Abstract
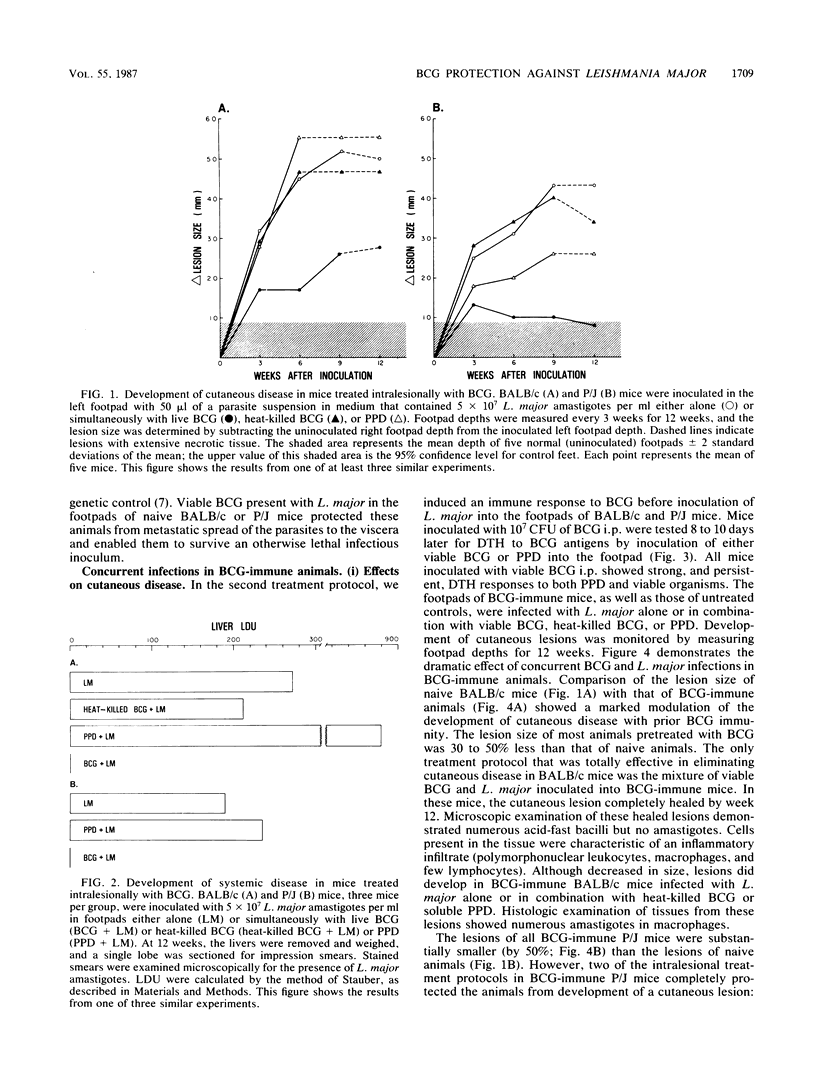
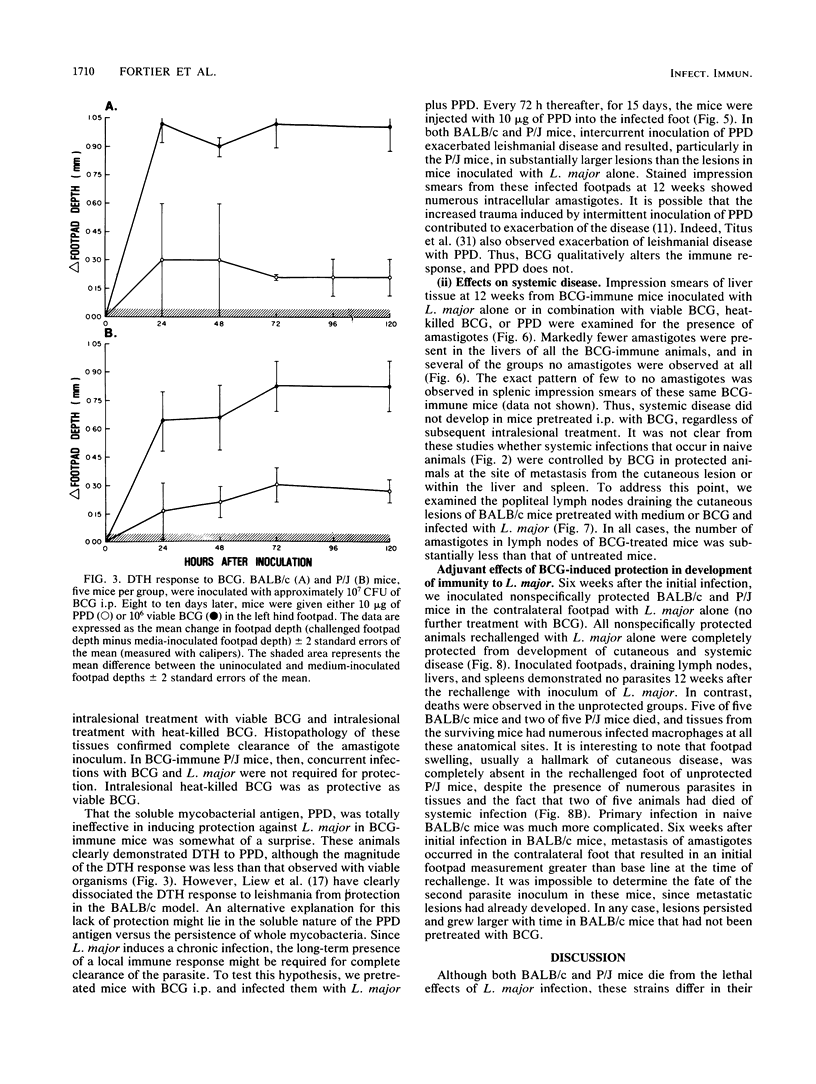
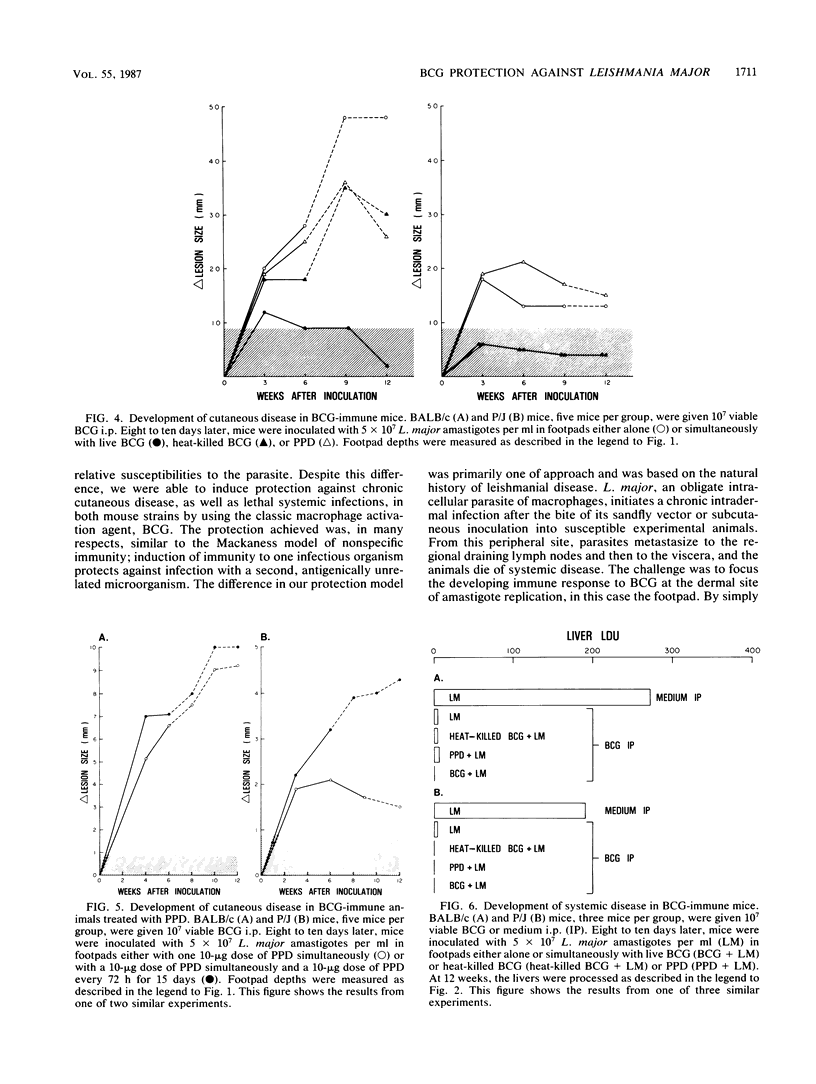
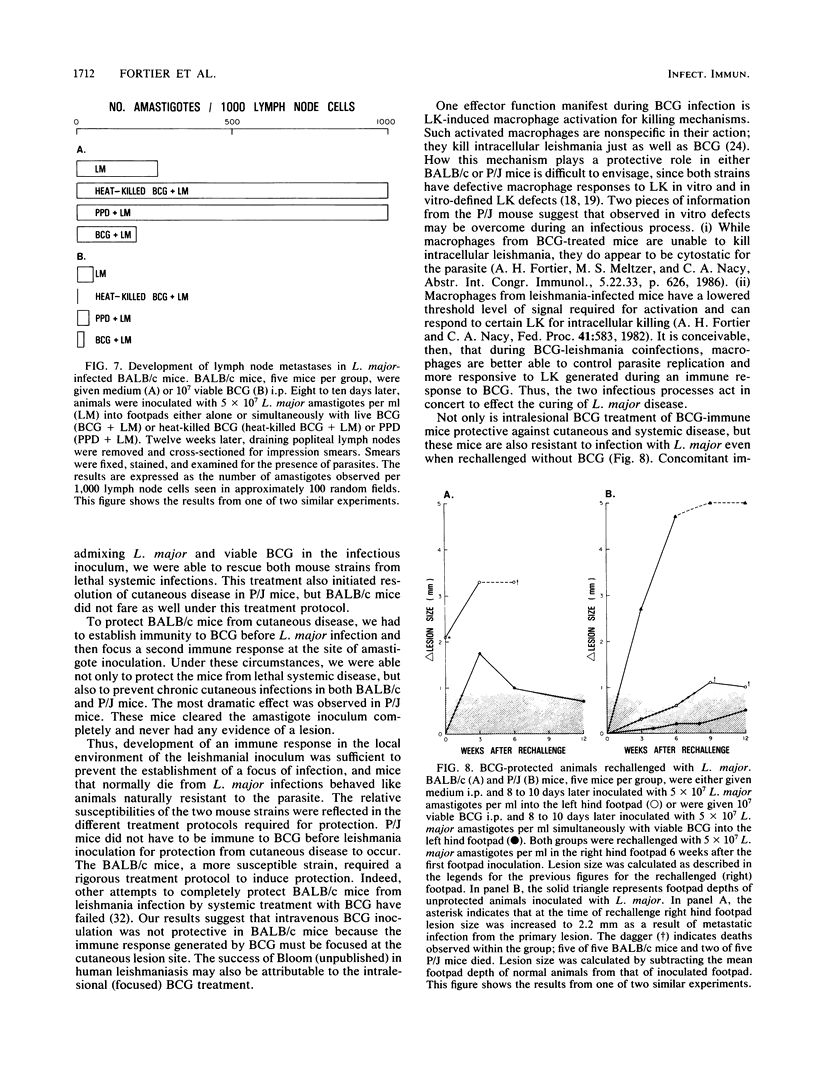
We examined the protective effects of Mycobacterium bovis bacillus Calmette-Guérin (BCG) administration on Leishmania major infections of BALB/c and P/J mice. There were two treatment protocols. In the first, the footpads of naive animals were inoculated with mixtures of L. major and BCG (viable or heat killed) or the soluble mycobacterial antigen, purified protein derivative. Viable BCG, but not heat-killed BCG or purified protein derivative, inoculated with L. major amastigotes into the footpads of naive BALB/c or P/J mice protected these animals from the metastatic spread of parasites to the viscera and from ensuing lethal systemic infection. This treatment also induced cures of the cutaneous lesions of P/J mice but not of BALB/c mice. In the second protocol, we induced an immune response to BCG before inoculation of L. major. BCG given intraperitoneally 10 days before infection of footpads with leishmania offered protection against the metastatic spread of amastigotes in both P/J and BALB/c mice, regardless of intralesional treatment, and modulated the severity of cutaneous infection by 30 to 50%. Inoculation of a mixture of viable BCG and L. major amastigotes into BCG-immune mice completely protected both BALB/c and P/J strains from cutaneous disease; we recovered no parasites from the inoculated footpads of these animals. Furthermore, each of the nonspecifically protected mice of both the BALB/c and P/J strains developed immunity to rechallenge with viable L. major. Injection of amastigotes at a site remote from the original lesion, the contralateral footpad, resulted in the complete clearance of parasites in the inoculum with no evidence of either cutaneous or systemic disease over an extended observation period.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mode of action of immunological adjuvants. J Reticuloendothel Soc. 1979 Dec;26(Suppl):619–630. [PubMed] [Google Scholar]
- Bjorvatn B., Neva F. A. A model in mice for experimental leishmaniasis with a West African strain of Leishmania tropica. Am J Trop Med Hyg. 1979 May;28(3):472–479. doi: 10.4269/ajtmh.1979.28.472. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from P/J mice: characterization of the macrophage cytotoxic defect after in vivo and in vitro activation stimuli. J Immunol. 1980 Aug;125(2):771–776. [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from P/J mice: tumoricidal defect involves abnormalities in lymphokine-derived activation stimuli and in mononuclear phagocyte responsiveness. J Immunol. 1980 Aug;125(2):777–782. [PubMed] [Google Scholar]
- Convit J., Aranzazu N., Ulrich M., Pinardi M. E., Reyes O., Alvarado J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda-negative contacts. Int J Lepr Other Mycobact Dis. 1982 Dec;50(4):415–424. [PubMed] [Google Scholar]
- Fortier A. H., Meltzer M. S., Nacy C. A. Susceptibility of inbred mice to Leishmania tropica infection: genetic control of the development of cutaneous lesions in P/J mice. J Immunol. 1984 Jul;133(1):454–459. [PubMed] [Google Scholar]
- Glasgow L. A., Fischbach J., Bryant S. M., Kern E. R. Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille calmette-guérin. J Infect Dis. 1977 May;135(5):763–770. doi: 10.1093/infdis/135.5.763. [DOI] [PubMed] [Google Scholar]
- Grimaldi G. F., Moriearty P. L., Hoff R. Leishmania mexicana in C3H mice: BCG and levamisole treatment of established infections. Clin Exp Immunol. 1980 Aug;41(2):237–242. [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A. Macrophage activation to kill Leishmania tropica: defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J Immunol. 1984 Mar;132(3):1487–1493. [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Nicklin S., Hale C., Liew F. Y. Prophylactic immunization against experimental leishmaniasis: I. Protection induced in mice genetically vulnerable to fatal Leishmania tropica infection. J Immunol. 1982 Nov;129(5):2206–2212. [PubMed] [Google Scholar]
- Jackson P. R., Pappas M. G., Hansen B. D. Fluorogenic substrate detection of viable intracellular and extracellular pathogenic protozoa. Science. 1985 Jan 25;227(4685):435–438. doi: 10.1126/science.2578226. [DOI] [PubMed] [Google Scholar]
- James S. L., Correa-Oliveira R., Leonard E. J. Defective vaccine-induced immunity to Schistosoma mansoni in P strain mice. II. Analysis of cellular responses. J Immunol. 1984 Sep;133(3):1587–1593. [PubMed] [Google Scholar]
- Liew F. Y., Hale C., Howard J. G. Prophylactic immunization against experimental leishmaniasis. IV. Subcutaneous immunization prevents the induction of protective immunity against fatal Leishmania major infection. J Immunol. 1985 Sep;135(3):2095–2101. [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Pappas M. G., Henry R. R. Susceptibility of inbred mice to Leishmania tropica infection: correlation of susceptibility with in vitro defective macrophage microbicidal activities. Cell Immunol. 1983 Apr 15;77(2):298–307. doi: 10.1016/0008-8749(83)90030-8. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Fortier A. H. Macrophage activation to kill Leishmania tropica: characterization of P/J mouse macrophage defects for lymphokine-induced antimicrobial activities against Leishmania tropica amastigotes. J Immunol. 1984 Dec;133(6):3344–3350. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- OLD L. J., CLARKE D. A., BENACERRAF B. Effect of Bacillus Calmette-Guerin infection on transplanted tumours in the mouse. Nature. 1959 Jul 25;184(Suppl 5):291–292. doi: 10.1038/184291a0. [DOI] [PubMed] [Google Scholar]
- Pappas M. G., Oster C. N., Nacy C. A. Intracellular destruction of Leishmania tropica by macrophages activated in vivo with Mycobacterium bovis strain bcg. Adv Exp Med Biol. 1983;162:425–431. doi: 10.1007/978-1-4684-4481-0_38. [DOI] [PubMed] [Google Scholar]
- Preston P. M., Carter R. L., Leuchars E., Davies A. J., Dumonde D. C. Experimental cutaneous leishmaniasis. 3. Effects of thymectomy on the course of infection of CBA mice with Leishmania tropica. Clin Exp Immunol. 1972 Feb;10(2):337–357. [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Scott P. A., Farrell J. P. Experimental cutaneous leishmaniasis. I. Nonspecific immunodepression in BALB/c mice infected with Leishmania tropica. J Immunol. 1981 Dec;127(6):2395–2400. [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Weintraub J., Weinbaum F. I. The effect of BCG on experimental cutaneous leishmaniasis in mice. J Immunol. 1977 Jun;118(6):2288–2290. [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]
- Zbar B., Bernstein I., Tanaka T., Rapp H. J. Tumor immunity produced by the intradermal inoculation of living tumor cells and living Mycobacterium bovis (strain BCG). Science. 1970 Dec 11;170(3963):1217–1218. doi: 10.1126/science.170.3963.1217. [DOI] [PubMed] [Google Scholar]



