Abstract
Increasing species diversity typically increases biomass in experimental assemblages. But there is uncertainty concerning the mechanisms of diversity effects and whether experimental findings are relevant to ecological process in nature. Hosts for parasites provide natural, discrete replicates of parasite assemblages. We considered how diversity affects standing-stock biomass for a highly interactive parasite guild: trematode parasitic castrators in snails. In 185 naturally occurring habitat replicates (individual hosts), diverse parasite assemblages had greater biomass than single-species assemblages, including those of their most productive species. Additionally, positive diversity effects strengthened as species segregated along a secondary niche axis (space). The most subordinate species—also the most productive when alone—altered the general positive effect, and was associated with negative diversity effects on biomass. These findings, on a previously unstudied consumer class, extend previous research to illustrate that functional diversity and species identity may generally both explain how diversity influences biomass production in natural assemblages of competing species.
Keywords: diversity, productivity, biomass, parasitic castrators, parasite communities, Cerithidea californica
1. Introduction
At first glance, the equation, 1A+1B>1A, might appear to be self-evident. But this is the contested and basic premise behind a questioned value of biodiversity, namely whether multi-species assemblages outperform single-species assemblages for some aggregate ecosystem functions. One potential function of biodiversity is the production of biomass (Schlapfer & Schmid 1999; Loreau et al. 2001; Hooper et al. 2005; Balvanera et al. 2006; Cardinale et al. 2006; Stachowicz et al. 2007). For several free-living trophic groups, diverse assemblages have higher standing-stock biomass than does the average single species when alone (Cardinale et al. 2006; Stachowicz et al. 2007). However, it is not completely clear what mechanisms underlie these positive effects of species diversity. Indeed, diverse assemblages frequently do not have greater total standing-stock biomass than does the single species with the greatest standing stock when it is alone (Cardinale et al. 2006). Thus, the diversity–production relationship might be explained by the simple effect of diverse assemblages tending to include ecologically important species (Aarssen 1997; Huston 1997; Tilman et al. 1997; Loreau 2000; Cardinale et al. 2006). Here, we examine diversity versus production for a natural assemblage of parasitic castrators. We study how castrator diversity within a host affects the production of castrator standing-stock biomass. We do this to test the universality of patterns uncovered in the studies of free-living communities (no parasitic groups have previously been adequately studied), as well as to examine whether the study of these simple parasite assemblages can clarify the mechanisms by which species diversity influences ecological process in general.
Trematode parasitic castrators in individual snails provide unexplored assemblages very suitable for investigating the effect of diversity on biomass production. The natural replication provided by individual hosts (as discrete habitat patches) has the simplicity that is typically only available in laboratory settings. In their first intermediate host snail, trematodes are parasitic castrators, a distinctive consumer strategy (Lafferty & Kuris 2002). Following infection, a single trematode reproduces asexually and exploits the entire reproductive budget of the host, as is typical for castrators (Kuris 1974; Baudoin 1975). Therefore, when more than one trematode species infects the same host, resource limitation drives strong interspecific competition, usually leading to the exclusion of competitively subordinate species (Lim & Heyneman 1972; Lie 1973; Combes 1982).
In general, functional differences between species should drive the effects of species diversity on their aggregate function (Tilman et al. 1997; Hooper et al. 2002; Naeem & Wright 2003). Because a single trematode infection theoretically uses all the resources (host reproductive effort) available for a parasitic castrator, it is unclear whether complementary mechanisms of resource acquisition among multiple trematode species could cause diversity to increase parasite biomass. However, recent analyses have indicated that trematode species differ in how they allocate resources (McCarthy et al. 2002; Hechinger et al. 2008). Also, trematode species can vary in spatial distribution within their host, i.e. they vary in what host tissues they infect (e.g. Cable 1956; Holliman 1961; DeCoursey & Vernberg 1974; Yoshino 1975; Kuris 1990; Sousa 1993; Hechinger et al. 2008). Variation in host tissue use by trematodes might reflect variation in resource use or acquisition. If so, then any variation in the effects of species diversity on productivity might be best explained by functional diversity, reflecting the degree of resource use complementarity.
Here, we examine the patterns and mechanisms underlying how naturally occurring trematode parasitic castrator diversity affects parasite standing-stock biomass. This allows a test of the generality of diversity effects on ecosystem function. This is because secondary consumers have scarcely been considered in diversity versus ecosystem function studies (Balvanera et al. 2006), and, to our knowledge, there have been no studies examining the effects of parasite diversity on parasite biomass among individual hosts. We found that diverse trematode castrator assemblages had greater levels of biomass and impacted resources more than did single-species assemblages, and that this was not simply a result of including the most productive species. Additionally, our findings indicate that functional traits of species—in this case, complementary use of space—can drive the effects of species diversity.
2. Material and methods
As presented in detail in Hechinger et al. (2008), we studied the biomass of trematode parasite assemblages that castrate the California horn snail, Cerithidea californica (Haldeman 1840). We dissected 594 snails that were haphazardly collected from tidal channels and intertidal flats at Carpinteria Salt Marsh, CA, USA (34.40° N, 119.53° W), in winter and summer 2005. We quantified standing-stock biomass for each encountered trematode assemblage except for those single-species assemblages that we frequently encountered. For such assemblages, we stopped quantifying biomass after sampling at least 20 of that species assemblage. Additionally, we excluded those assemblages with immature infections (those that have only begun to grow). We identified the trematode species following Martin (1972) and R. F. Hechinger & T. Huspeni (unpublished manuscript).
We quantified parasite standing-stock biomass and snail tissue mass in 185 replicate habitats (i.e. individual host snails): 159 single-species and 26 mixed-species assemblages. Single-species assemblages included 15 species of larval trematode parasites. Mixed-species assemblages included 17 types of species-pair combinations involving 13 species (table 1). We did not encounter any triple- and quadruple-species assemblages, which are exceedingly rare (Martin 1955; Yoshino 1975; Sousa 1993).
Table 1.
Diversity effects of the particular mixed-species parasitic castrator assemblages on parasite standing stock and impact on resources (host snail tissue) for the various encountered trematode species-pair combinations and details of spatial resource use.
| mixed-species assemblagea | n | proportional change ofb | spatial complementarityc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| standing-stock mass compared with | impact on resource compared with | tissue sitesd | |||||||
| average sp. | largest sp. | average sp. | largest sp. | sp. 1 | sp. 2 | mixed | |||
| ACAN–AUST | 1 | −0.019 | −0.077 | −0.007 | −0.017 | g | g+dg+bvm | g+dg+bvm | 0.67 |
| ACAN–LGXI | 1 | 0.378 | 0.314 | 0.067 | 0.065 | g | g+dg | g+dg | 0.50 |
| AUST–EUHA | 2 | 0.059 | −0.040 | −0.001 | −0.009 | g+dg+bvm | g | g+dg+bvm | 0.67 |
| AUST–HIMA | 4 | 0.110 | 0.129 | 0.027 | 0.023 | g+dg+bvm | g | g+dg+bvm | 0.67 |
| AUST–HIMB | 3 | −0.017 | −0.011 | −0.029 | −0.055 | g+dg+bvm | g+bvm | g+dg+bvm | 0.33 |
| AUST–PROB | 1 | 0.282 | −0.025 | 0.087 | −0.074 | g+dg+bvm | g+dg+bvm | g+dg+bvm | 0.00 |
| AUST–STIC | 2 | 0.783 | 0.569 | 0.212 | 0.192 | g+dg+bvm | g | g+dg+bvm | 0.67 |
| CATA–LGXI | 1 | 0.635 | 0.453 | 0.164 | 0.147 | m | g+dg | m+g+d | 1.00 |
| EUHA–SMCY | 1 | 0.419 | 0.288 | 0.065 | 0.044 | g | g+dg+bvm | g+dg+bvm | 0.67 |
| HIMA–LGXI | 1 | 0.275 | 0.164 | 0.059 | 0.044 | g | g+dg | g+dg | 0.50 |
| HIMA–PROB | 1 | −0.078 | −0.307 | −0.051 | −0.230 | g | g+dg+bvm | g+dg+bvm | 0.67 |
| HIMB–PROB | 3 | 0.219 | −0.077 | 0.049 | −0.086 | g+bvm | g+dg+bvm | g+dg+bvm | 0.33 |
| HIMB–RENB | 1 | 0.950 | 0.566 | 0.140 | 0.093 | g+bvm | m | m+g+bvm | 1.00 |
| PARO–PROB | 1 | −0.211 | −0.377 | −0.124 | −0.281 | g+bvm | g+dg+bvm | g+dg+bvm | 0.33 |
| PROB–SMCY | 1 | 0.263 | −0.150 | 0.122 | −0.069 | g+dg+bvm | g+dg+bvm | g+dg+bvm | 0.00 |
| RENB–SMCY | 1 | 1.082 | 0.982 | 0.133 | 0.131 | m | g+dg+bvm | m+g+dg+bvm | 1.00 |
| SMCY–SMMI | 1 | 0.842 | 0.413 | 0.187 | 0.129 | g+dg+bvm | g | g+dg+bvm | 0.67 |
| sum or mean | 26 | 0.351 | 0.165 | 0.065 | 0.003 | ||||
| mean without PROBe | 0.458 | 0.312 | 0.085 | 0.065 | |||||
Species codes: ACAN, Acanthoparyphium spinulosum; AUST, Austrobilharzia sp.; CATA, Catatropis johnstoni; EUHA, Euhaplorchis californiensis; HIMA, Himasthla rhigedana; HIMB, Himasthla sp. B; LGXI, large xiphiocercaria; PARO, Parorchis acanthus; PROB, Probolocoryphe uca; RENB, Renicola buchanani; SMCY, small cyathocotylid; SMMI, small microphallid; STIC, Stictodora hancocki.
Effects of mixed-species assemblages are expressed relative to the effects characterizing their particular constituent species when in single-species assemblages (the average of their constituents and to the single constituent with the largest effect). The absolute values for mixed- and single-species assemblages were least-squares means from general linear models (tables 2 and 3).
Spatial niche complementarity=(no. of tissues not shared by the constituent species)/(total no. of tissues used by the constituent species).
g, gonad; dg, digestive gland; bvm, basal visceral mass; m, mantle; tissue use from (Hechinger et al. 2008).
PROB is the outlier species (by far the largest when alone, yet the most competitively inferior) excluded for these calculations.
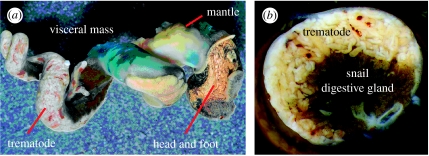
We quantified trematode biomass (wet weight) in snails as detailed in Hechinger et al. (2008). Briefly, we did so by (i) separating and weighing the infected regions of each snail (figure 1a), (ii) using serial cross sections (figure 1b) to quantify trematode tissue proportional volume in the infected regions, (iii) multiplying the trematode proportion by the mass of the infected region, and (iv) ensuring that tissue densities for trematode and snail tissue were at least approximately equal. For mixed-species assemblages, we calculated aggregate mass. Because infections are intermixed, we were unable to separately calculate each species' individual contribution to aggregate mass. We determined snail tissue mass by subtracting the trematode mass from the entire infected snail's directly weighed soft tissue mass.
Figure 1.
Trematode parasitic castrator infections in the snail, Cerithidea californica. (a) Snail (shell removed) infected with the trematode, Himasthla rhigedana (photo credit: Todd Huspeni). The trematode has completely castrated the snail, replacing the snail gonadal tissue. (b) Cross section through the visceral mass of a snail infected by the trematode, Cloacitrema michiganensis.
To compare average biomass for mixed- and single-species assemblages, we used a general linear model (GLM; table 2) that statistically controlled for any effects of snail size, habitat and season (variables previously determined to affect trematode relative mass in snails; Hechinger et al. 2008). We incorporated assemblage (the particular species or species combination) as a random factor nested within the fixed-factor diversity level (mixed- or single-species assemblage). We used the ‘expected mean square’ approach (Quinn & Keough 2002) to incorporate random effects. This approach had the advantage of allowing us to test the diversity effect using the appropriate denominator mean squared error and degrees of freedom, while also providing unbiased least-squares means (LSMs) for the particular species and species combinations (which we were also particularly interested in, used below). All two-way interactions were non-significant (all p>0.17) and not used in the final GLMs. To determine the effect of trematode diversity on resource levels (snail tissue), we performed the parallel analysis with snail tissue mass as the response variable (table 3). We used JMP v. 7 for these analyses.
Table 2.
Results of GLM assessing how trematode standing stocka is affected by trematode assemblage diversity and other predictor variables.
| predictor | d.f. | SS | F-ratio | r2 | p |
|---|---|---|---|---|---|
| diversity (mixed or single species) | 1 | 0.032 | 12.42b | 0.15 | 0.0004c |
| assemblage (diversity)d | 30 | 0.151 | 4.21 | 0.46 | <0.0001 |
| season (summer or winter) | 1 | 0.028 | 23.08 | 0.13 | <0.0001 |
| habitat (channel or flat) | 1 | 0.012 | 10.31 | 0.06 | 0.0016 |
| total tissue weight | 1 | 0.170 | 141.95 | 0.49 | <0.0001 |
| full model | 34 | 0.838 | 20.61 | 0.82 | <0.0001 |
| residual | 150 | 0.179 |
Trematode mass was fourth-root transformed in analysis to meet assumptions of normality and homogeneity of variance.
The synthesized denominator mean squared error for this test was 0.0026, with 59.1 d.f.
One-tailed p-value.
The particular species (15 spp.) or species-pair combination (17 types) as a random factor nested within if an assemblage was mixed or single species.
Table 3.
Results of GLM assessing how trematode resource level (snail tissue) is affected by trematode assemblage diversity and other predictor variables.
| predictor | d.f. | SS | F-ratio | r2 | p |
|---|---|---|---|---|---|
| diversity (mixed or single species) | 1 | 0.009 | 7.55a | 0.09 | 0.0039b |
| assemblage (diversity)c | 30 | 0.070 | 3.86 | 0.44 | <0.0001 |
| season (summer or winter) | 1 | 0.008 | 13.24 | 0.08 | 0.0004 |
| habitat (channel or flat) | 1 | 0.003 | 5.42 | 0.03 | 0.0212 |
| total tissue weight | 1 | 0.564 | 936.67 | 0.86 | <0.0001 |
| full model | 34 | 0.932 | 45.51 | 0.91 | <0.0001 |
| residual | 150 | 0.090 |
The synthesized denominator mean squared error for this test was 0.0012, with 62 d.f.
One-tailed p-value.
The particular species (15 spp.) or species-pair combination (17 types) as a random factor nested within if an assemblage was mixed or single species.
To examine whether any positive diversity effects were solely due to mixed-species assemblages including more productive species, we expressed the effect of diversity as the proportional change in the standing stock of each of the 17 types of mixed-species assemblages compared with (i) the average of the standing stocks characterizing their constituent species when in single-species assemblages and (ii) the standing stock characterizing the constituent species that achieved the largest mass when it was alone. The second is a test for ‘transgressive overyielding’ (Trenbath 1974; Fridley 2001; Hector et al. 2002), and directly examines whether mixed-species assemblages produce more biomass than any of the constituent species when alone. We used the GLM LSMs for the standing stock characterizing each type of mixed- and single-species assemblage. We used the LSMs for each pair combination as replicates (n=17) instead of each encountered mixed-species assemblage (n=26). Although this decreased our sample size, it removed pseudo-replication issues.
To examine whether functional traits of the trematode species could explain the positive effects of species diversity, we calculated an index for ‘functional diversity’. This index was based upon the degree of complementary use of space by trematodes in mixed-species assemblages (table 1). Most of the trematode species occupy the gonadal space of the snail host visceral mass (Yoshino 1975; Kuris 1990; Sousa 1993; Hechinger et al. 2008). However, they differ to the extent that they also use surrounding tissues in the snail visceral mass (Hechinger et al. 2008). Furthermore, some trematode species do not use the visceral mass at all and occupy a completely disjunct area—the mantle (Martin 1955; Yoshino 1975; Sousa 1993; Hechinger et al. 2008). Therefore, we calculated functional diversity as the number of tissues not shared by the constituent species divided by the total number of tissues used by the constituent species. We used ordinary least-squares regression to determine whether functional diversity explained species diversity effects on trematode standing-stock biomass.
Because one trematode species (Probolocoryphe uca) was particularly associated with negative effects of diversity on standing-stock biomass, we examined diversity effects of mixed-species assemblages both with and without this species.
We ensured that we met assumptions regarding normality and variance homogeneity by inspecting plots of residuals versus predicted values and normal quantile plots with Lilliefors' curves (Kutner et al. 2005). All tests concerning diversity effects are one tailed, commensurate with the directional hypothesis of positive diversity effects. This is further justified by two recent, thorough and quantitative meta-analyses (Balvanera et al. 2006; Cardinale et al. 2006), which found that, across the board, diversity effects on the standing stock range from positive to zero. Because our tests were based on a priori defined hypotheses, we present nominal p values. But we note that all tests deemed significant at the 0.05 level remain so when controlled for multiple comparisons by holding the false discovery rate (Benjamini & Hochberg 1995) to 0.05 for our family of 10 diversity tests.
3. Results
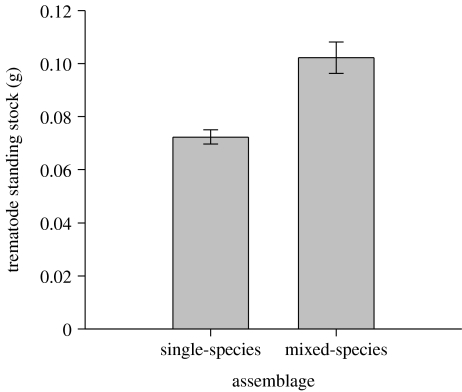
Trematode standing stock varied among single-species assemblages and also among the particular combinations of mixed-species assemblages (GLM, F30,150=4.2, p<0.0001; tables 1 and 2). The average mixed-species assemblage in our sample had 41.3 per cent greater trematode standing stock than did the average single-species assemblage in our sample (t-test on GLM LSMs, t59=3.5, pone-tailed=0.0004; figure 2; table 2).
Figure 2.
Average trematode standing-stock biomass of 159 naturally occurring single- and 26 mixed-species parasite assemblages. Data are LSMs (±1 s.e.m.) from a GLM controlling for the effects of season, habitat and snail mass.
The combined trematode and host mass did not differ significantly between mixed- and single-species assemblages (GLM, F1,150=1.8, p=0.18). But hosts containing mixed-species assemblages had 8.3 per cent less snail tissue than did hosts with single-species assemblages (t-test on GLM LSMs, t62=2.7, pone-tailed=0.0039; table 3).
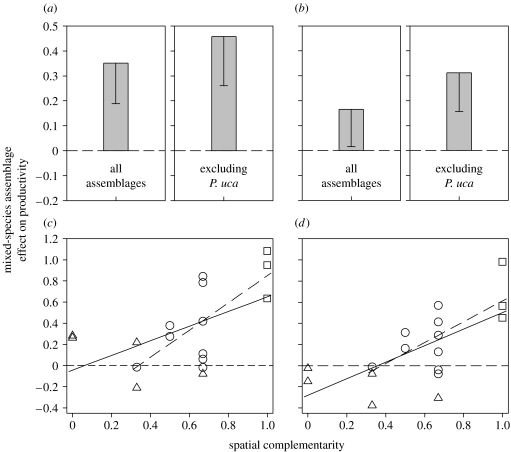
We then compared the standing stock of mixed-species assemblages directly to the standing stock achieved by their constituent species when in single-species assemblages. On average, mixed-species assemblages had 35.1 per cent greater standing stock than the average of their constituent species when alone (one-sample t-test, t16=3.8, pone-tailed=0.0008; figure 3a). Furthermore, mixed-species assemblages ‘transgressively overyielded’ (Trenbath 1974; Fridley 2001; Hector et al. 2002), having on average 16.5 per cent greater standing stock than that characterizing their single largest constituent species when it was alone (t16=1.9, pone-tailed=0.036; figure 3b).
Figure 3.
Effects of trematode parasite diversity on trematode standing-stock biomass. (a,b) Means and lower 95% confidence bounds for the proportional change in the standing stock of mixed-species assemblages compared with the standing stock characterizing their constituent species when alone. Data are shown for all types of encountered mixed-species assemblages (n=17) and for mixed-species assemblages not involving Probolocoryphe uca (n=12). (c,d) Stronger effects of species diversity on standing stock with increasing functional diversity. (a,c) display diversity effects on standing stock relative to the average standing stock characterizing the constituent species when alone, while (b,d) show diversity effects on standing stock expressed relative to the constituent species with the maximum standing stock when alone. Triangles in (c,d) assemblages involving P. uca, and squares indicate those involving one mantle-dwelling species and one visceral mass dweller (i.e. those with maximum spatial complementarity). The solid regression lines were fit using all mixed-species assemblages ((c) y=0.69x−0.04 and (d) y=0.78x−0.28) and the dashed regression lines are fit excluding assemblages with P. uca ((c) y=1.29x−0.44 and (d) y=0.99x−0.37). The horizontal dashed line in all plots represents the null hypothesis of no diversity effect.
Sometimes, mixed-species assemblages had a lower standing stock than did their most productive species when alone (figure 3d). This effect was most strong and common for mixed-species assemblages including the trematode, P. uca (figure 3d; table 1). Probolocoryphe uca is an outlier species in terms of productivity, taking a remarkable 39 per cent of the infected snail tissue mass when it is alone (Hechinger et al. 2008). Excluding assemblages with P. uca from analysis, diversity more strongly affected parasite standing stock (figure 3a,b). In this case, mixed-species assemblages had 45.8 per cent greater standing stock than the average of their constituent species when alone (one-sample t-test, t11=4.0, pone-tailed=0.0010; figure 3a). Additionally, when compared with the standing stock characterizing their single largest constituent when alone, mixed-species assemblages not including P. uca had 31.3 per cent greater standing stock (t11=3.5, pone-tailed=0.0024; figure 2b).
As predicted, the effects of trematode diversity on production increased with the degree of spatial complementarity characterizing mixed-species assemblages (figure 3c,d). This occurred if the effects of diversity were expressed relative to the average of the constituent species (r2=0.30, slope=0.70, t15=2.51, pone-tailed=0.012) or relative to the single largest constituent species (r2=0.44, slope=0.78, t15=3.44, pone-tailed=0.0018), and also when P. uca was excluded and the diversity effects were compared with the average constituent (r2=0.48, slope=1.29, t10=3.1, pone-tailed=0.0062) or compared with the largest constituent (r2=0.46, slope=0.99, t10=2.91, pone-tailed=0.0078; figure 3c,d). The effects of diversity approached complete additivity for those mixed-species assemblages with the maximal spatial complementarity (figure 3c,d; table 1). Specifically, on average, mixed-species assemblages involving both mantle and visceral mass dwellers had 1.9 times the average standing stock characterizing their constituent species when alone and 1.7 times the standing stock of their single largest constituent species when it was alone.
4. Discussion
To our knowledge, this is the first examination of the effects of parasite diversity on parasite standing-stock biomass in individual hosts for any type of parasitic functional group (but see below). Thus, the positive diversity effects we uncovered support the generality of positive diversity effects on productivity.
Because trematode standing stock varied among single-species assemblages, the positive effects of species diversity could have arisen by highly productive parasite species being over-represented in mixed-species assemblages (akin to the issue of non-random selection of highly productive species in diversity experiments; Huston 1997). However, on average, a mixed-species assemblage had greater standing stock than either the average of its constituent species when alone or the largest constituent species when it was alone. This last result (transgressive overyielding) provides strong evidence that the positive effects of diversity on trematode standing stock are caused by something other than simply including highly productive species in mixed-species assemblages (Loreau 1998; Fridley 2001).
The greater biomass of most mixed-species assemblages compared with their most productive constituents indicates that these parasites have partially complementary mechanisms of resource acquisition or use. The increase in biomass with functional diversity (spatial complementarity) supports this hypothesis. This is surprising considering that each of these trematode species fully castrate their host when in single-species assemblages. Although single infections target and take the entirety of the same resource, diversity still had a positive effect on standing-stock biomass. Specific differences in the mechanism of resource extraction or strategy of resource use are probably directly related to the spatial partitioning that we quantified, and which explained the positive diversity effects on standing-stock biomass.
One trematode, P. uca, was strongly associated with negative diversity effects on standing-stock biomass. Although P. uca is by far the most productive species when alone (Hechinger et al. 2008), this parasite is also the most subordinate trematode in the competitive dominance hierarchy of this parasite guild (Kuris 1990). Thus, there is a simple potential explanation for the decrease in the standing stock for mixed-species assemblages involving P. uca: in such mixed-species infections, the dominant co-occurring species was in the process of competitively excluding P. uca, lowering the standing stock from the high level characterizing P. uca to the level characterizing the dominant species. This phenomenon—a competitive inferior being the most productive when alone—can also occur in plant (Hector et al. 2002) and microalgal assemblages (Weis et al. 2007).
High diversity is usually a transient state within individual snails for these trematode assemblages. The strong interspecific competition characterizing these mixed-species assemblages typically leads to competitive exclusion of the subordinate species (Kuris 1990; Sousa 1993). Thus, in individual snails, mixed-species assemblages are relatively ephemeral. So, their higher standing stock may not reflect longer-term resource constraints. As competition is resolved, the higher biomass will decline to the level characterizing that of the dominant species when it is alone. In fact, some of the unexplained variations in trematode diversity effects may be due to quantifying standing stock for mixed-species assemblages that are at different points in the competitive exclusion process.
The initial increase and decline in the standing stock characterizing these parasite assemblages warrants comparison with findings from previous work on microalgae and plants. Weis et al. (2007) found that niche partitioning can increase biomass production in diverse microalgal cultures, but only when comparisons were restricted to early successional time periods. Similar to the trematode assemblages of our study, dominant species ultimately exclude subordinates, forming monocultures. Unlike the trematodes we studied, these equilibrial-state monocultures had more biomass than did early successional diverse assemblages, even though dominants were not the most productive species. To more thoroughly compare the processes operating in microalgal and trematode castrator assemblages, experiments could determine whether niche partitioning can increase biomass production by adding dominant microalgae to subordinate monocultures at carrying capacity. Transient positive effects of diversity do not appear to be the rule for plant assemblages, where the positive diversity effects tend to increase over time (e.g. Tilman et al. 2001; van Ruijven & Berendse 2005; and see meta-analysis by Cardinale et al. 2007). However, most plant biodiversity experiments are not conducted for more than a few years (van Ruijven & Berendse 2005; Cardinale et al. 2007). Perhaps the positive diversity effects would also be transient over the longer time scales potentially necessary for competitive exclusion to take place in these assemblages.
Although mixed-species assemblages may frequently be short lived within individual snails, such assemblages are continually reformed in snail populations. Mixed-species assemblages appear to be always present in California horn snail populations, comprising from less than 2 per cent to over 20 per cent of encountered infections (Hunter 1942; Martin 1955; Sousa 1993; Lafferty et al. 1994). Thus, the ubiquitous presence of mixed-species assemblages, in conjunction with the large biomass that these trematodes contribute to estuaries (Kuris et al. 2008), indicates that the positive effect of diversity on trematode biomass within snails may scale up to have considerable influence at the ecosystem level.
Our findings bear on a general issue in parasitology and medicine concerning how variation in resource overlap can influence the production and pathology of a parasite species in mixed-species infections. In our study system, parasite assemblages are strongly interactive, with each species contending for the same ultimate resource (host reproductive effort). The production of one or both parasites was depressed by the presence of the other (apparent from the sub-additivity of mixed-species assemblages). Importantly, the severity of these negative effects was at least temporarily mediated by the degree of overlap in spatial resource use. This fits with the findings of a recent meta-analysis of mouse pathogen–parasitic worm co-infections. Although she did not measure total production, Graham (2008) found that the density of pathogens consuming red blood cells (RBCs) tends to decrease when in co-infections with parasitic worms expected to deplete the RBC resource (by blood feeding or causing haemorrhage). However, RBC-feeding pathogen density is not lower in co-infections with parasitic worm species that are thought not to decrease RBCs. Thus, ecological rules regarding how niche overlap affects competition may have broad implications for medicine and the dynamics of infectious disease.
In a previous comparative study of helminth parasites across vertebrate hosts, Poulin et al. (2003) considered whether productivity influences parasite species richness. They found that the mean diversity of parasites in a host species increased with the mean parasite biovolume (which was used to represent the productivity offered to parasites by a host species). This pattern is consistent with complementarity among parasite species (Poulin et al. 2003). However, complementarity may not be necessary because mean parasite biomass for the typical macroparasites used in that study does not represent the maximal parasite biomass supportable by a host (Poulin & George-Nascimento 2007). This is because such parasites do not grow to commandeer available resources (as opposed to parasitic castrators and pathogens; Lafferty & Kuris 2002) and mean numbers in a host are typically far lower than maximum numbers (Poulin & George-Nascimento 2007). Because mean measures for those parasites do not necessarily characterize assemblages experiencing competition, they are less subject to study focused on how niche complementarity influences diversity effects on productivity.
5. Conclusion
While much of the best work on the diversity–production relationship has been experimental, it is also important to examine the diversity patterns and processes in naturally occurring species assemblages (Chapin et al. 1997; Huston 1997; Loreau et al. 2001; Giller et al. 2004, and see Bracken et al. 2008). Unfortunately, the complexity of most natural systems makes them difficult to replicate and analyse. The simple, replicated assemblages in individual hosts that we studied allowed consideration of naturally occurring patterns and the mechanisms by which species diversity influences ecological process. Including assemblages of differing species composition at our ‘high’ diversity level allowed us to examine functional diversity. This provided insight into the aspects of resource use that may drive the positive effects of diversity on standing-stock biomass. Future studies should manipulate trematode diversity to further clarify the mechanisms by which diversity influences standing stock and to confirm its causality. However, these findings provide further evidence that the effects of diversity on productivity are universal and rich—complementarity (functional diversity) and species identity probably both contribute to the influence of diversity on the aggregate function of assemblages.
Acknowledgments
We are grateful to F. Mancini, L. Mababa, E. Abe and V. Frankel for field and/or laboratory work. We are indebted to B. Cardinale, M. Rigby and the anonymous reviewers whose comments improved the manuscript. We thank the UCNRS for access to field sites. Financial support came from a US NSF/NIH Ecology of Infectious Diseases Program grant (DEB-0224565). Any use of trade, product or firm names in this publication is for descriptive purposes only and does not imply endorsement by the US government.
References
- Aarssen L.W. High productivity in grassland ecosystems: effected by species diversity or productive species? Oikos. 1997;80:183–184. doi:10.2307/3546531 [Google Scholar]
- Balvanera P, Pfisterer A.B, Buchmann N, He J.S, Nakashizuka T, Raffaelli D, Schmid B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. doi:10.1111/j.1461-0248.2006.00963.x [DOI] [PubMed] [Google Scholar]
- Baudoin M. Host castration as a parasitic strategy. Evolution. 1975;29:335–352. doi: 10.1111/j.1558-5646.1975.tb00213.x. doi:10.2307/2407221 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B: Methodol. 1995;57:289–300. [Google Scholar]
- Bracken M.E.S, Friberg S.E, Gonzalez-Dorantes C.A, Williams S.L. Functional consequences of realistic biodiversity changes in a marine ecosystem. Proc. Natl Acad. Sci. USA. 2008;105:924–928. doi: 10.1073/pnas.0704103105. doi:10.1073/pnas.0704103105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cable, R. M. 1956 Marine cercariae of Puerto Rico. In Scientific survey of Porto Rico and the Virgin Islands, vol. XVI-Part 4 (ed. R. W. Miner), pp. 491–577. New York, NY: New York Academy of Sciences.
- Cardinale B.J, Srivastava D.S, Duffy J.E, Wright J.P, Downing A.L, Sankaran M, Jouseau C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. doi:10.1038/nature05202 [DOI] [PubMed] [Google Scholar]
- Cardinale B.J, Wright J.P, Cadotte M.W, Carroll I.T, Hector A, Srivastava D.S, Loreau M, Weis J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl Acad. Sci. USA. 2007;104:18 123–18 128. doi: 10.1073/pnas.0709069104. doi:10.1073/pnas.0709069104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin F.S, Walker B.H, Hobbs R.J, Hooper D.U, Lawton J.H, Sala O.E, Tilman D. Biotic control over the functioning of ecosystems. Science. 1997;277:500–504. doi:10.1126/science.277.5325.500 [Google Scholar]
- Combes C. Trematodes: antagonism between species and sterilizing effects on snails in biological control. Parasitology. 1982;84:151–175. [Google Scholar]
- DeCoursey P.J, Vernberg W.B. Double infections of larval trematodes: competitive interactions. In: Vernberg W.B, editor. Symbiosis in the sea. University of South Carolina Press; Columbia, SC: 1974. pp. 93–109. [Google Scholar]
- Fridley J.D. The influence of species diversity on ecosystem productivity: how, where, and why? Oikos. 2001;93:514–526. doi:10.1034/j.1600-0706.2001.930318.x [Google Scholar]
- Giller P.S, et al. Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. Oikos. 2004;104:423–436. doi:10.1111/j.0030-1299.2004.13253.x [Google Scholar]
- Graham A.L. Ecological rules governing helminth–microparasite coinfection. Proc. Natl Acad. Sci. USA. 2008;105:566–570. doi: 10.1073/pnas.0707221105. doi:10.1073/pnas.0707221105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldeman S.S. J. Dobson; Philadelphia, PA: 1840. A monograph of the Limniades and other freshwater univalve shells of North America. [Google Scholar]
- Hechinger, R. F., Lafferty, K. D., Mancini III, F. T., Warner, R. R. & Kuris, A. M. 2008 How large is the hand in the puppet? Ecological and evolutionary factors affecting on body mass of 15 trematode parasitic castrators in their snail host. Evol. Ecol. (doi:10.1007/s10682-008-9262-4).
- Hector A, Bazeley-White E, Loreau M, Otway S, Schmid B. Overyielding in grassland communities: testing the sampling effect hypothesis with replicated biodiversity experiments. Ecol. Lett. 2002;5:502–511. doi:10.1046/j.1461-0248.2002.00337.x [Google Scholar]
- Holliman R.B. Larval trematodes from the Apalachee Bay area, Florida, with a checklist of known marine cercariae arranged in a key to their superfamilies. Tulane Stud. Zool. 1961;9:2–74. [Google Scholar]
- Hooper D, et al. Species diversity, functional diversity and ecosystem functioning. In: Loreau M, Naeem S, Inchausti P, editors. Biodiversity and ecosystem functioning. Oxford University Press; Oxford, UK: 2002. pp. 195–208. [Google Scholar]
- Hooper D.U, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 2005;75:3–35. doi:10.1890/04-0922 [Google Scholar]
- Hunter W.S. University of California; Los Angeles, CA: 1942. Studies on cercariae of the common mud-flat snail, Cerithidea californica. [Google Scholar]
- Huston M.A. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. doi:10.1007/s004420050180 [DOI] [PubMed] [Google Scholar]
- Kuris A.M. Trophic interactions—similarity of parasitic castrators to parasitoids. Q. Rev. Biol. 1974;49:129–148. doi:10.1086/408018 [Google Scholar]
- Kuris A.M. Guild structure of larval trematodes in molluscan hosts: prevalence, dominance and significance of competition. In: Esch G.W, Bush A.O, Aho J.M, editors. Parasite communities: patterns and processes. Chapman and Hall; London, UK: 1990. pp. 69–100. [Google Scholar]
- Kuris A.M, et al. Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature. 2008;454:515–518. doi: 10.1038/nature06970. doi:10.1038/nature06970 [DOI] [PubMed] [Google Scholar]
- Kutner M.H, Nachtsheim C.J, Neter J, Li W. McGraw-Hill/Irwin; Boston, MA: 2005. Applied linear statistical models. [Google Scholar]
- Lafferty K.D, Kuris A.M. Trophic strategies, animal diversity and body size. Trends Ecol. Evol. 2002;17:507–513. doi:10.1016/S0169-5347(02)02615-0 [Google Scholar]
- Lafferty K.D, Sammond D.T, Kuris A.M. Analysis of larval trematode communities. Ecology. 1994;75:2275–2285. doi:10.2307/1940883 [Google Scholar]
- Lie K.J. Larval trematode antagonism: principles and possible application as a control method. Exp. Parasitol. 1973;33:343–349. doi: 10.1016/0014-4894(73)90038-6. doi:10.1016/0014-4894(73)90038-6 [DOI] [PubMed] [Google Scholar]
- Lim H.K, Heyneman D. Intramolluscan inter-trematode antagonism: a review of factors influencing the host–parasite system and its possible role in biological control. Adv. Parasitol. 1972;10:191–268. doi: 10.1016/s0065-308x(08)60175-x. doi:10.1016/S0065-308X(08)60175-X [DOI] [PubMed] [Google Scholar]
- Loreau M. Separating sampling and other effects in biodiversity experiments. Oikos. 1998;82:600–602. doi:10.2307/3546381 [Google Scholar]
- Loreau M. Biodiversity and ecosystem functioning: recent theoretical advances. Oikos. 2000;91:3–17. doi:10.1034/j.1600-0706.2000.910101.x [Google Scholar]
- Loreau M, et al. Ecology—biodiversity and ecosystem functioning: current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. doi:10.1126/science.1064088 [DOI] [PubMed] [Google Scholar]
- Martin, W. E. 1955 Seasonal infections of the snail, Cerithidea californica Haldeman, with larval trematodes. In Essays in Natural Science in Honor of Captain Alan Hancock on the occasion of his birthday, pp. 203–210. Los Angeles, CA: University of Southern California Press.
- Martin W.E. An annotated key to the cercariae that develop in the snail Cerithidea californica. Bull. South. Calif. Acad. Sci. 1972;71:39–43. [Google Scholar]
- McCarthy H.O, Fitzpatrick S, Irwin S.W.B. Life history and life cycles: production and behavior of trematode cercariae in relation to host exploitation and next-host characteristics. J. Parasitol. 2002;88:910–918. doi: 10.1645/0022-3395(2002)088[0910:LHALCP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Naeem S, Wright J.P. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecol. Lett. 2003;6:567–579. doi:10.1046/j.1461-0248.2003.00471.x [Google Scholar]
- Poulin R, George-Nascimento M. The scaling of total parasite biomass with host body mass. Int. J. Parasitol. 2007;37:359–364. doi: 10.1016/j.ijpara.2006.11.009. doi:10.1016/j.ijpara.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Poulin R, Mouillot D, George-Nascimento M. The relationship between species richness and productivity in metazoan parasite communities. Oecologia. 2003;137:277–285. doi: 10.1007/s00442-003-1343-z. doi:10.1007/s00442-003-1343-z [DOI] [PubMed] [Google Scholar]
- Quinn G.P, Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental design and data analysis for biologists. [Google Scholar]
- Schlapfer F, Schmid B. Ecosystem effects of biodiversity: a classification of hypotheses and exploration of empirical results. Ecol. Appl. 1999;9:893–912. doi:10.1890/1051-0761(1999)009[0893:EEOBAC]2.0.CO;2 [Google Scholar]
- Sousa W.P. Interspecific antagonism and species coexistence in a diverse guild of larval trematode parasites. Ecol. Monogr. 1993;63:103–128. doi:10.2307/2937176 [Google Scholar]
- Stachowicz J.J, Bruno J.F, Duffy J.E. Understanding the effects of marine biodiversity on communities and ecosystems. Annu. Rev. Ecol. Syst. 2007;38:739–766. doi:10.1146/annurev.ecolsys.38.091206.095659 [Google Scholar]
- Tilman D, Lehman C.L, Thomson K.T. Plant diversity and ecosystem productivity: theoretical considerations. Proc. Natl Acad. Sci. USA. 1997;94:1857–1861. doi: 10.1073/pnas.94.5.1857. doi:10.1073/pnas.94.5.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Reich P.B, Knops J, Wedin D, Mielke T, Lehman C. Diversity and productivity in a long-term grassland experiment. Science. 2001;294:843–845. doi: 10.1126/science.1060391. doi:10.1126/science.1060391 [DOI] [PubMed] [Google Scholar]
- Trenbath B.R. Biomass productivity of mixtures. Adv. Agron. 1974;26:177–210. doi:10.1016/S0065-2113(08)60871-8 [Google Scholar]
- van Ruijven J, Berendse F. Diversity–productivity relationships: Initial effects, long-term patterns, and underlying mechanisms. Proc. Natl Acad. Sci. USA. 2005;102:695–700. doi: 10.1073/pnas.0407524102. doi:10.1073/pnas.0407524102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J.J, Cardinale B.J, Forshay K.J, Ives A.R. Effects of species diversity on community biomass production change over the course of succession. Ecology. 2007;88:929–939. doi: 10.1890/06-0943. doi:10.1890/06-0943 [DOI] [PubMed] [Google Scholar]
- Yoshino T.P. A seasonal and histologic study of larval digenea infecting Cerithidea californica (Gastropoda: Prosobranchia) from Goleta Slough, Santa Barbara County, California, USA. Veliger. 1975;18:156–161. [Google Scholar]