Abstract
Sex ratios have important evolutionary consequences and are often biased by environmental factors. The effect of developmental temperature on offspring sex ratios has been widely documented across a diverse range of taxa but has rarely been investigated in birds and mammals. However, recent field observations and artificial incubation experiments have demonstrated that the hatching sex ratio of a megapode, the Australian brush-turkey (Alectura lathami), varied with incubation temperature; more females hatched at high incubation temperatures and more males hatched at low temperatures. Here, we investigated the causes of this temperature-dependent sex-biasing system. Molecular sexing of chicks and embryos confirmed that male embryo mortality was greater at high temperatures while female embryo mortality is greater at low temperatures, with mortality in both sexes similar at intermediate incubation temperatures. Temperature-dependent sex-biased embryo mortality represents a novel mechanism of altering sex ratios in birds. This novel mechanism, coupled with the unique breeding biology of the brush-turkey, offers a potentially unparalleled opportunity in which to investigate sex allocation theory in birds.
Keywords: temperature, sex ratios, megapode, Alectura lathami, embryo mortality
1. Introduction
Sex can be influenced by temperature during embryonic development in many species, including fishes (Oldfield 2005; Piferrer et al. 2005), amphibians (Eggert 2004; Sakata et al. 2005) and many species of reptiles (Bull 1980; Ewert & Nelson 1991; Pieau et al. 1999). In those fish and reptile species where sex remains undetermined at fertilization, this process is known as temperature-dependent sex determination (TSD). In some amphibians (Eggert 2004; Sakata et al. 2005) and lizard species (Shine et al. 2002; Quinn et al. 2007), extreme temperatures during embryonic development can override genetic sex determination (GSD) in a process known as sex reversal. In birds, Ferguson's (1994) early work on chickens showed that pulsing eggs with abnormally high or low temperatures produced sex reversal in 10 per cent of the hatchlings, that is, their sexual phenotype differed from their sexual genotype. However, further experiments failed to replicate this outcome. So in birds, despite considerable investigation and debate over possible mechanisms causing sex ratio variation (Ankney 1982), there is no empirical evidence of TSD or sex reversal. Convincing evidence that temperature could influence avian offspring sex ratios was lacking until recent work on a megapode bird, the Australian brush-turkey (Alectura lathami), showed this to be the case (Göth & Booth 2005; Göth 2007).
Alectura lathami are members of the family Megapodiidae, a small group of birds distributed throughout the broader Australasian region, and are the only avian species that are not brood incubators (Frith 1956). All megapodes employ environmental heat to incubate their eggs. Specifically, male brush-turkeys construct large mounds from soil and leaf litter, exploiting the heat produced by decomposing organic material to incubate their eggs (Jones et al. 1995). Although males use a range of behaviours to regulate mound temperature, eggs in natural mounds experience and tolerate prolonged fluctuations in incubation temperature (Eiby & Booth 2008). Females test mound temperatures regularly and lay a single egg every 3 or more days over the 6 month breeding period (Jones 1990). So there can be eggs from several females at various developmental stages in a single mound and the temporal and spatial variability of mound temperatures results in different thermal regimes for each egg. Conversely, non-megapode avian embryos typically develop at a relative constant temperature owing to the attentive behaviour of their brooding parent(s), making it extremely difficult to explore the effect of different incubation temperatures on hatchling attributes. Therefore, brush-turkeys provide a unique opportunity to investigate the impact of incubation temperature on the sex ratios of avian hatchlings.
Two recent studies involving both field observations of wild mounds and artificial incubation experiments have reported the first evidence that temperature does influence the sex ratio of brush-turkey chicks (Göth & Booth 2005; Göth 2007). These initial studies suggested the occurrence of a thermally sensitive period during the first two weeks of incubation whereby high temperature results in more female chicks hatching and low temperature results in more male chicks hatching, with a 1 : 1 sex ratio at the intermediate temperature (34°C; Göth & Booth 2005). In megapodes, as in all other non-ratite birds, sex is determined by heteromorphic Z and W sex chromosomes (Belterman & Deboer 1984), so it was hypothesized that the observed skewed sex ratios of hatchlings in brush-turkeys were caused by temperature-dependent sex-biased embryo mortality (TDSEM). Despite Göth & Booth (2005) and Göth (2007) providing strong evidence of TDSEM, the primary sex ratio was not determined and only the sex of successfully hatched chicks was recorded. Recent research shows that disparities between the primary and secondary sex ratios should be viewed cautiously and that sexing of all offspring is necessary to allow the effects of mortality to be directly observed (Krakow & Neuhuser 2008). Therefore, the failure to identify the sex of abortive embryos meant that TDSEM could not be conclusively confirmed. Here, we repeat the artificial incubation experiments previously undertaken by those studies and use molecular sexing methods to determine the sex of both the embryos that failed to complete development and the chicks that hatched successfully. This allows for the calculation of both the primary (at fertilization of eggs) and the secondary (at hatching of chicks) sex ratios. By comparing the mortality of male and female embryos at each temperature treatment we can determine whether TDSEM is the underlying mechanism driving the skewed sex ratios observed in brush-turkey hatchlings.
2. Material and methods
(a) Egg collection and artificial incubation
Brush-turkey eggs (n=148) were collected in Brisbane, Australia from 10 mounds in both the 2004–2005 and 2005–2006 breeding seasons and 11 mounds in the 2006–2007 season. Two methods of egg collection were used. The first method involved digging out entire mounds, which resulted in the collection of eggs with embryos at various developmental stages. However, the critical stage for temperature affecting sex ratio of chicks is during the first two weeks of embryonic development, so these eggs were incubated at 34°C and only used in primary sex ratio calculations. The second method collected freshly laid eggs for testing the TDSEM hypothesis. Mounds were monitored for females displaying laying behaviour (Jones 1988) and the eggs were collected immediately after being laid. All eggs were buried in mound material contained in closed boxes with air-permeable lids. Freshly collected eggs were assigned to constant incubation treatments of 32, 34 or 36°C. Embryo viability was determined weekly by holding a torch to the surface of the egg in a dark room, allowing visualization (candling). If an embryo died, as indicated by the retreat of the chorioallantoic membrane, the embryo was removed and preserved in 70 per cent ethanol.
(b) Molecular sexing
Non-ratite birds have chromosomal sex determination (females WZ; males ZZ). Polymerase chain reaction (PCR) can be used to discriminate between the intron lengths of the highly conserved CHD1 genes on each of the sex chromosomes, with females displaying two fragments (CHD1W and CHD1Z), while males show only one fragment (CHD1Z; Fridolfsson & Ellegren 1999). Tissue samples were collected from chicks (breast muscle and secondary feathers) that hatched successfully and from dead embryos (leg muscle or whole embryo). Total genomic DNA was extracted from tissue homogenates using either DNeasy Tissue Kits (Qiagen) or NucleoSpin Tissue Kits (Macherey-Nagel). All PCRs were performed in 10 μl volumes using 0.25 U HotMaster Taq (5 Prime), 0.8 mM dNTPs and 1 μM of primers 2550F and 2718R (Fridolfsson & Ellegren 1999). The thermal profile consisted of an initial denaturing step of 94°C for 2 min followed by a ‘touch-down’ scheme (Don et al. 1991; Fridolfsson & Ellegren 1999) where the annealing temperature was lowered 1°C per cycle, starting from 60°C, until a temperature of 51°C was reached. Then, 30 additional cycles were run at a constant annealing temperature of 51°C. Denaturation was at 94°C for 30 s, annealing for 30 s and extension at 65°C for 30 s. A final extension step of 2 min was added after the last cycle. PCR products were separated in 2 per cent agarose gels and visualized by ethidium bromide staining.
(c) Statistical analysis
A non-parametric Χ2 test was used to determine whether the observed primary sex ratio differed from the expected 1 : 1 ratio. To statistically test the hypothesis (HA) that temperature affects embryo mortality such that males and females at 34°C had the lowest mortality; males at 32°C and females at 36°C had intermediate mortality; and males at 36°C and females at 32°C had the highest mortality, a post hoc order–heterogeneity test was applied (Rice & Gaines 1994). In this procedure, Pc was taken from a binomial GLM procedure (McCullagh & Nelder 1989) to test for a significant interaction between temperature and sex, with the analysis performed in R (R Development Core Team 2008) and Rs calculated according to Rice & Gaines (1994) assigning expected ranks of 1.5, 3.5 and 5.5 for survivorship of males and females from 34°C; males from 32°C and females from 36°C; and males from 36°C and females from 32°C, respectively.
3. Results
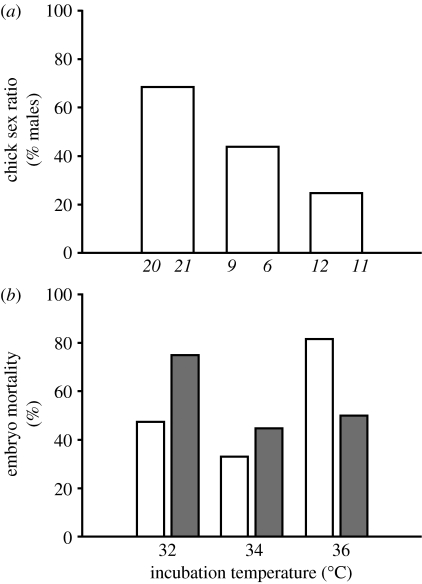
A total of 148 eggs were collected from 31 brush-turkey mounds over three breeding seasons (2004–2007). Molecular sexing assays of all eggs (successful and failed) showed that 79 were female and 69 were male, providing strong evidence that brush-turkeys have a 1 : 1 primary sex ratios over all seasons sampled (Χ12=0.676, p=0.411) and within any particular season (Χ52=1.164, p=0.948). Confirmation of a 1 : 1 primary sex ratio removes the likelihood that any observed skew towards a female ratio indicated for embryos may be an artefact brought about by sampling the vitelline membrane of the egg and thereby amplifying maternal rather than embryonic DNA (Arnold et al. 2003). Because the thermally sensitive period for sex-biased embryonic mortality is thought to occur within the first two weeks of development (Göth & Booth 2005), only those eggs that were collected immediately after laying (n=79) were used in our test for TDSEM. As previously reported (Göth & Booth 2005), chick sex ratios were skewed at 32 and 36°C incubation temperatures (figure 1a). The order–heterogeneity test found a significant difference in mortality between the groups (RsPc=0.902, k=6, p<0.001). Therefore, temperature does affect mortality consistent with HA and sex-biased embryo mortality occurred (figure 1b). Eggs incubated at 32°C not only produced more male chicks but also had higher female embryo mortality (female mortality=75.0%, male mortality=47.6%), whereas eggs incubated at 36°C produced more female chicks and indicated a higher male embryo mortality (female mortality=50.0%, male mortality=81.8%). Hatching success and embryo mortality were similar for both sexes at the intermediate temperature of 34°C (female mortality=44.4%, male mortality=33.3%).
Figure 1.
(a) Chick sex ratios (i.e. the number of males hatched/total number of chicks hatched) and (b) embryo mortality (the proportion of male (white bars) and female (grey bars) embryos that died) of the Australian brush-turkey (A. lathami) when artificially incubated at three different constant temperatures. All eggs were incubated at the test temperature from the day they were laid. Numbers in italics (n) indicate the number of eggs incubated. Note the close to 1 : 1 sex ratio and similar mortality of male and female embryos at the optimum test temperature of 34°C, and the greater relative hatching success of male embryos at low temperatures and greater relative hatching success of female embryos at high temperatures as found in a previous study (Göth & Booth 2005). We conclude that the sex-biased differential mortality of embryos observed at 32 and 36°C (b) is the mechanism behind the temperature-dependent skewing of sex ratio observed in hatchling brush-turkeys (a), with high and low temperatures having opposite effects on males and females.
4. Discussion
The sexing of failed embryos and the discovery of differential embryonic mortality between the sexes substantiate the hypothesis that the mechanism driving the observed skew of secondary sex ratios is TDSEM: a mechanism previously unknown to occur in birds. TDSEM is rare but has been demonstrated in pine snakes, where females suffer higher mortality at high temperatures and males at low temperatures (Burger & Zappalorti 1988), opposite to the pattern seen in brush-turkeys. What the potential adaptive significance of TDSEM, if any, could be in brush-turkeys is currently unclear.
The unusual breeding biology of brush-turkeys coupled with the discovery of TDSEM provides biologists with a unique avian system for investigating vertebrate sex allocation biology. Unlike other birds, brush-turkeys (and other megapodes) provide no parental care to hatchlings and there is the potential for either parent to influence hatching sex ratios; females through the selection of egg placement (laying locality) within mounds and males through the monitoring and adjustment of mound temperatures by changing their mound-tending behaviour. In this regard, brush-turkeys have a breeding strategy similar to that of many lizards and turtles, where parental manipulation of sex ratio, if present, is exerted during embryonic development rather than post-hatching. Because estimates of reproductive effort and the way parents may divide their investment unequally into male or female offspring are usually confounded by post-hatching parental care in birds, brush-turkeys with no post-hatching parental care may provide a potentially unrivalled avian model species for research in this field.
According to the Charnov & Bull (1989) model, in reptiles, species with TSD have an adaptive advantage over species with GSD because females can target, through egg placement, nest temperatures that simultaneously determine the sex of offspring and maximize the fitness of that sex. Empirical evidence has been elusive but recent work on a short-lived lizard (Amphibolurus muricatus) showed that the temperature which produced each sex also maximized lifetime fitness for each sex (Warner & Shine 2008). This is unlike brush-turkeys, where temperature does not determine the sex of offspring, but rather produces skewed sex ratios through mortality. Here, temperature imposes a physiological constraint to embryonic development, reflecting a difference in optimal development temperatures between the sexes.
Other mechanisms capable of manipulating sex ratios through differential mortality in birds include the influence of maternal hormones. Research focused on the links between maternal and yolk androgen testosterone and offspring phenotype has suggested the possibility of maternal modifications of secondary sex ratios (Groothuis & Schwabl 2008). Stress and metabolic hormones may also determine sex ratios. For example, in European starlings (Sturnus vulgaris), maternal elevation of the stress hormone corticosterone resulted in increased mortality of male embryos (Love et al. 2005). Because chicks are altricial in this species and most parental energetic investment is in post-hatching care rather than in the eggs, embryonic mortality may not represent a major loss of resources for the parents. By contrast, although female brush-turkeys may invest considerable energy into large, yolk-rich eggs (approx. 200 g; Eiby & Booth 2008), they do not invest any energy during egg incubation or through post-hatching care. Consequently, embryonic mortality resulting from TDSEM may represent a significant loss of investment for the parents. Any adaptive advantage of this mechanism may be coupled with maternal effects, such as control of the primary sex ratio, yolk hormones and incubation temperature, which may be operating to optimize sex-specific fitness.
For the first time, TDSEM has been shown to influence the sex ratios of offspring in a bird, the (A. lathami). Because variable environmental heat sources are used by all megapodes to incubate their eggs, it would be of interest to determine whether the TDSEM mechanism is operating in the other 18 extant megapode species in order to establish its evolutionary origins in this bird family. As many megapode species are threatened by human habitat modification (Jones et al. 1995), an understanding of the influence of environmental factors on reproductive success is also essential to conservation and management of these species.
Our data suggest that developmental temperature optima are different between the sexes, with optimal temperatures in one sex resulting in elevated mortality in the other. If different developmental optima are present between the sexes, then we would predict that incubation temperature would have other effects post-hatching, such as differences in growth rates, size, energy reserves, behaviour and survival (Burger 1998). Further investigation is required to determine whether individual female brush-turkeys can control secondary sex ratios and influence sex-specific development through the interactive effects of yolk hormones and incubation temperatures.
Acknowledgments
The study was conducted under the University of Queensland animal ethics approval no. ZOO/ENT/200/04/URG and the eggs were collected under Queensland EPA scientific permit no. WIEP01995604.
References
- Ankney C.D. Sex ratio varies with egg sequence in Lesser Snow Geese. Auk. 1982;99:662–666. [Google Scholar]
- Arnold K.E, Orr K.J, Griffiths R. Primary sex ratios in birds: problems with molecular sex identification of undeveloped eggs. Mol. Ecol. 2003;12:3451–3458. doi: 10.1046/j.1365-294x.2003.02007.x. doi:10.1046/j.1365-294X.2003.02007.x [DOI] [PubMed] [Google Scholar]
- Belterman R.H.R, Deboer L.E.M. A karyological study of 55 species of birds, including karyotypes of 39 species new to cytology. Genetica. 1984;65:39–82. doi:10.1007/BF00056765 [Google Scholar]
- Bull J.J. Sex determination in reptiles. Q. Rev. Biol. 1980;55:3–21. doi:10.1086/411613 [Google Scholar]
- Burger J. Effects of incubation temperature on hatchling pine snakes: implications for survival. Behav. Ecol. Sociobiol. 1998;43:11–18. doi:10.1007/s002650050461 [Google Scholar]
- Burger J, Zappalorti R.T. Effects of incubation temperature on sex-ratios in pine snakes: differential vulnerability of males and females. Am. Nat. 1988;132:492–505. doi:10.1086/284867 [Google Scholar]
- Charnov E.L, Bull J.J. The primary sex ratio under environmental sex determination. J. Theor. Biol. 1989;139:431–436. doi: 10.1016/s0022-5193(89)80063-3. doi:10.1016/S0022-5193(89)80063-3 [DOI] [PubMed] [Google Scholar]
- Don R.H, Cox P.T, Wainwright B.J, Baker K, Mattick J.S. Touchdown PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991;19:4008. doi: 10.1093/nar/19.14.4008. doi:10.1093/nar/19.14.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert C. Sex determination: the amphibian models. Reprod. Nutr. Dev. 2004;44:539–549. doi: 10.1051/rnd:2004062. doi:10.1051/rnd:2004062 [DOI] [PubMed] [Google Scholar]
- Eiby Y.A, Booth D.T. Embryonic thermal tolerance and temperature fluctuations in mounds of the Australian brush-turkey (Alectura lathami) Auk. 2008;125:594–599. [Google Scholar]
- Ewert M.A, Nelson C.E. Sex determination in turtles: diverse patterns and some possible adaptive values. Copeia. 1991;4:50–69. doi:10.2307/1446248 [Google Scholar]
- Ferguson, M. W. J. 1994 Temperature dependent sex determination in reptiles and manipulation of poultry sex by incubation temperature. In Proc. 9th European Poultry Conf., Glasgow, pp. 380–382.
- Fridolfsson A.K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. doi:10.2307/3677252 [Google Scholar]
- Frith H.J. Breeding habits in the family Megapodiidae. Ibis. 1956;98:620–640. doi:10.1111/j.1474-919X.1956.tb01453.x [Google Scholar]
- Göth A. Incubation temperatures and sex ratios in Australian brush-turkey (Alectura lathami) mounds. Austral Ecol. 2007;32:378–385. doi:10.1111/j.1442-9993.2007.01709.x [Google Scholar]
- Göth A, Booth D.T. Temperature-dependent sex ratio in a bird. Biol. Lett. 2005;1:31–33. doi: 10.1098/rsbl.2004.0247. doi:10.1098/rsbl.2004.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G.G, Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil. Trans. R. Soc. B. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. doi:10.1098/rstb.2007.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.N. Construction and maintenance of the incubation mounds of the Australian brush-turkey Alectura lathami. Emu. 1988;88:210–218. [Google Scholar]
- Jones D.N. Social-organization and sexual interactions in Australian brush-turkeys (Alectura lathami): implications of promiscuity in a mound-building megapode. Ethology. 1990;84:89–104. [Google Scholar]
- Jones D.N, Dekker R.W.R.J, Roselaar C.S. Oxford University Press; Oxford, UK: 1995. The Megapodes: Megapodiidae. [Google Scholar]
- Krakow S, Neuhuser M. Insights from complete-incomplete brood sex-ratio disparity. Behav. Ecol. Sociobiol. 2008;62:469–477. doi:10.1007/s00265-007-0466-3 [Google Scholar]
- Love O.P, Chin E.H, Wynne-Edwards K.E, Williams T.D. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 2005;166:751–766. doi: 10.1086/497440. doi:10.1086/497440 [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder J.A. Chapman and Hall; London, UK: 1989. Generalized linear models. [Google Scholar]
- Oldfield R.G. Genetic, abiotic and social influences on sex differentiation in cichlid fishes and the evolution of sequential hermaphroditism. Fish Fish. 2005;6:93–110. doi:10.1111/j.1467-2979.2005.00184.x [Google Scholar]
- Pieau C, Dorizzi M, Richard-Mercier N. Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell Mol. Life Sci. 1999;55:887–900. doi: 10.1007/s000180050342. doi:10.1007/s000180050342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piferrer F, Blazquez M, Navarro L, Gonzalez A. Genetic, endocrine, and environmental components of sex determination and differentiation in the European sea bass (Dicentrarchus labrax L.) Gen. Comp. Endocrinol. 2005;142:102–110. doi: 10.1016/j.ygcen.2005.02.011. doi:10.1016/j.ygcen.2005.02.011 [DOI] [PubMed] [Google Scholar]
- Quinn A.E, Georges A, Sarre S.D, Guarino F, Ezaz T, Graves J.A.M. Temperature sex reversal implies sex gene dosage in a reptile. Science. 2007;316:411. doi: 10.1126/science.1135925. doi:10.1126/science.1135925 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2008. R: a language and environment for statistical computing. [Google Scholar]
- Rice W.R, Gaines S.D. The order-heterogeneity family of tests. Biometrics. 1994;50:746–752. doi:10.2307/2532788 [Google Scholar]
- Sakata N, Tamori Y, Wakahara M. P450 aromatase expression in the temperature-sensitive sexual differentiation of salamander (Hynobius retardatus) gonads. Int. J. Dev. Biol. 2005;49:417–425. doi: 10.1387/ijdb.041916ns. doi:10.1387/ijdb.041916ns [DOI] [PubMed] [Google Scholar]
- Shine R, Elphick M.J, Donnellan S. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecol. Lett. 2002;5:486–489. doi:10.1046/j.1461-0248.2002.00351.x [Google Scholar]
- Warner D.A, Shine R. The adaptive significance of temperature-dependent sex determination in a reptile. Nature. 2008;451:566–568. doi: 10.1038/nature06519. doi:10.1038/nature06519 [DOI] [PubMed] [Google Scholar]