated, but no studies have considered whether allelopathic compounds are modified by biotic interactions. Here, we show that compounds exuded during Karenia brevis blooms were allelopathic to the cosmopolitan diatom Skeletonema costatum, but that bloom allelopathy varied dramatically among collections and years. We investigated several possible causes of this variability and found that neither bloom density nor concentrations of water-borne brevetoxins correlated with allelopathic potency. However, when we directly tested whether the presence of competing phytoplankton influenced bloom allelopathy, we found that S. costatum reduced the growth-inhibiting effects of bloom exudates, suggesting that S. costatum has a mechanism for undermining K. brevis allelopathy. Additional laboratory experiments indicated that inducible changes to K. brevis allelopathy were restricted to two diatoms among five sensitive phytoplankton species, whereas five other species were constitutively resistant to K. brevis allelopathy. Our results suggest that competitors differ in their responses to phytoplankton allelopathy, with S. costatum exhibiting a previously undescribed method of resistance that may influence community structure and alter bloom dynamics.
Keywords: chemical ecology, competition, harmful algal bloom, plankton, variability
1. Introduction
Competition, one of the dominant processes structuring ecological communities, can occur through differential exploitation of limiting resources, but also through direct inhibition of competing organisms (Morin 1999; Krebs 2000). The direct inhibition of competitors via chemical compounds, allelopathy (Rice 1974; Lambers et al. 1998), can influence community-wide processes. For example, in microbial communities, allelopathic interactions can promote biodiversity if weak exploitation competitors persist in a community by directly inhibiting strong exploitation competitors (Czaran et al. 2002). Allelopathy has also been implicated in patterns of succession in both terrestrial (Gant & Clebsch 1975) and planktonic communities (Keating 1977; Vardi et al. 2002). Recent studies have indicated that allelopathy may also facilitate the spread of invasive species (Bais et al. 2003; Figueredo et al. 2007), if naive native competitors have not evolved resistance to allelopathic compounds of exotic competitors (Vivanco et al. 2004).
Recent studies have shown that phytoplankton allelopathy can alter aquatic community structure, including patterns of species dominance. For example, while allelopathic compounds produced by the haptophyte Prymnesium parvum suppressed the overall phytoplankton assemblage, dinoflagellates and cyanobacteria increased in relative abundance (Fistarol et al. 2003). Similarly, after exposure to allelopathic compounds exuded by the dinoflagellate Alexandrium tamarense, the dominant species within a natural phytoplankton community changed from the dinoflagellate Scrippsiella trochoidea to the diatom Leptocylindrus sp. (Fistarol et al. 2004b). However, how some phytoplankton resist allelopathic compounds is largely unknown.
Although rarely studied, competitors may fight back against allelopathy. For example, upon exposure to the allelopathic plant Centaurea maculosa, two resistant plants produce oxalate that prevents oxidative damage from the allelopathic compound (±)-catechin by scavenging the reactive oxygen species it produces (Weir et al. 2006). Resistant phytoplankton may also respond to allelopathic compounds from competitors; however, reports of such reciprocal interactions are rare, possibly because these interactions are difficult to detect in laboratory experiments. If we are to understand the dynamic process and consequences of chemical signalling in competition, we need to design experiments that allow detection of reciprocal interspecific interactions.
In the Gulf of Mexico, the red tide dinoflagellate Karenia brevis can form nearly monospecific blooms of thousands to millions of cells per litre (Tester & Steidinger 1997). Karenia brevis produces brevetoxins, polyketide-based natural products that cause neurotoxic shellfish poisoning in humans and massive fish kills (Landsberg 2002). Previous studies with K. brevis have shown that although both blooms and cultures exude compounds that inhibit the growth of competing phytoplankton, brevetoxins are rarely responsible for these effects (E. K. Prince, K. Poulson & J. Kubanek 2008, unpublished data; Kubanek et al. 2005; Prince et al. 2008). Only the diatom Skeletonema costatum was modestly but significantly suppressed by a mixture of three common brevetoxins at natural concentrations (Kubanek et al. 2005), yet these effects were variable (E. K. Prince, K. Poulson & J. Kubanek 2008, unpublished data). However, no previous studies have considered how the presence of competitors changes the allelopathic effects of K. brevis on those competitors, or indeed whether competitors alter allelopathy in any planktonic system. We hypothesized that some susceptible competitors may have a mechanism for dealing with K. brevis allelopathy, distinct from the constitutive resistance observed among some other phytoplankters (Kubanek et al. 2005). We chose S. costatum as a target competitor for testing this hypothesis since it is sensitive to K. brevis allelopathy, yet occasionally co-occurs with K. brevis and is generally abundant and widespread enough in the Gulf of Mexico to potentially influence plankton community structure (Saunders & Glenn 1969; Badylak et al. 2007).
2. Material and methods
(a) Phytoplankton culturing
Experiments were performed using 10 species of phytoplankton whose growth was previously shown to be affected by K. brevis live cells or filtrates (Kubanek et al. 2005). The following non-axenic (i.e. bacteria containing) clones were obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton (CCMP): the dinoflagellates Akashiwo cf. sanguinea (CCMP 1740), Peridinium sp. (CCMP 626), Prorocentrum mexicanum (CCMP 687) and Prorocentrum minimum (CCMP 695); the diatoms Amphora sp. (CCMP 129), Asterionellopsis glacialis (CCMP 137), Rhizosolenia cf. setigera (CCMP 1694), S. costatum (CCMP 775) and Thalassiosira sp. (CCMP 1055); and the cryptophyte Rhodomonas lens (CCMP 739). All species are known to co-occur with K. brevis in the Gulf of Mexico, were isolated from the Gulf of Mexico or the Caribbean (except R. cf. setigera available as an isolate from the Arabian Sea) and tolerate similar light, nutrient and temperature conditions. Cultures were maintained as described in Kubanek et al. (2005). Experiments were also conducted in L1+silicate media (CCMP) made with filtered Maine seawater (36 ppt).
(b) Collection of field samples
Field samples of K. brevis blooms (‘bloom samples’) and nearby field samples containing no K. brevis (‘non-bloom samples’) were collected during 2005 and 2006. Owing to the spatial and temporal variabilities associated with K. brevis blooms, all bloom samples in a given year were collected from a single bay. In October 2005, three bloom samples (3 l each) were collected during a red tide from St Joseph's Peninsula near the town of Port St Joe in Florida, USA. Three non-bloom samples (3 l each) were collected on the same day from Apalachicola Bay near the town of Apalachicola, FL. In September 2006, eight bloom samples (2 l each) were collected during a red tide at Long Boat Key beach, near the city of Bradenton, FL. Seven non-bloom samples (2 l each) were collected on the same day from the nearby beach at Green Key, near New Port Richey, FL. Collections at each site were approximately 10 m apart. Karenia brevis concentrations, assessed as described in Prince et al. (2008), ranged from 2.1×102 to 4.4×102 cells ml−1 in the bloom samples. Karenia brevis was not detected in the non-bloom samples, indicating concentrations less than 7×10−2 cells ml−1, the limit of detection for this study. We also identified common species from aliquots using the samples stained with Lugol's solution. We allowed 5 ml of each of three bloom and three non-bloom samples from each year to settle in a Palmer–Maloney settling chamber and identified all morphologically distinct species to the lowest taxonomic level possible by comparison with descriptions, pictures and drawings available in several taxonomic guides (Tomas et al. 1997; Horner 2002). Exuded organic compounds were extracted from each field sample using the method described in Prince et al. (2008). Type II brevetoxins (e.g. PbTx-2, PbTx-3 and PbTx-9) in K. brevis cultures were quantified by competitive ELISA (Naar et al. 2002) using the method described in Kubanek et al. (2005), and brevetoxin B (PbTx-2) was quantified from all field samples using liquid chromatography/mass spectrometry (LC–MS) (Kubanek et al. 2007).
(c) Variability in bloom allelopathy (experiment 1)
We assessed allelopathic effects of K. brevis blooms on the growth of S. costatum, by exposing cultured S. costatum to extracellular extracts of bloom and non-bloom field samples. The experimental design and extract addition was conducted as described in Prince et al. (2008), except that extracts were added on the day the cultures were inoculated and again after 7 d to counteract the probable decomposition of allelopathic compounds. In 2005, culture tubes of S. costatum initially contained 6.0 ml of media and were inoculated with 250 μl of S. costatum culture, and in 2006 the culture tubes initially contained 5.0 ml of media. Final concentration of S. costatum in each tube was (1.8±0.3)×104 (n=10 in 2005, n=3 in 2006). One additional 2006 bloom sample was tested using six replicates. Relative cell growth of each culture was assessed as described in Kubanek et al. (2005).
(d) Effect of S. costatum on bloom allelopathy (experiment 2)
We tested how the competitor S. costatum affected K. brevis bloom allelopathy by exposing field bloom samples to S. costatum before extractions. After collection, half of each bloom sample was exposed to live cultured S. costatum cells (filtered onto GF/F filter paper and resuspended in filtered seawater to avoid adding nutrients to field samples), and the other half was exposed only to filtered seawater (collected from Boothbay Harbor, Maine). In 2005, 100 ml of S. costatum was added to each of three bloom and three non-bloom samples. In 2006, 50 ml of S. costatum was added to each of seven bloom and seven non-bloom samples (controls received the same volume of filtered seawater). The final density of S. costatum in treated field samples was (1.9±0.8)×104 cells ml−1 in 2005 and (1.6±0.2)×104 cells ml−1 in 2006. The samples to which S. costatum was added were referred to as either ‘bloom+S. costatum samples’ or ‘non-bloom+S. costatum samples’. Because one 2006 bloom sample (no. 11) was not exposed to S. costatum, the total number of ‘bloom+S. costatum’ samples was 10, even though the total number of ‘bloom’ samples was 11. After 36 h of exposure to S. costatum, the samples were extracted using the methods described previously.
The growth of S. costatum exposed to bloom, bloom+S. costatum, non-bloom+S. costatum and non-bloom extracts was measured using the methods described for experiment 1. Data for each treatment were normalized to those of non-bloom controls using the following equation: per cent growth of S. costatum relative to non-bloom control=(1−([S.c.]ctrl−[S.c.]trt)/([S.c.]ctrl))×100%, where [S.c.] ctrl is the average maximum fluorescence of S. costatum exposed to non-bloom controls and [S.c.] trt is the maximum fluorescence of S. costatum exposed to each of the other treatments. Cell concentrations in cultures of S. costatum exposed to non-bloom+S. costatum extracts were never significantly different from the cell concentrations of S. costatum exposed to non-bloom extract controls (data not shown; nonlinear regression, p=0.88 in 2005 and p=0.47 in 2006).
(e) Effect of competitors on allelopathy of K. brevis cultures (experiment 3)
Experiment 3 was conducted with cultured phytoplankton to determine whether the ability to alter K. brevis allelopathy was common among phytoplankton or specific to S. costatum. The experimental design is presented in the electronic supplementary material. Initially, two 1 l cultures were inoculated with K. brevis, and two 1 l seawater control flasks were set up on the same day for each of 10 phytoplankton competitor species. Once the K. brevis cultures reached exponential growth stage (5–10 d), 150 ml of either a target competitor culture or seawater was added to each culture. All flasks remained in the incubator for 36 h and then extracted using the method described above. Concentrations of K. brevis cells in cultures were 7.4×103 to 17×103 cells ml−1, and the competitor cell concentrations ranged from 3.6×102 cells ml−1 for the slow-growing A. cf. sanguinea to 6.4×104 cells ml−1 for Thalassiosira sp.
To test the allelopathic activity of each extract, 10 species of competing phytoplankton (A. cf. sanguinea, Amphora sp., A. glacialis, Peridinium sp., P. mexicanum, P. minimum, R. cf. setigera, R. lens, S. costatum and Thalassiosira sp.) were each grown in 60 ml culture tubes. For each species except A. cf. sanguinea and P. mexicanum, the tubes containing 29.0 ml of L1+silicate media were inoculated with 1.0 ml of phytoplankton. A 2.5 ml inoculum plus 27.5 ml media was used for the slow-growing A. cf. sanguinea and P. mexicanum. Each tube received extracts generated from 30.0 ml of one of the four treatments described earlier (each dissolved in 50 μl of DMSO) (n=8). Extracts were added initially on the day the experiment began and again after 7 d. Relative cell concentration of each tube was measured as described above. Experiment 3 was repeated five additional times for S. costatum at a smaller scale (using the same experimental design used for experiment 1, 2005; n=5), with independent extracts generated for each experiment.
We normalized allelopathic effects using an equation similar to the that used in experiment 1: per cent growth relative to control=(1−([C.P.]ctrl−[C.P.]trt)/([C.P.]ctrl))×100%, where [C.P.] ctrl is the average maximum fluorescence of the competing phytoplankter exposed to the seawater extract control and [C.P.] trt is the maximum fluorescence of the competing phytoplankter exposed to either K. brevis, K. brevis+competitor or competitor extracts.
(f) Statistical analysis
Before testing for differences in allelopathic effects among treatments and collections, data were tested for normality using both Kolmogorov–Smirnov and D'Agostino–Pearson tests (Dagostino et al. 1990). Although the Kolmogorov–Smirnov test suggested that data from all experiments were distributed normally, the D'Agostino–Pearson test indicated that data from experiment 2 had a non-normal distribution. Experiments 1 and 2 were analysed using nested ANOVA (Systat 9), with the random effects of collection nested within the fixed effects of treatment (Zar 1999). For experiments 1 and 2, we analysed the data from the 2005 and 2006 blooms separately, and pairwise comparisons between treatments were made using Tukey's post hoc test. Because we cannot be sure that data from experiment 2 were normally distributed, we also analysed the combined 2005 and 2006 results (i.e. the average effect of each collection) using the non-parametric Mann–Whitney test (GraphPad Prism 4.0). Differences were accepted as significant when p<0.05.
For experiment 3, growth curves were compared among treatments using nonlinear regression with an F-test (GraphPad Prism 4.0). A Gompertz (bacterial growth) equation successfully fits the data in most cases. All treatments were compared with the ‘seawater’ control, and ‘K. brevis’ treatments were compared with ‘K. brevis+target competitor’ treatments with respect to maximum cell concentration. Differences were accepted as significant when p<0.05. In the cases where nonlinear regression failed to fit the data, the maximum relative cell concentration data were compared for treatment versus seawater control via two-tailed t-tests. When this led to two contrasts using a single control dataset, we used a Bonferroni adjustment to determine that differences were accepted as significant when p<0.025.
We used regression analysis to test for a correlation between concentrations of brevetoxins and allelopathic potency of K. brevis blooms and cultures, as well as for a correlation between K. brevis cell concentrations and allelopathic potency of blooms and cultures (GraphPad Prism 4.0). We accepted a trend as significantly non-zero when p<0.05.
3. Results
(a) Phytoplankton community composition of bloom and non-bloom samples
In both 2005 and 2006, the vast majority of phytoplankton cells present in bloom samples were K. brevis, and no species other than K. brevis made up more than 5 per cent of phytoplankton cells in the community. In 2005, the remainder of the bloom community was largely composed of unidentified species of pennate diatoms. Other dinoflagellate species including Ceratium furca and P. mexicanum were also present in low numbers. By contrast, the 2005 non-bloom community did not have a single dominant species, and included at least 20 morphologically distinct species, including nearly all of the species present in the bloom community except K. brevis. The centric diatom, S. costatum (or other members of this species complex; Sarno et al. 2005), was not observed in the 2005 bloom samples nor in the 2005–2006 non-bloom samples. The 2006 bloom samples contained dinoflagellates Oxyphysis oxytoxoides and Scrippsiella sp. and diatoms of the Chaetoceros and Bacteriastrum genera. Skeletonema costatum was present at 67±25 cells ml−1 in the 2006 bloom samples (n=8). The composition of the 2006 non-bloom community was quite different from the 2006 bloom community; nearly half of the cells present belonged to one of a few species of pennate diatoms, including Nitzschia longissima. One dinoflagellate, P. mexicanum, also made a significant contribution to the 2006 non-bloom community, as did several other species at lesser abundance, including the dinoflagellate C. furca and a small number of unidentified centric diatoms.
(b) Variability in bloom allelopathy (experiment 1)
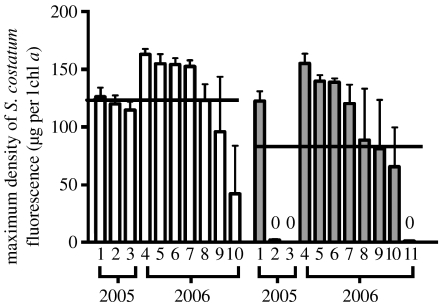
The allelopathic effects of K. brevis blooms were highly variable. Although on average, S. costatum exposed to bloom extracts grew only to 73 per cent of the density of controls, final densities ranged from less than 1 to 122 per cent of controls (figure 1). In 2005, the overall allelopathic effect of K. brevis on S. costatum was quite strong, with bloom extracts suppressing S. costatum by an average of 66 per cent relative to non-bloom controls (p<0.001, n=3). However, in 2006, the allelopathic effects of K. brevis bloom samples were only marginally significant, with bloom extracts suppressing S. costatum by 13 per cent relative to non-bloom controls (p=0.063, n=7–8). The allelopathic potency of K. brevis was highly variable even within years: in both 2005 and 2006, allelopathy varied significantly among bloom collections (p<0.001).
Figure 1.
Effects of extracellular extracts of K. brevis blooms (open bars) and non-bloom controls (filled bars) on the growth of S. costatum (experiment 1). Each bar represents the average maximum growth of S. costatum treated with the extract of an independent field sample and measured after 14–18 d. Black lines indicate the grand mean of each treatment. Error bars indicate standard error. The grand means are significantly different between treatments in 2005 (p<0.001; n=3) and marginally significant between treatments in 2006 (p=0.063; n=7–8). For both years, the effect of sample within treatments is significant (p<0.001; n=3–10).
(c) Correlation of allelopathy with K. brevis cell density and with brevetoxin concentration
We detected no quantitative relationship between the magnitude of inhibition of S. costatum by K. brevis and the concentration of K. brevis cells present in cultures (r2=0.26, p=0.13) or the 2005–2006 blooms (r2=0.053, p=0.50) over the range of K. brevis cell concentrations present in these samples (i.e. 8.5×103 to 17×103 cells ml−1 present in cultures and 2.1×102 to 4.4×102 cells ml−1 present in blooms). We detected no relationship between inhibition of S. costatum by K. brevis blooms and concentrations of water-borne brevetoxin B (PbTx-2) in the 2005–2006 samples (r2=0.19, p=0.18) or PbTx-3 in the 2006 samples (r2=0.24, p=0.26). PbTx-3 concentrations were not measured in 2005. Allelopathic potency and the concentration of all water–borne type II brevetoxins (which include PbTx-2, PbTx-3 and PbTx-9) were marginally inversely related over the range of brevetoxins present in K. brevis cultures, 9.0–35.5 ng ml−1 (r2=0.32, p=0.054).
(d) Effects of S. costatum on bloom allelopathy (experiment 2)
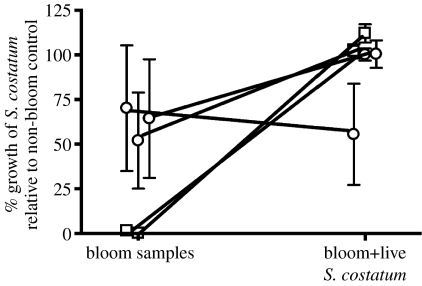
Allelopathic effects of K. brevis blooms decreased when exposed to live S. costatum (figure 2). Extracts of five allelopathic bloom samples from 2005 to 2006 (samples 2–3 and 8–10, figure 1) all inhibited the growth of S. costatum cultures by more than 30 per cent, with an average suppression of 62 per cent. When each of these five bloom samples was exposed for 36 h to S. costatum (i.e. bloom+S. costatum samples), the resulting extracts suppressed the growth of S. costatum by only 6 per cent, significantly less than the extracts of bloom samples that were incubated without S. costatum for this same period of time (p=0.032, Mann–Whitney test; figure 2). In 2005, reduction in K. brevis allelopathic potency by S. costatum was dramatic, with the allelopathic potency of bloom samples reduced by more than 99 per cent (samples 2–3, figure 2; p<0.001, nested ANOVA). When the 2006 bloom data were considered alone (i.e. samples 8–10, figure 1), the trend was towards a reduction in allelopathy, with the allelopathic potency of bloom samples reduced by 53 per cent; however, the effect was not significant (p=0.29).
Figure 2.
Effects of live S. costatum cells on the allelopathic effects of K. brevis bloom extracts (experiment 2). Paired samples are connected by a line. Error bars indicate standard error. The mean is significantly different between treatments (p=0.032; n=5 bloom samples; n=3–10 allelopathy assays per sample). Squares, 2005; circles, 2006.
(e) Effects of competitors on allelopathy of K. brevis cultures (experiment 3)
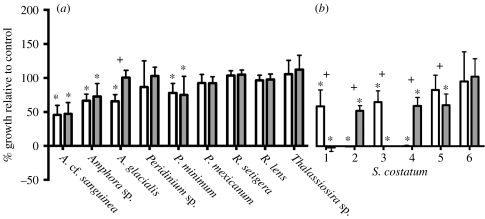
Of the 10 competitor species tested, only A. glacialis and S. costatum significantly altered the allelopathic potency of cultured K. brevis (figure 3). Three species, A. cf. sanguinea, Amphora sp. and P. minimum, were significantly inhibited by the extracts of K. brevis cultures and K. brevis+competitor cultures, indicating susceptibility to K. brevis allelopathy (p<0.01 for all, using contrasts of maximum cell concentration). Five species, Peridinium sp., P. mexicanum, R. cf. setigera, R. lens and Thalassiosira sp., were not inhibited by K. brevis or K. brevis+competitor extracts, suggesting constitutive resistance to K. brevis allelopathy (p=0.097–0.87). Asterionellopsis glacialis, by contrast, was significantly inhibited by K. brevis extracts (p<0.001), but not by K. brevis+A. glacialis extracts (p=0.70), indicating loss of K. brevis allelopathy caused by exposure to A. glacialis. No species was significantly inhibited by its own exudates (p=0.083–0.83), although R. cf. setigera and P. mexicanum were stimulated by their own exudates (data not shown).
Figure 3.
(a) The effects of extracellular extracts of K. brevis cultures exposed to seawater or competitor culture on the growth of nine competitor species. Each bar represents the maximum cell density of a phytoplankton species normalized to the growth of the same species exposed to a seawater control. White bars, extracellular extract of K. brevis; grey bars, extracellular extract of K. brevis and competitor. (b) Six independent tests of the growth of S. costatum exposed to the same treatments as (a). Asterisks indicate a significant allelopathic effect relative to seawater control, and plus signs indicate a significant difference between treatments for a given species (n=5–8).
The effects of S. costatum on cultured K. brevis allelopathy were extremely variable. In two separate experiments, S. costatum strongly undermined the allelopathic potency of K. brevis cultures (p<0.001 for both; 2 and 4, figure 3b). However, in two other experiments, S. costatum strongly induced allelopathic potency in K. brevis cultures (p<0.001 for both; 1 and 3, figure 3b), and in one case weakly induced K. brevis allelopathy (p=0.038; 5, figure 3b). In one case, K. brevis cultures were not allelopathic to S. costatum, whether or not K. brevis was exposed to S. costatum (p=0.30; 6, figure 3b).
4. Discussion
(a) Skeletonema costatum undermines allelopathy of K. brevis blooms
In this study, we provide the first example of allelopathy undermined by a phytoplankton competitor. Karenia brevis allelopathic effects decreased significantly when bloom samples were exposed to live S. costatum cells (figure 2), indicating that S. costatum possesses a mechanism to undermine the allelopathic potency of K. brevis blooms. Skeletonema costatum also undermined allelopathy in two independent experiments with cultured K. brevis (figure 3b), but there was substantial variability in this process for both natural bloom and cultured K. brevis (figures 2 and 3).
Although rare, other studies have proposed mechanisms by which phytoplankton resist or lessen the effects of allelopathy. Reports of co-occurring phytoplankton reciprocally inhibited by each other's exudates suggest that some phytoplankton may counter allelopathic effects by producing their own allelopathic compounds (e.g. Vardi et al. 2002; Yamasaki et al. 2007). If S. costatum was allelopathic to K. brevis, we would have expected that live S. costatum added to blooms would affect the number, morphology or behaviour of K. brevis cells. However, we observed no such effects in bloom samples over 36 h. Using a different mechanism to resist allelopathy, the dinoflagellate S. trochoidea formed temporary cysts in response to exudates from potentially allelopathic phytoplankton (including dinoflagellates Alexandrium spp. and Karenia mikimotoi), which may have enabled S. trochoidea to escape mortality from allelopathic compounds (Fistarol et al. 2004a; Tillmann et al. 2007). Other competitors appear to be constitutively resistant to allelopathic compounds, by unknown mechanisms (Kubanek et al. 2005; figure 3a). However, the direct interference of K. brevis allelopathy by S. costatum (figure 2) appears to be markedly different from these previously observed instances of allelopathy resistance.
Skeletonema costatum is known to exhibit higher growth rates and lower nutrient requirements than K. brevis (Furnas 1990; Steidinger et al. 1998), suggesting that it may outcompete K. brevis for resources and indirectly undermine allelopathy by depriving K. brevis of the resources needed for the production of allelopathic compounds. Alternatively, S. costatum may stop biosynthesis or exudation of K. brevis allelopathic compounds, a mechanism used by pathogenic bacteria to avoid defences of terrestrial plants (Abramovitch & Martin 2004; Bais et al. 2005). S. costatum may also metabolize or degrade allelopathic compounds released by K. brevis, a strategy used by the fungal pathogen Septoria lycopersici in response to the defensive compounds of the tomato plant (Bouarab et al. 2002). Finally, S. costatum may produce compounds counteracting the physiological damage of allelopathy, observed for two plants responding to the allelopathic plant Centaurea maculosa (Weir et al. 2006).
Because diffusion should be the dominant process carrying allelopathic compounds away from individual cells, the cells are expected to be surrounded by a halo of their exuded compounds with much of the surrounding water and exudate moving with each cell as it swims (Purcell 1977; Dusenbery 1992). On the scale of individual cells, it is likely that there exists a complex chemical landscape, with patches of high concentrations of allelopathic compounds surrounded by areas of comparatively low concentration. Thus, an individual allelopathic cell should be able to benefit from poisoning an individual competitor cell. However, we expect a serious limitation to the evolution by natural selection of an induced resistance strategy that takes up to 36 h to manifest, since during that period of time a resisting cell may have moved away from an allelopathic cell, which therefore may not benefit from its own resistance strategy. One plausible exception to this time limitation would be if target cells cooperatively launched their undermining of allelopathy within a spatial area no smaller than a cell's daily range, such that these individuals (or their daughter clones) could directly benefit from the induced resistance. On the other hand, it is possible that the induced resistance we observed happened more quickly than our 36 h incubation allowed us to detect, which could make undermining allelopathy adaptive to competitors. We intend to test this in future studies.
(b) The ability of competitors to influence K. brevis allelopathy is rare
When we tested 10 species of phytoplankton common to the Gulf of Mexico for their ability to influence the allelopathy of cultured K. brevis, we found that competitor-mediated changes in allelopathic potency were rare. Two dinoflagellates and a diatom had no effect on K. brevis allelopathic potency, and two dinoflagellates, two diatoms and a cryptophyte were constitutively resistant to K. brevis allelopathy (figure 3a). Only two diatoms, A. glacialis and S. costatum, affected the allelopathic potency of K. brevis cultures (figure 3), suggesting that like S. costatum, A. glacialis possesses a mechanism for undermining K. brevis allelopathy. Although the concentration and biovolume of competitor cells added to K. brevis cultures varied by several orders of magnitude among the 10 competitor species tested, concentrations and biovolumes of A. glacialis and S. costatum represented neither the highest nor the lowest values, indicating that the ability to undermine K. brevis allelopathy was not dependent on cell abundance alone.
Exposure to S. costatum affected the allelopathic potency of K. brevis cultures in a manner that was dramatic but highly variable. The factors that influence allelopathy may differ between cultured versus field phytoplankton, since we occasionally observed induction of allelopathic potency of cultured K. brevis by exposure to S. costatum (1 and 3, figure 3b), but allelopathic induction was not observed with natural bloom samples (figure 2). However, the variability in the allelopathic potency of K. brevis blooms and cultures indicates that although competitors significantly influence K. brevis allelopathy, other factors are probably at play.
(c) Allelopathic potency of K. brevis blooms and cultures is variable
As previously established, compounds exuded during natural blooms of K. brevis and from the cultures of K. brevis were significantly allelopathic to the diatom S. costatum (Prince et al. 2008), but the magnitude of allelopathic effects varied widely among field samples and cultures of K. brevis (p<0.001; figures 1 and 3b). We tested a total of 11 samples from K. brevis blooms over 2 years, of which 3 suppressed growth of S. costatum by more than 95 per cent, 3 suppressed growth of S. costatum by at least 30 per cent and 5 did not suppress S. costatum growth (figure 1). Similarly, of six extracts of K. brevis cultures, two suppressed the growth of S. costatum by more than 95 per cent, two modestly but significantly suppressed S. costatum and two had no effect (figure 3b). However, the variability of allelopathic potency in K. brevis blooms and cultures was not related to concentration of water-borne brevetoxins (see §3), indicating that other K. brevis compounds were responsible for the majority of the observed allelopathic effects. We are currently working to characterize the multiple allelopathic compounds of K. brevis, which appear to be low-molecular-weight, polar, unstable organic compounds unrelated to the brevetoxin class of secondary metabolites (E. K. Prince, K. Poulson & J. Kubanek 2008, unpublished data).
While published reports indicate that allelopathy varies with cell concentration (e.g. Vardi et al. 2002; Yamasaki et al. 2007), we found no direct relationship between cell density and allelopathic potency for either bloom or cultures of K. brevis (see §3). All of the bloom samples contained hundreds of cells ml−1, considered the low end of a medium-density bloom (Florida Fish and Wildlife Research Institute; http://research.myfwc.com/). Cell concentrations of K. brevis cultures were approximately 10 000 cells ml−1, corresponding to high-density blooms. However, K. brevis concentrations in the Gulf of Mexico range from undetectable to thousands of cells ml−1 (Steidinger & Haddad 1981). It is possible that K. brevis blooms with concentrations of thousands of cells ml−1 are more consistently allelopathic than the less concentrated blooms used in this study, or that there is an approximate cell density threshold, above which blooms are more likely to be allelopathic.
In a previous study of K. brevis, Kubanek et al. (2005) found that allelopathy of cultured K. brevis did vary with growth stage, as previously observed in other systems (e.g. Schmidt & Hansen 2001; Wang et al. 2006). However, in the current study, all cultures of K. brevis were extracted during exponential growth stage yet had widely variable allelopathic effects (figure 3b), indicating that K. brevis growth stage alone cannot explain variance in allelopathic potency among cultures. Because allelopathic potency of field samples has not been previously assessed, it is difficult to determine whether allelopathy varies with bloom development stage. In this study, all samples of K. brevis blooms were taken from established blooms during ‘maintenance’ stage, a time characterized by low growth rates and probable nutrient limitation (Steidinger et al. 1998). Bloom samples from the same year were collected within 1 h of each other, no more than 70 m apart, suggesting that samples of a given year contained K. brevis of approximately the same growth stage. However, allelopathy varied dramatically among the field samples (figure 1), indicating that factors other than K. brevis bloom stage must play a role in allelopathy.
The observed variability in the allelopathic effects may have been caused by a number of factors. Plankton community interactions are known for their variable and even chaotic outcomes (Beninca et al. 2008). It has previously been noted that competitor physiological status affects susceptibility to allelopathy (Fistarol et al. 2005). Skeletonema costatum used in experiment 2 was in stationary phase but S. costatum was growing exponentially at the time of experiment 3, indicating that the physiological status of S. costatum was different in these two experiments. However, these differences cannot explain the high variance within experiments 2 and 3. Although the production of secondary metabolites has also been shown to vary with time of day for some phytoplankton (Taroncher-Oldenburg et al. 1997), this factor could not have been responsible because we started and terminated laboratory experiments at the same time of day for all tests. By contrast, S. costatum was added to the field samples of K. brevis blooms at different times of day in 2005 versus 2006, which may have been partially responsible for the differences in allelopathy (figure 2). However, because allelopathy of bloom samples also varied within years, localized differences in field conditions (e.g. nutrient concentrations, light levels, mixing intensity, presence of other community members) probably affected allelopathic potency of K. brevis blooms. Bacteria associated with K. brevis in bloom samples and cultures may have influenced allelopathy, either by directly producing allelopathic compounds or by mediating allelopathic effects of K. brevis. Associated bacteria can, by unknown mechanisms, increase the production of secondary metabolites (including toxins) by eukaryotic phytoplankton (Bates et al. 1995), whereas other marine bacteria are algicidal to phytoplankton including K. brevis (Mayali & Doucette 2002). Because we used natural bloom samples and non-axenic cultured strains of K. brevis, the involvement of bacteria in allelopathy remains a viable hypothesis, along with the possibility that the observed undermining could be due to antibiotic effects of S. costatum against an allelopathic bacterium. However, our field samples did not contain noticeable quantities of colonial cyanobacteria such as Trichodesmium spp., which bloom in the Gulf of Mexico (Lenes et al. 2001). If bacteria are responsible for K. brevis allelopathy, they would more likely be non-colonial, symbiotic organisms found both in blooms and non-axenic cultures. Future experiments will test this further.
(d) Chemically mediated interactions among competitors may have community-wide consequences
In addition to the effects on community composition caused by the differential susceptibility of competitor species to allelopathy, the dynamics of K. brevis blooms may be influenced by the presence of a few specific competitors, such as S. costatum and A. glacialis, which occasionally undermine bloom allelopathy (figures 2 and 3). We focused on the cosmopolitan diatom, Skeletonema (potentially composed of several cryptic species; Sarno et al. 2005), because it is one of the dominant phytoplankters present in the Gulf of Mexico (Saunders & Glenn 1969) and routinely found at densities greater than 1000 cells per ml during the autumn (Turner & Hopkins 1974; Badylak et al. 2007) when K. brevis blooms frequently occur (Tester & Steidinger 1997). We found that while S. costatum was not present in any 2005 samples nor in the 2006 non-bloom samples, the concentration of S. costatum was 67±25 cells ml−1 in the 2006 bloom samples, making it one of the most abundant phytoplankters other than K. brevis. Although a number of factors, including the levels of light, nutrients and turbulence, may have influenced the allelopathic potency of K. brevis bloom samples, the presence of S. costatum in field samples may explain differences in allelopathic potency between 2005 and 2006 samples. If S. costatum undermines K. brevis allelopathy in the field, then its presence in 2006 may help explain why these bloom samples were only marginally allelopathic (figure 1) and why the addition of S. costatum cells to K. brevis blooms did not significantly decrease the allelopathic potency of 2006 samples (figure 2), since live K. brevis cells in bloom samples had already been exposed to S. costatum in the field. This needs to be explored further, since so far we have only sampled two K. brevis blooms (2005 and 2006), and the samples within each bloom were collected within 70 m of each other. The fact that S. costatum was relatively common in the 2006 bloom compared with other, non-Karenia species, suggests that the undermining of K. brevis allelopathy by S. costatum is likely to be ecologically meaningful: the ability of S. costatum to coexist with K. brevis may provide it with a competitive advantage over species that are more consistently sensitive to K. brevis allelopathy, potentially altering the composition of the bloom community.
In conclusion, we found K. brevis blooms to be allelopathic, but the magnitude of allelopathy varied dramatically between collections and years (figure 1). Neither brevetoxin concentration nor K. brevis cell density explained the variance in allelopathy. However, when we directly tested whether competitive interactions influenced allelopathy, we found that the diatom S. costatum was able to substantially undermine the allelopathic potency of K. brevis blooms (figure 2). This ability is not common to most Gulf of Mexico phytoplankton, suggesting that S. costatum may be able to occur during K. brevis blooms partly because it can overcome allelopathy, although some other competitors appear to be constitutively resistant, a strategy expected to be even more effective than induced resistance (figure 3a). Our results indicate that phytoplankton competitors may play an important role in K. brevis bloom dynamics and underscore the importance of reciprocal competitive interactions and the complexity of species interactions in planktonic communities.
Acknowledgments
This research was supported by National Science Foundation (NSF) grants OCE-0134843 and OCE-0726689 to J.K. E.K.P. was additionally supported by an NSF Integrative Graduate Education and Research Traineeship fellowship. We would like to thank the owners of Presnell's Marina in Port St Joe, Florida, R. Bartleson and S. Bartone of the Sanibel Captiva Conservation Foundation Marine Laboratory and C. Heil and E. Truby of Florida Fish and Wildlife Conservation Commission for field assistance. We would also like to thank C. Pirkle for laboratory assistance, and W. Morrison and two anonymous reviewers for their comments that substantially improved the manuscript.
References
- Abramovitch R.B, Martin G.B. Strategies used by bacterial pathogens to suppress plant defenses. Curr. Opin. Plant Biol. 2004;7:356–364. doi: 10.1016/j.pbi.2004.05.002. doi:10.1016/j.pbi.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Badylak S, Phlips E.J, Baker P, Fajans J, Boler R. Distributions of phytoplankton in Tampa Bay Estuary, USA 2002–2003. Bull. Mar. Sci. 2007;80:295–317. [Google Scholar]
- Bais H.P, Vepachedu R, Gilroy S, Callaway R.M, Vivanco J.M. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science. 2003;301:1377–1380. doi: 10.1126/science.1083245. doi:10.1126/science.1083245 [DOI] [PubMed] [Google Scholar]
- Bais H.P, Prithiviraj B, Jha A.K, Ausubel F.M, Vivanco J.M. Mediation of pathogen resistance by exudation of antimicrobials from roots. Nature. 2005;434:217–221. doi: 10.1038/nature03356. doi:10.1038/nature03356 [DOI] [PubMed] [Google Scholar]
- Bates S.S, Douglas D.J, Doucette G.J, Leger C. Enhancement of domoic acid production by reintroducing bacteria to axenic cultures of the diatom Pseudo-nitzchia multiseries. Nat. Toxins. 1995;3:428–435. doi: 10.1002/nt.2620030605. doi:10.1002/nt.2620030605 [DOI] [PubMed] [Google Scholar]
- Beninca E, Huisman J, Heerkloss R, Johnk K.D, Branco P, Van Nes E.H, Scheffer M, Ellner S.P. Chaos in a long-term experiment with a plankton community. Nature. 2008;451:822–825. doi: 10.1038/nature06512. doi:10.1038/nature06512 [DOI] [PubMed] [Google Scholar]
- Bouarab K, Melton R, Peart J, Baulcombe D, Osbourn A. A saponin-detoxifying enzyme mediates suppression of plant defences. Nature. 2002;418:889–892. doi: 10.1038/nature00950. doi:10.1038/nature00950 [DOI] [PubMed] [Google Scholar]
- Czaran T.L, Hoekstra R.F, Pagie L. Chemical warfare between microbes promotes biodiversity. Proc. Natl. Acad. Sci. USA. 2002;99:786–790. doi: 10.1073/pnas.012399899. doi:10.1073/pnas.012399899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagostino R.B, Belanger A, Dagostino R.B. A suggestion for using powerful and informative tests of normality. Am. Stat. 1990;44:316–321. doi:10.2307/2684359 [Google Scholar]
- Dusenbery D.B. W.H. Freeman; New York, NY: 1992. Sensory ecology: how organisms acquire and respond to information. [Google Scholar]
- Figueredo C.C, Giani A, Bird D.F. Does allelopathy contribute to Cylindrospermopsis raciborskii (cyanobacteria) bloom occurrence and geographic expansion? J. Phycol. 2007;43:256–265. doi:10.1111/j.1529-8817.2007.00333.x [Google Scholar]
- Fistarol G.O, Legrand C, Graneli E. Allelopathic effect of Prymnesium parvum on a natural plankton community. Mar. Ecol. Prog. Ser. 2003;255:115–125. doi:10.3354/meps255115 [Google Scholar]
- Fistarol G.O, Legrand C, Rengefors K, Graneli E. Temporary cyst formation in phytoplankton: a response to allelopathic competitors? Environ. Microbiol. 2004;6:791–798. doi: 10.1111/j.1462-2920.2004.00609.x. doi:10.1111/j.1462-2920.2004.00609.x [DOI] [PubMed] [Google Scholar]
- Fistarol G.O, Legrand C, Selander E, Hummert C, Stolte W, Graneli E. Allelopathy in Alexandrium spp.: effect on a natural plankton community and on algal monocultures. Aquat. Microb. Ecol. 2004;35:45–56. doi:10.3354/ame035045 [Google Scholar]
- Fistarol G.O, Legrand C, Graneli E. Allelopathic effect on a nutrient-limited phytoplankton species. Aquat. Microb. Ecol. 2005;41:153–161. doi:10.3354/ame041153 [Google Scholar]
- Furnas M.J. In situ growth rates of marine phytoplankton—approaches to measurement, community and species growth rates. J. Plankton Res. 1990;12:1117–1151. doi:10.1093/plankt/12.6.1117 [Google Scholar]
- Gant R.E, Clebsch E.E.C. Allelopathic influences of Sassafras albidum in old-field succession in Tennessee. Ecology. 1975;56:604–615. doi:10.2307/1935494 [Google Scholar]
- Horner R.A. Biopress Limited; Bristol: 2002. A taxonomic guide to some common marine phytoplankton. [Google Scholar]
- Keating K.I. Allelopathic influence of blue–green bloom sequences in a eutrophic lake. Science. 1977;196:885–887. doi: 10.1126/science.196.4292.885. doi:10.1126/science.196.4292.885 [DOI] [PubMed] [Google Scholar]
- Krebs C.J. Benjamin-Cummings Publishing Company; San Francisco, CA: 2000. Ecology: the experimental analysis of distribution and abundance. [Google Scholar]
- Kubanek J, Hicks M.K, Naar J, Villareal T.A. Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton? Limnol. Oceanogr. 2005;50:883–895. [Google Scholar]
- Kubanek J, Snell T.W, Pirkle C. Chemical defense of the red tide dinoflagellate Karenia brevis against rotifer grazing. Limnol. Oceanogr. 2007;52:1026–1035. [Google Scholar]
- Lambers H, Chapin F.S, Pons T.L. Springer-Verlag; Berlin: 1998. Plant physiological ecology. [Google Scholar]
- Landsberg J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002;10:113–390. doi:10.1080/20026491051695 [Google Scholar]
- Lenes J.M, Darrow B.P, Cattrall C, Heil C.A, Callahan M, Vargo G.A, Byrne R.H, Prospero J.M, Bates D.E, Fanning K.A, Walsh J.J. Iron fertilization and the Trichodesmium response on the West Florida shelf. Limnol. Oceanogr. 2001;46:1261–1277. [Google Scholar]
- Mayali X, Doucette G.J. Microbial community interactions and population dynamics of an algicidal bacterium active against Karenia brevis (Dinophyceae) Harmful Algae. 2002;1:277–293. doi:10.1016/S1568-9883(02)00032-X [Google Scholar]
- Morin P.J. Blackwell Science, Inc; Malden, MA: 1999. Community ecology. [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney P.L, Flewelling L, Steidinger K, Lancaster J, Baden D.G. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ. Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince E.K, Myers T.L, Kubanek J. Effects of harmful algal blooms on competitors: allelopathic mechanisms of the red tide dinoflagellate Karenia brevis. Limnol. Oceanogr. 2008;53:531–541. [Google Scholar]
- Purcell E.M. Life at low Reynolds number. Am. J. Phys. 1977;45:3–11. doi:10.1119/1.10903 [Google Scholar]
- Rice E.L. Academic Press; New York: 1974. Allelopathy. [Google Scholar]
- Sarno D, Kooistra W.H.C.F, Medlin L.K, Percopo I, Zingone A. Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species with the description of four new species. J. Phycol. 2005;41:151–176. doi:10.1111/j.1529-8817.2005.04067.x [Google Scholar]
- Saunders R.P, Glenn D.A. Memoirs of the hourglass cruises. vol. 1. Florida Department of Natural Resources, Marine Research Laboratory; St Petersburg, FL: 1969. Diatoms; pp. 1–119. [Google Scholar]
- Schmidt L.E, Hansen P.J. Allelopathy in the prymnesiophyte Chrysochromulina polylepis: effect of cell concentration, growth phase and pH. Mar. Ecol. Prog. Ser. 2001;216:67–81. doi:10.3354/meps216067 [Google Scholar]
- Steidinger K.A, Haddad K. Biologic and hydrographic aspects of red tides. Bioscience. 1981;31:814–819. doi:10.2307/1308678 [Google Scholar]
- Steidinger K.A, Vargo G.V, Tester P.A, Tomas C.R. Bloom dynamics and physiology of Gymnodinium breve with emphasis on the Gulf of Mexico. In: Anderson D.M, Cembella A.D, Hallengraef G.M, editors. Physiological ecology of harmful algal blooms. vol. 41. Springer-Verlag; Berlin, Germany: 1998. pp. 133–153. [Google Scholar]
- Taroncher-Oldenburg G, Kulis D.M, Anderson D.M. Toxin variability during the cell cycle of the dinoflagellate Alexandrium fundyense. Limnol. Oceanogr. 1997;42:1178–1188. [Google Scholar]
- Tester P.A, Steidinger K.A. Gymnodinium breve red tide blooms: initiation, transport, and consequences of surface circulation. Limnol. Oceanogr. 1997;42:1039–1051. [Google Scholar]
- Tillmann U, John U, Cembella A. On the allelochemical potency of the marine dinoflagellate Alexandrium ostenfeldii against heterotrophic and autotrophic protists. J. Plankton Res. 2007;29:527–543. doi:10.1093/plankt/fbm034 [Google Scholar]
- Tomas C.R, Hasle G.R, Syvertsen E.E, Steidinger K.A, Tangen K, Throndsen J, Heimdal B.R. Academic Press; San Diego, CA: 1997. Identifying marine phytoplankton. [Google Scholar]
- Turner J.T, Hopkins T.L. Phytoplankton of the Tampa Bay system, Florida. Bull. Mar. Sci. 1974;24:101–121. [Google Scholar]
- Vardi A, Schatz D, Beeri K, Motro U, Sukenik A, Levine A, Kaplan A. Dinoflagellate–cyanobacterium communication may determine the composition of phytoplankton assemblage in a mesotrophic lake. Curr. Biol. 2002;12:1767–1772. doi: 10.1016/s0960-9822(02)01217-4. doi:10.1016/S0960-9822(02)01217-4 [DOI] [PubMed] [Google Scholar]
- Vivanco J.M, Bais H.P, Stermitz F.R, Thelen G.C, Callaway R.M. Biogeographical variation in community response to root allelochemistry: novel weapons and exotic invasion. Ecol. Lett. 2004;7:285–292. doi:10.1111/j.1461-0248.2004.00576.x [Google Scholar]
- Wang Y, Yu Z.M, Song X.X, Zhang S.D. Interactions between the bloom-forming dinoflagellates Prorocentrum donghaiense and Alexandrium tamarense in laboratory cultures. J. Sea Res. 2006;56:17–26. doi:10.1016/j.seares.2006.04.002 [Google Scholar]
- Weir T.L, Bais H.P, Stull V.J, Callaway R.M, Thelen G.C, Ridenour W.M, Bhamidi S, Stermitz F.R, Vivanco J.M. Oxalate contributes to the resistance of Gaillardia grandiflora and Lupinus sericeas to a phytotoxin produced by Centaurea maculosa. Planta. 2006;223:785–795. doi: 10.1007/s00425-005-0192-x. doi:10.1007/s00425-005-0192-x [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Nagasoe S, Matsubara T, Shikata T, Shimasaki Y, Oshima Y, Honjo T. Allelopathic interactions between the bacillariophyte Skeletonema costatum and the raphidophyte Heterosigma akashiwo. Mar. Ecol. Prog. Ser. 2007;339:83–92. doi:10.3354/meps339083 [Google Scholar]
- Zar J.H. Prentice-Hall; Upper Saddle River, NJ: 1999. Biostatistical analysis. [Google Scholar]