Abstract
Sea ice loss will indirectly alter energy transfer through the pelagic food web and ultimately impact apex predators. We quantified spring-time trends in sea ice recession around each of 46 thick-billed murre (Uria lomvia) colonies in west Greenland across 20° of latitude and investigated the magnitude and timing of the associated spring-time primary production. A geographical information system was used to extract satellite-based observations of sea ice concentration from the Nimbus-7 scanning multichannel microwave radiometer (SMMR, 1979–1987) and the Defence Meteorological Satellite Programs Special Sensor Microwave/Imager (SSMI, 1987–2004), and satellite-based observations of chlorophyll a from the moderate resolution imaging spectroradiometer (MODIS: EOS-Terra satellite) in weekly intervals in circular buffers around each colony site (150 km in radius). Rapid recession of high Arctic seasonal ice cover created a temporally predictable primary production bloom and associated trophic cascade in water gradually exposed to solar radiation. This pattern was largely absent from lower latitudes where little to no sea ice resulted in a temporally variable primary production bloom driven by nutrient cycling and upwelling uncoupled to ice. The relationship between the rate and variability of sea ice recession and colony size of thick-billed murres shows that periodical confinement of the trophic cascade at high latitudes determines the carrying capacity for Arctic seabirds during the breeding period.
Keywords: Arctic, chlorophyll a, thick-billed murre, Greenland, primary production, sea ice
1. Introduction
Identifying environmental forcing variables responsible for population patterns has long been an important component of ecology (Andrewartha & Birch 1954). In the Arctic marine system, the most influential environmental variable is the annual formation and recession of sea ice. This feature exerts broad-scale control on energy flux and primary and secondary production, ultimately reaching the top of the food chain (Sakshaug & Skjoldal 1989; Heide-Jørgensen & Laidre 2004; Heide-Jørgensen et al. 2007). Studies across latitudinal gradients with varying environmental conditions provide insight into effects of physical forcing of sea ice on biological production and population processes (Post & Forchhammer 2002). One of these gradients is the coastline of Greenland, the world's largest island, which spans 20° of latitude (approx. 60° N–80° N). This gradient is the longest continuous stretch of subarctic to high Arctic coastline in the world.
The thick-billed murre (Uria lomvia) is an Arctic seabird species that breeds in colonies throughout the circumpolar region, including the entire west coast of Greenland (Gaston & Jones 1998). Approximately 1700 km separate the northernmost (77° N) and southernmost (60° N) of 46 current and historical breeding colonies in west Greenland (Boertmann et al. 1996; figure 1). Colony size varies markedly across this range, with some colonies containing a few hundred birds and others supporting over 200 000 individuals (Boertmann et al. 1996). The temporal progression of the arrival of breeding birds is strongly correlated with latitude, with a maximum three-week difference in phenology (i.e. arrival, egg-laying, hatching) between the southernmost and northernmost colonies (Falk & Kampp 1997).
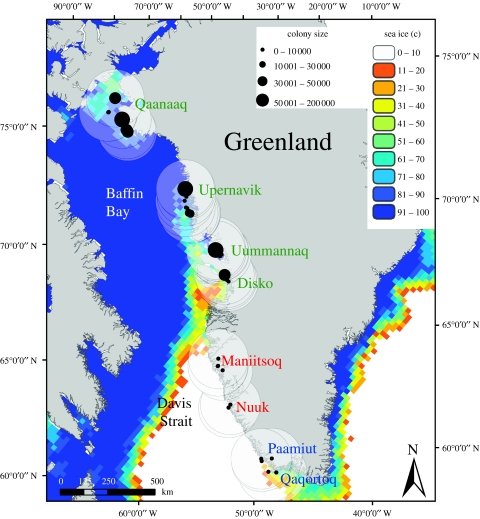
Figure 1.
Current and historical thick-billed murre breeding colonies in west Greenland (maximum historical population size shown) with sea ice concentration in the last week of May (Nimbus-7 scanning multichannel microwave radiometer (SMMR)/Defence Meteorological Satellite Programs Special Sensor Microwave/Imager (SSMI) annual mean, 1979–2003). A potential maximum foraging range of 150 km around each colony was assumed during the breeding season.
The effects of climate and the environment on seabirds are broad reaching and have large geographical variation (Durant et al. 2006; Irons et al. 2008; Sandvik et al. 2008). Thick-billed murres experience marked differences in environmental conditions in spring across their breeding distribution in west Greenland. Waters north of 67° N are more or less completely covered with ice between December and April, with break-up occurring in May and June. Waters south of 67° N are covered with loose pack ice until March and April, and those below 66° N are entirely ice-free year round (figure 1). Thus, in effect, the sizes of these bird colonies represent a large-scale natural spatial experiment offering insight into how variations in physical forcing and biological production influence top marine predators.
Seabirds are constrained to central place foraging during their reproductive period when tied to a breeding site on land. This increases the effects of the temporal and spatial variation in the environment around the breeding site. In spring, thick-billed murres migrate up to several thousand kilometres from wintering grounds in Davis Strait, the Labrador Sea and Newfoundland to breeding sites in the high Arctic (Lyngs 2003). The energy cost of the migration is substantial and local feeding conditions at breeding sites before egg-laying are critical to survival and reproductive success (Gaston & Hipfner 1998; Hipfner et al. 2005). Energetic theory predicts that any increased cost from distance migrated should be offset by benefits gained for long-term survival and reproductive success (Drent et al. 2003). Therefore, birds that migrate to the northernmost areas of Greenland should obtain equivalent, if not potentially more optimal or abundant prey resources, than those at more southern latitudes.
We quantified spring-time trends in sea ice recession around each of 46 current and historical thick-billed murre colonies in west Greenland across a 20° latitudinal gradient and investigated the magnitude and timing of the associated spring-time primary production (figure 1). The relationship between population size of thick-billed murre breeding colonies (Boertmann et al. 1996; Falk & Kampp 1997) and year-to-year variation in sea ice break-up was examined to understand how large-scale latitudinal gradients influence Arctic sea bird colony size.
2. Material and methods
(a) Thick-billed murre data
The study area included 46 current and historical colonies along the west coast of Greenland, ranging from 60°68′ N to 77°44′ N (table 1). Maximum historical population size was obtained from Falk & Kampp (1997) and the Danish National Environmental Research Institute (Boertmann et al. 1996). During the last century, thick-billed murre colonies in Greenland have been counted on a semi-regular and regular basis (Falk & Kampp 1997). Initial counts were made in 1936, primarily by visual assessment (Salmonsen 1943, 1967). Since then, methods used for estimating colony size have been primarily photographic counts or direct counts and have increased in precision and accuracy (Falk & Kampp 1997).
Table 1.
Thick-billed murre colonies in west Greenland obtained from Falk & Kampp (1997) and the Danish National Environmental Research Institute (Boertmann et al. 1996). (Large regions are northwest Greenland (NW), central west Greenland (CW) and southwest Greenland (SW).)
| large region | small region | colony name | breeders arrive (median Julian day) | latitude (deg. N) | historical colony size |
|---|---|---|---|---|---|
| NW | Qaanaaq | Apparsuit | 166 | 77.4 | 37 000 |
| NW | Qaanaaq | Kitsissut I | 166 | 76.7 | 1250 |
| NW | Qaanaaq | Kitsissut II | 166 | 76.7 | 80 |
| NW | Qaanaaq | Kitsissut III | 166 | 76.7 | 5730 |
| NW | Qaanaaq | Appat/Saunders Island | 166 | 76.6 | 143 000 |
| NW | Qaanaaq | Issuvissuup Appai | 166 | 76.2 | 50 000 |
| NW | Qaanaaq | Appat Appai | 166 | 76.1 | 48 000 |
| NW | Upernavik | Kippaku | 163 | 73.7 | 30 000 |
| NW | Upernavik | Apparsuit | 163 | 73.8 | 200 000 |
| NW | Upernavik | Toqqussaaq | 163 | 73.4 | 2150 |
| NW | Upernavik | Kingittuarsuk I | 163 | 73.2 | 1000 |
| NW | Upernavik | Kingittuarsuk II | 157 | 72.9 | 3500 |
| NW | Upernavik | Uummannaq | 157 | 72.6 | 50 |
| NW | Upernavik | Qoornoq | 157 | 72.7 | 525 |
| NW | Upernavik | Saqqarsuaq | 157 | 72.7 | 65 |
| NW | Upernavik | Appallit | 157 | 72.7 | 200 |
| NW | Upernavik | Umiasussuk | 157 | 72.8 | 100 |
| NW | Upernavik | Angissoq | 157 | 72.9 | 1500 |
| NW | Upernavik | Timmiakulussuit | 157 | 72.7 | 6800 |
| NW | Upernavik | Appatsiaat | 157 | 72.7 | 8700 |
| NW | Upernavik | Apparsuit/Kingittoq | 157 | 72.7 | 17 000 |
| NW | Upernavik | Apparsuit | 157 | 72.7 | 25 000 |
| NW | Uummannaq | Salleq | 148 | 71.0 | 150 000 |
| NW | Uummannaq | Appatsiaat | 148 | 71.1 | 200 |
| NW | Uummannaq | Innarsuaq | 148 | 70.9 | 200 |
| NW | Uummannaq | Agguarfik | 148 | 70.9 | 200 |
| NW | Uummannaq | Umiasussuk | 148 | 70.8 | 1000 |
| NW | Uummannaq | Qingartarsuaq | 148 | 70.7 | 100 |
| NW | Uummannaq | Qingaarsuaq | 148 | 70.7 | 20 |
| NW | Uummannaq | Innarsuaq | 148 | 70.7 | 500 |
| NW | Disko Bay | Innaq/Ritenbenk | 147 | 69.8 | 50 000 |
| NW | Disko Bay | Oqaatsut Nuuat | 147 | 69.9 | 150 |
| NW | Disko Bay | Qaqulluit | 147 | 69.6 | 15 |
| NW | Disko Bay | Nuuluk | 147 | 69.6 | 10 |
| NW | Disko Bay | Nuunnguaq | 147 | 69.5 | 50 |
| CW | Maniitsoq | Taateraat | 140 | 66.0 | 10 000 |
| CW | Maniitsoq | Sermilinnguaq I | 140 | 65.7 | 11 000 |
| CW | Maniitsoq | Sermilinnguaq II | 140 | 65.7 | 2600 |
| CW | Maniitsoq | Innarsuaq | 140 | 65.5 | 2200 |
| CW | Nuuk | Nunngarussuit | 140 | 63.8 | 3000 |
| CW | Nuuk | Qeqertarsuaq | 140 | 63.9 | 120 |
| SW | Paamiut | Appat | 140 | 61.3 | 1000 |
| SW | Paamiut | Siggiit | 140 | 61.4 | 4000 |
| SW | Paamiut | Taateraarunnerit | 140 | 61.3 | 5000 |
| SW | Qaqortoq | Kitsissut Avalliit | 162 | 60.8 | 9000 |
| SW | Qaqortoq | Qiioqit | 162 | 60.7 | 1000 |
Latitude and longitude coordinates for each breeding colony were used as the centre of a potential maximum foraging range of 150 km (Swartz 1967; Brown 1980). This was characterized by a circular buffer (150 km radius) using a geographical information system (GIS) (ESRI Arc9) (figure 1). Colonies less than 12 km from each other were assigned the same foraging buffer due to proximity, thus 17 separate buffers were created. Colonies were categorized into three regions: northwest Greenland (NW); central west Greenland (CW); and southwest Greenland (SW).
Analyses were restricted to the breeding season using the median dates of arrival of breeders at the colonies. Timelines of breeding phenology (i.e. arrival of breeders, egg-laying and hatching) were obtained (Falk & Kampp 2002) for each geographical area in Greenland.
(b) SMMR/SSMI sea ice data
We used a GIS to extract satellite-based observations of sea ice from the Nimbus-7 scanning multichannel microwave radiometer (SMMR, 1979–1987) and the Defence Meteorological Satellite Programs Special Sensor Microwave/Imager (SSMI, 1987–2004) in weekly intervals in foraging buffers between March and July. Sea ice concentration (1% resolution) was derived using the bootstrap algorithm following Comiso (1995), where daily sea ice concentrations for the Northern Hemisphere were mapped to a polar stereographic projection (true at 70° N) at a 25 km2 resolution. Sea ice data obtained from the National Snow and Ice Data Center were converted from raw binary to ASCII format using a program written in Compaq Visual Fortran 90 and imported into ESRI Arc9 as raster grids. The centre of each cell was assigned the estimate of average sea ice concentration, in that 625 km area and pixels were consistently classified as land or sea ice across all years. A weekly average composite was created as the product of the vertical spatial and temporal average ice concentration for each cell for all days and years between the months of March and July. The fraction (or percentage) of open water, the ‘ice-free’ portion of each potential colony foraging area, (F) was modelled as
where i indexes the lowest sea ice concentration in the foraging area to h, the highest sea ice concentration; IC is the specific sea ice concentration calculated in full integer units and recorded as a per cent; PC is the pixel count for each specific sea ice concentration; and FA is the foraging area in number of pixels.
(c) Ocean colour data
Data were obtained from the moderate resolution imaging spectroradiometer (MODIS: EOS-Terra satellite) using chlorophyll a (MOD26, SeaWifs analogue OC3M) in level 3 mapped product format for 8 day periods (weekly mean) between the fourth week of May and the first week of July 2001–2003 (4 km2 resolution). Data were obtained in hierarchical data format from the Goddard Earth Sciences Distributed Active Archive Center and converted to ESRI Arc9 grids for each weekly mean, where the centre of each cell received the estimate of average (or maximum) chlorophyll a concentration (mg m−3) for that week. Only good quality data (quality code 0) were used.
Chlorophyll a pixels within buffers (figure 1) were extracted from each 8 day time series between 2001 and 2003. Weekly chlorophyll a density was calculated based on the number of pixels and unique chlorophyll a concentrations observed during that week, or weekly mean density (Dw),
where i indexes the lowest chlorophyll a value observed for the week to h, the highest chlorophyll a value observed for the week; Ca is a specific chlorophyll a value (to 0.01 resolution); PC is the pixel count for each specific chlorophyll a value; and PNw is the total number of pixels observed within the study area for that week (see also Heide-Jørgensen et al. 2007). Pixels within the foraging buffer that fell on land were excluded from the analysis and the exclusion of land pixels was consistent across years, weeks and data type. The number of marine pixels varied based on the location of the colony with respect to the shape of the coastline.
The weekly fraction of open water and density of primary production was quantified in each foraging buffer and linked to the median date for the arrival of breeders in each colony. Time-series trends of sea ice recession in each colony were estimated. The year-to-year variation in sea ice break-up was measured by the slope of the rate of sea ice recession and deviations from interannual means.
3. Results
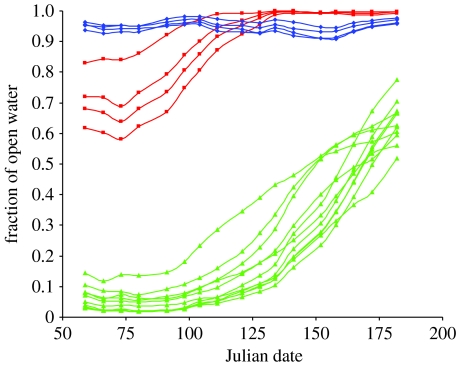
Breeding thick-billed murres arrived at the breeding colonies between 60° N and 80° N between 21 May and 16 June (day of the year 140 and 166). The temporal progression of the median date of arrival of breeders was correlated with latitude in all areas of Greenland (figure 2) with one known outlier (the colony of Kitsissut in South Greenland; see §4). Sea ice break-up around colonies could be grouped into distinct regional patterns upon arrival of breeders (figure 3). Colonies in NW had less than 20 per cent open water in March and a rapid exponential increase in the amount of open water between mid-April and July. Colonies in CW initially had 60–80 per cent open water in March and a slower progression to ice-free conditions (approx. 95% open water) by early May. Colonies in SW had negligible sea ice cover and the changes in the fraction of open water fluctuated between 90 and 100 per cent. Egg-laying in NW occurred when the open water fraction was between 40 and 78 per cent (day of the year 159–186), while, in SW and CW, open water levels were more than 90 per cent at all colonies during egg-laying.
Figure 2.
Timing of the arrival of breeding thick-billed murres (median date of arrival as day of the year) at each colony relative to latitude. The full range in arrival dates is shown with error bars. Note the trend of later arrival with increasing latitude with the exception of an outlier at 60.8° N (Kitsissut in Qaqortoq), a colony influenced by the multi-annual pack ice that travels around the southern tip of Greenland.
Figure 3.
Sea ice trends from March through to July (weekly mean, 1979–2003) categorized into regional trends. Green lines represent trends around colonies in NW (Qaanaaq, Upernavik, Uummannaq and Disko), red lines represent trends around colonies in CW (Maniitsoq and Nuuk) and blue lines represent trends around colonies in SW (Paamiut and Qaqortoq).
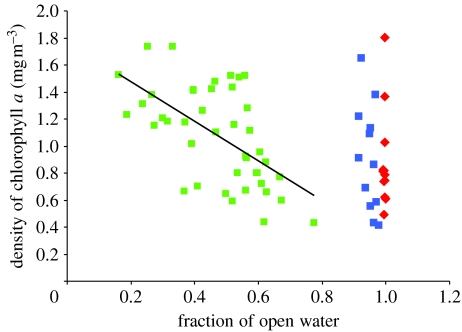
When birds arrived at breeding colonies in NW, primary production (mean density of chlorophyll a) was negatively correlated with the fraction of open water (figure 4). The highest production levels were found early in the season when the fraction of open water was 20–30 per cent. As break-up proceeded through the spring, primary production levels declined. By contrast, in central and SW, where sea ice cover is negligible or patterns of break-up are less distinct, there were no correlations in the timing of the peak primary production and sea ice.
Figure 4.
Spring-time primary production in NW (green squares, i.e. Qaanaaq, Upernavik, Uummannaq and Disko), CW (red diamonds, i.e. Maniitsoq and Nuuk) and SW (blue squares, i.e. Paamiut and Qaqortoq) relative to the fraction of open water. The values shown are weekly chlorophyll a densities at bird colonies each year between 2001 and 2003. A linear regression is fit for NW (y=−1.4694x+1.7726, R2=0.3508).
Spatial patterns between the spring-time sea ice break-up and primary production cascade northwards along the west coast. An intense and large primary production bloom occurs after the ice recedes north of Maniitsoq extending to Upernavik. In addition, a large and consistent production bloom occurs around the northernmost colonies in Qaanaaq in the North Water polynya. The bloom along the central and northwest coast continues through the third week of June, but intensity and coverage declines rapidly and the bloom is over by the first week of July.
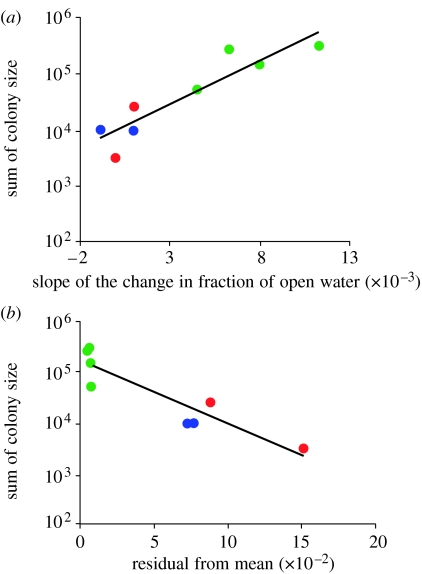
There was a clear relationship between the slope of the recession of sea ice (i.e. increasing fraction of open water) and the colony size of thick-billed murres (figure 5). Small colonies (less than 25 000 birds) were located in waters where sea ice cover was less than 50 per cent when breeders arrived and spring-time ice recession was slow. Large colonies (i.e. 25 000–200 000 birds) were located where the fraction of open water was between 50 and 80 per cent when the breeders arrived and spring-time ice recession was rapid. Furthermore, annual deviations from the mean interannual slope of sea ice recession showed that predictability drove the trend in colony size. The largest thick-billed murre colonies were distinctly clumped together in areas where the deviation from the annual mean of sea ice recession was low (figure 5), or when initial conditions at the arrival of the breeders were predictable.
Figure 5.
(a) Relationship between maximum historical colony size of thick-billed murres and the speed of the decay in spring-time sea ice, measured as the slope of the linear regression of the fraction of open water on week. Colony sizes (green, north; red, central; blue, south) were pooled into regions and are shown on logarithmic scales. (b) Relationship between maximum historical colony size of thick-billed murres and residual mean square error of the mean interannual slope of sea ice recession.
4. Discussion
The general distribution of the thick-billed murre breeding population and the location of large colonies in west Greenland south of 74° N have been known since the late 1700s (Winge 1898). However, colony sizes were first assessed by visual estimates in the 1930s (Salomonsen 1943, 1950). Each colony was counted by the same scientist and colony sizes estimated during this period are internally comparable despite the lack of strict census methods including estimates of variance. These estimates are the best pre-exploitation population sizes available in Greenland and are in agreement with recent estimates for the northern colonies, which have not been heavily influenced by human exploitation.
Today, the thick-billed murre constitutes the largest subsistence seabird harvest in Greenland, with approximately 171 000 birds reported to the hunting register Piniarneq per year (annual average between 1993 and 2000). After the introduction of modern technology such as speed boats and rifles, the total breeding population of thick-billed murres was reduced in west Greenland by 35–50 per cent. During this period (between 1930 and 2000), 20 minor colonies and one major colony were rendered extinct (Evans & Kampp 1991). New legislation and catch reporting has reduced the harvest and recent statistics show that the post-2001 harvest level is 46 per cent of the pre-2001 level (Piniarneq, Greenland Home Rule hunting statistics 2006, unpublished data). The pre-exploitation thick-billed murre colony size estimates used in this study pre-date the intense harvest period and are considered to be pristine. Thus, these historical estimates are the best available data on potential colony carrying capacities along west Greenland.
The availability of suitable nesting habitat along the west Greenland coast is important to the conclusions drawn in this study, particularly in the areas with smaller or fewer colonies south of 64° N. In general, there are many potential breeding sites along the coastline of west Greenland. These sites mostly consist of gneiss, with only the westernmost central Greenland (69° N–72° N) and the northernmost (above 76° N) being volcanic or sedimentary. Although no comprehensive large-scale geological surveys of cliff structure have been conducted, there is generally considered an abundance of coastline with steep cliffs and flat ledges suitable for thick-billed murre nesting in south Greenland. Thus, habitat availability was not assumed to be a limiting explanatory factor in the colony sizes in the south.
The arrival at the breeding colonies was strongly correlated with latitude in all areas, suggesting that the timing of ice break-up is an important determining factor in when birds begin to breed. The outlier in the correlation between latitude and arrival of breeders was the colony of Kitsissut in south Greenland (figures 1 and 2). Kitsissut (latitude 60.8° N) is a known deviant from the general pattern of thick-billed murre phenology due to the influence of the east Greenland multi-annual pack ice (‘Storis’), which travels around the southern tip of Greenland and north into west Greenland waters in April–June (Falk & Kampp 2002).
The thick-billed murre is a pelagic feeder that dives to more than 100 m to feed on fishes and invertebrates (Gaston & Jones 1998; Falk et al. 2002). Birds primarily feed on Arctic cod (Boreogadus saida), capelin (Mallotus villosus) and amphipods (e.g. Parathemisto libelulla) during the breeding period at sea ice edges (Bradstreet 1980; Bradstreet & Cross 1982; Gaston & Bradstreet 1993 ). It is generally agreed that seabirds foraging in the Arctic require sufficient available open water for diving and feeding, and it has been proposed that obstruction by sea ice cover negatively affects reproduction (Gaston & Hipfner 1998; Gaston et al. 2005a,b). In this study, we demonstrate that the total amount of sea ice cover within a colony foraging range is not the determining factor in the quality of habitat. Conversely, breeding habitats with higher sea ice cover and less open water in the beginning of the season may offer better foraging opportunities due to the predictable spring-time trophic cascade closely timed with the recession of sea ice and the breeding cycle.
The observed pattern in the relationship between sea ice cover and chlorophyll a is a reflection of the two different mechanisms that drive the production cascade in subarctic and high Arctic ecosystems. In SW, primary production is driven and restricted by solar radiation, stratification and nutrient upwelling. The timing of the peak is variable and can be bimodal when it is sometimes regenerated by wind in July. Prey species available to thick-billed murres in SW (i.e. capelin or young North Atlantic cod, Gadus morhua) typically come from subarctic regions or from east Greenland on the warm Irminger current and are strongly affected by interannual changes in oceanographic conditions and temperature regimes (Rose 2005; Stige et al. 2006). In NW, the phase lag between sea ice break-up and solar radiation controls the primary production and ice cover is the most important variable (Hansen et al. 2002; Smayda et al. 2004). The temporally predictable sequence ultimately supports high densities of ice-associated spatially constrained prey. Amphipods, copepods and polar cod feed intensively on the sub-ice stratum and are located close to the surface where they are accessible and abundant food sources for pre-laying thick-billed murres (Bradstreet 1980; Bradstreet & Cross 1982; Cross 1982). Later in the spring or after sea ice has disappeared, these prey species disperse over larger areas and descend to greater depths similar to conditions at subarctic latitudes.
The importance of seasonality in the Arctic cannot be overemphasized and top predator phenologies are often timed with optimal environmental conditions. Periodical confinement of the high Arctic primary production has a straightforward connection to feeding options for thick-billed murres. Birds at high latitudes depend on the strict development of primary production and the predictable peak of this event offers reliable and abundant ice-related foraging opportunities, ultimately supporting large colonies. Birds at lower latitudes, where little to no sea ice is present or where ice recession is more variable, experience a greater variability in the timing of the primary production bloom and greater dispersal of prey resources. Although, in general, seabirds rely on open water in close proximity to colonies for feeding opportunities (Gaston et al. 2005a,b), our results suggest larger colonies can be supported at high Arctic latitudes, where the overall fraction of open water may be lower than subarctic latitudes yet the predictability of primary production bloom is high. Abundant and reliable food concentrations near the colonies in the entire breeding season are necessary for the large colonies located in the high Arctic where the adults work at the maximum foraging efforts feeding chicks (Falk et al. 2002). Furthermore, in seabirds with a clutch size of 1, the quality and quantity of food delivered to the chick by the parents has a large effect on reproductive success (Durant et al. 2006).
The conclusions drawn here are focused on identifying the long-term and large-scale environmental influence of the latitudinal gradient and division of production regimes in Greenland on colony sizes of thick-billed murres. This is in contrast to short-term studies of colony dynamics as a function of local environmental variability. Large colonies exist in several other Arctic and subarctic areas (Gaston & Jones 1998), yet colony sizes in these regions are influenced by different production regimes and divergent oceanographic forcing on prey composition. Large thick-billed murre colonies in north and northwest Iceland (estimated to 1.3 million individuals including non-breeders) rely heavily on the large stocks of subarctic and north Atlantic fish species, prey whose occurrence and abundance is entirely independent of sea ice dynamics (Lilliendahl & Solmundson 1997; Vilhjalmsson 2002; Astthorsson et al. 2007).
Prevailing climate change models predict earlier break-up and large-scale reduction of Arctic ice cover (ACIA 2005; Comiso 2006; Holland et al. 2006). This will alter the timing, magnitude and breadth of primary production and reduce the sub-ice substrate habitat for invertebrate grazers (e.g. amphipods, copepods) and near-surface schools of polar cod. These alternations will directly impact food availability for sea birds and may reduce carrying capacity. The reliance on sympagic prey production is not limited to thick-billed murres. Several other seabirds and marine mammals depend on the predictable ice-associated trophic cascade that ensures prey availability in spring at high latitudes (Stirling 1997). A comprehensive mechanistic understanding of the impacts of climate change on top predators has so far been lacking, and, with predictions of severe declines in seasonal ice coverage in the high Arctic, there is an urgent need for understanding the trophic impacts of the coupling between sea ice, primary production and marine food webs.
Acknowledgments
K.L.L. was supported by the US National Science Foundation IRFP grant no. 0401077 and the Greenland Institute of Natural Resources. Thanks to N. Hamel and S. Zador for their productive discussions and two anonymous reviewers who improved this paper. Some data in this study were acquired as part of the NASA's Earth Science Enterprise with algorithms developed by the MODIS Science Teams. The data were processed by the MODIS Adaptive Processing System (MODAPS) and Goddard Distributed Active Archive Center (DAAC), archived and distributed by the Goddard DAAC. SMMR/SSMI sea ice data were obtained from the US NSIDC.
References
- ACIA. Cambridge University Press; Cambridge, UK: 2005. Impacts of a warming Arctic: Arctic climate impact assessment (ACIA) [Google Scholar]
- Andrewartha H.G, Birch L.C. University of Chicago Press; Chicago, IL: 1954. The distribution and abundance of animals. [Google Scholar]
- Astthorsson O.S, Gislason A, Jonsson S. Climate variability and the Icelandic marine ecosystem. Deep-Sea Res. II. 2007;54:2456–2477. doi:10.1016/j.dsr2.2007.07.030 [Google Scholar]
- Boertmann D, Mosbech A, Falk K, Kampp K. NERI Technical report no. 170. National Environmental Research Institute; Denmark: 1996. Seabird colonies in western Greenland (60*°–79° 30′ N. lat.) pp. 148. [Google Scholar]
- Bradstreet M.S.W. Thick-billed murres and Black Guillemots in the Barrow Strait area, N.W.T., during spring: diets and food availability along ice edges. Can. J. Zool. 1980;58:2120–2140. [Google Scholar]
- Bradstreet M.S.W, Cross W.E. Trophic relationships at high Arctic ice edges. Arctic. 1982;35:1–12. [Google Scholar]
- Brown, R. G. B. 1980 Seabirds as marine animals. In Behaviour of marine animals, vol. 4 (eds J. Burger, B. L. Olla, H. Wien), pp. 1–39. New York, NY: Plenum Publishing.
- Comiso J.C. SSMI concentration using the bootstrap algorithm. NASA Report 1380. Ann. Glaciol. 1995;34:441–446. [Google Scholar]
- Comiso J.C. Abrupt decline in the Arctic winter sea ice cover. Geophys. Res. Lett. 2006;33:L18504. doi:10.1029/2006GL027341 [Google Scholar]
- Cross W.E. Under Ice biota at the Pond Inlet ice edge and in adjacent fast ice areas during spring. Arctic. 1982;35:13–27. [Google Scholar]
- Drent R, Both C, Green M, Madsen J, Piersma T. Pay-offs and penalties of competing migratory schedules. Oikos. 2003;103:274–292. doi:10.1034/j.1600-0706.2003.12274.x [Google Scholar]
- Durant J.M, Anker-Nilssen T, Stenseth N.C. Ocean climate prior to breeding affects the duration of the nestling period in the Atlantic puffin. Biol. Lett. 2006;2:628–631. doi: 10.1098/rsbl.2006.0520. doi:10.1098/rsbl.2006.0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P.G.H, Kampp K. Recent changes in thick-billed Murre populations in west Greenland. In: Gaston A.J, Elliot R.D, editors. Studies of high-latitude seabirds. 2. Conservation biology of thick-billed murres in the Northwest Atlantic. Canadian Wildlife Service Occasional Paper no. 69, Ottawa, Canada. 1991. pp. 7–14. [Google Scholar]
- Falk K, Kampp K. Technical Report no. 7. Greenland Institute of Natural Resources; Pinngortitaleriffik, Greenland: 1997. A manual for monitoring thick-billed Murre populations in Greenland. pp. 90. [Google Scholar]
- Falk K, Kampp K. Technical report no. 38, March 2001. Grønlands Naturinstitut; Pinngortitaleriffik, Greenland: 2001. Lomvien i Grønland: mulige effekter af forskellige bestands-påvirkende faktorer, og praktiske grænser for ressourceudnyttelse. (Thick-billed murres in Greenland: possible effects of different population factors and practical advice for resource utilization) [Google Scholar]
- Falk K, Benvenuti S, Antonia L.D, Gilchrist G, Kampp K. Foraging behaviour of thick-billed murres breeding in different sectors of the North Water polynya: an inter-colony comparison. Mar. Ecol. Prog. Ser. 2002;231:293–302. doi:10.3354/meps231293 [Google Scholar]
- Gaston A.J, Bradstreet M.S.W. Intercolony differences in the summer diet of thick-billed murres in the eastern Canadian Arctic. Can. J. Zool. 1993;7:1831–1840. doi:10.1139/z93-261 [Google Scholar]
- Gaston A.J, Hipfner M. The effect of ice conditions in northern Hudson Bay on breeding by thick-billed murres (Uria lomvia) Can. J. Zool. 1998;76:480–492. doi:10.1139/cjz-76-3-480 [Google Scholar]
- Gaston A.J, Jones I.L. Oxford University Press; Oxford, UK: 1998. The auks. [Google Scholar]
- Gaston A.J, Gilchrist H.G, Hipfner M. Climate change, ice conditions and reproduction in the Arctic nesting marine bird: Brünnich's Guillemot (Uria lomvia L.) J. Anim. Ecol. 2005a;74:832–841. doi:10.1111/j.1365-2656.2005.00982.x [Google Scholar]
- Gaston A.J, Gilchrist H.G, Mallory M.L. Variation in ice conditions has strong effects on the breeding of marine birds at Prince Leopold Island, Nunavut. Ecography. 2005b;28:331–344. doi:10.1111/j.0906-7590.2005.04179.x [Google Scholar]
- Hansen A.S, Nielsen T.G, Levinsen H, Madsen S.D, Thingstad T.F, Hansen B.W. Impact of changing ice cover on pelagic productivity and food web structure in Disko Bay, west Greenland: a dynamic model approach. Deep-Sea Res. I. 2002;50:171–187. [Google Scholar]
- Heide-Jørgensen M.P, Laidre K.L. Declining open water refugia for top predators in Baffin Bay and adjacent waters. Ambio. 2004;33:488–495. doi: 10.1579/0044-7447-33.8.487. doi:10.1639/0044-7447(2004)033[0487:DEOORF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Heide-Jørgensen M.P, Laidre K.L, Logsdon M.L, Nielsen T.G. Springtime coupling between chlorophyll a, sea ice and sea surface temperature in Disko Bay, west Greenland. Prog. Ocean. 2007;73:79–95. doi:10.1016/j.pocean.2007.01.006 [Google Scholar]
- Hipfner J.M, Gaston A.J, Gilchrist H.G. Variation in egg size and laying date in thick-billed murre populations breeding in the low Arctic and high Arctic. The Condor. 2005;107:657–664. doi:10.1650/0010-5422(2005)107[0657:VIESAL]2.0.CO;2 [Google Scholar]
- Holland M.M, Bitz C.M, Tremblay B. Future abrupt reductions in the summer Arctic sea ice. Geophys. Res. Lett. 2006;33:L23503. doi:10.1029/2006GL028024 [Google Scholar]
- Irons D.B, et al. Fluctuations in circumpolar seabird populations linked to climate oscillations. Global Change Biol. 2008;14:1455–1463. doi:10.1111/j.1365-2486.2008.01581.x [Google Scholar]
- Lilliendahl K, Solmundsson J. An estimate of summer food consumption of six seabird species in Iceland. ICES J. Mar. Sci. 1997;54:624–639. doi:10.1006/jmsc.1997.0240 [Google Scholar]
- Lyngs P. Migration and winter ranges of birds in Greenland. Dansk Ornithologisk Forenings Tidsskrift. 2003;97:167. [Google Scholar]
- Post E.P, Forchammer M.C. Synchronization of animal population dynamics by large-scale climate. Nature. 2002;420:168–171. doi: 10.1038/nature01064. doi:10.1038/nature01064 [DOI] [PubMed] [Google Scholar]
- Rose G.A. Capelin (Mallotus villosus) distribution and climate change: a sea “canary” for marine ecosystem change. ICES J. Mar. Sci. 2005;62:1524–1530. doi:10.1016/j.icesjms.2005.05.008 [Google Scholar]
- Sakshaug E, Skjoldal H.R. Life at the ice edge. Ambio. 1989;18:60–67. [Google Scholar]
- Salomonsen, F. 1943 The natural history expedition to Northwest Greenland 1936. Report on the expedition. Meddelelser om Grønland124, 38.
- Salomonsen F. Munksgaard; Copenhagen, Germany: 1950. The birds of Greenland. pp. 609. [Google Scholar]
- Sandvik H, Coulson T, Sæther B.-E. A latitudinal gradient in climate effects on seabird demography: results from interspecific analyses. Global Change Biol. 2008;14:1–11. doi:10.1111/j.1365-2486.2007.01533.x [Google Scholar]
- Swartz L.G. Distribution and movements of birds in the Bering and Chukchi Seas. Pacif. Sci. 1967;21:332–347. [Google Scholar]
- Smayda T.J, Borkman D.G, Beaugrand G, Belgrano A. Responses of marine phytoplankton to fluctuations in marine climate. In: Stenseth N.C, Ottersen G, Hurrell J.W, Belgrano A, editors. Marine ecosystems and climate variation. Oxford University Press; Oxford, UK: 2004. pp. 49–58. [Google Scholar]
- Stige L.C, Ottersen G, Brander K, Chan K.-S, Stenseth N.C. Cod and climate: effect of the North Atlantic Oscillation on recruitment in the North Atlantic. Mar. Ecol. Prog. Ser. 2006;325:227–241. doi:10.3354/meps325227 [Google Scholar]
- Stirling I. The importance of polynyas, ice edges, and leads to marine mammals and birds. J. Mar. Sci. 1997;10:9–21. [Google Scholar]
- Vilhjalmsson H. Capelin (Mallotus villosus) in the Iceland-East Greenland–Jan Mayen ecosystem. ICES J. Mar. Sci. 2002;59:870–883. doi:10.1006/jmsc.2002.1233 [Google Scholar]
- Winge, H. 1898 Grønlands Fugle. Meddelelser om Grønland21, 316.