Abstract
Global climate change has accelerated the pace of glacial retreat in high-latitude and high-elevation environments, exposing lands that remain devoid of vegetation for many years. The exposure of ‘new’ soil is particularly apparent at high elevations (5000 metres above sea level) in the Peruvian Andes, where extreme environmental conditions hinder plant colonization. Nonetheless, these seemingly barren soils contain a diverse microbial community; yet the biogeochemical role of micro-organisms at these extreme elevations remains unknown. Using biogeochemical and molecular techniques, we investigated the biological community structure and ecosystem functioning of the pre-plant stages of primary succession in soils along a high-Andean chronosequence. We found that recently glaciated soils were colonized by a diverse community of cyanobacteria during the first 4–5 years following glacial retreat. This significant increase in cyanobacterial diversity corresponded with equally dramatic increases in soil stability, heterotrophic microbial biomass, soil enzyme activity and the presence and abundance of photosynthetic and photoprotective pigments. Furthermore, we found that soil nitrogen-fixation rates increased almost two orders of magnitude during the first 4–5 years of succession, many years before the establishment of mosses, lichens or vascular plants. Carbon analyses (pyrolysis-gas chromatography/mass spectroscopy) of soil organic matter suggested that soil carbon along the chronosequence was of microbial origin. This indicates that inputs of nutrients and organic matter during early ecosystem development at these sites are dominated by microbial carbon and nitrogen fixation. Overall, our results indicate that photosynthetic and nitrogen-fixing bacteria play important roles in acquiring nutrients and facilitating ecological succession in soils near some of the highest elevation receding glaciers on the Earth.
Keywords: primary succession, nitrogen fixation, cyanobacteria, Peruvian Andes
1. Introduction
Ecological research conducted along deglaciated chronosequences has been pivotal in advancing our understanding of primary succession and ecosystem development. These studies employ space-for-time substitutions, such that distance from a receding glacier is used as a proxy for soil age, with older, more developed soils being further from the glacier's edge. The majority of these studies have focused on plant succession (Bormann & Sidle 1990; Matthews 1992; Chapin et al. 1994) and, while vegetation succession can be stochastic across different environments, research has demonstrated some consistent patterns of plant-mediated changes to ecosystem functioning such as nitrogen (N) fixation (Walker & del Moral 2003). For example, plant communities dominated by N-fixing species are common early in the primary succession of newly deglaciated soils in Glacier Bay, Alaska (Reiners et al. 1971). These N-fixing plants increase soil N pools and N-cycling rates (Bormann & Sidle 1990; Chapin et al. 1994) and affect soil development (Crocker & Major 1955). Such plant-driven changes to soil N cycling have significant effects on ecosystem functioning and on the establishment of subsequent plant communities (Chapin et al. 1994).
By contrast, the earliest pre-plant stages of primary succession have received little study, despite the fact that this stage of succession can last for many years in high-latitude and high-elevation environments (Hodkinson et al. 2003; Sigler & Zeyer 2004). However, with the application of modern molecular approaches, it is becoming clear that microbes rapidly colonize deglaciated soils long before vascular plants or lichens appear (Jumpponen 2003; Tscherko et al. 2003; Nicol et al. 2005; Bardgett et al. 2007). Yet we know little about the ecological or biogeochemical importance of these early colonists, or of the biological activity occurring within these seemingly barren soils. It is possible that micro-organisms follow predictable patterns of succession in particular ecosystems, and that microbial succession may play important roles in ecosystem development. Alternatively, it has been suggested that early microbial inhabitants of proglacial lands are mostly dormant (Jumpponen 2003), and that fluxes of nutrients and organic matter in these systems originate from aeolian inputs (Swan 1992; Hodkinson et al. 2002) or from ancient carbon (C) pools (Bardgett et al. 2007). However, our preliminary studies (Nemergut et al. 2007) indicated that C and N pools increased over the first 20 years of succession (significantly for N, but not for C), suggesting that these elements were being replenished and hinting at a role for N and C fixation within these soils.
Global climate change has hastened both the rate and extent of glacial melting in high-latitude and high-elevation environments (Barnett et al. 2005; Barry 2006). This is particularly true in high-elevation sites (5000 m.a.s.l.) in the Andes (Mark & Seltzer 2005; Bradley et al. 2006), where extreme aridity, low atmospheric partial pressures, low temperatures and high ultraviolet radiation hinder rapid plant colonization. These ostensibly barren sites contain diverse microbial communities (Nemergut et al. 2007) and thus offer a rare opportunity to investigate how life becomes established and develops in some of the most extreme terrestrial environments on Earth. In this paper, we describe the co-development of microbial communities and microbial biogeochemical processes in recently deglaciated soils in the high Andes of Peru. Repeated visits to the same receding glacier combined with isochrones of glacier termini from aerial photos allowed us to investigate a soil chronosequence devoid of plant life. We show that these seemingly barren soils rapidly developed a diverse photosynthetic and N-fixing microbial community many years before the development of lichens, mosses or plants, and that this community contributes substantially to the biogeochemical cycles of this system, even during the first 4–5 years following deglaciation.
2. Material and methods
(a) Study site and ice recession analysis
The terminus (elevation 5000 m.a.s.l. in 2005) of the Puca Glacier lies in the Laguna Sibinacocha Basin, in the Cordillera Vilcanota of the Peruvian Andes (figure 1). This basin was heavily glaciated during the Little Ice Age (LIA), resulting in a still-visible boundary above which there are no lichens (Seimon et al. 2007). Soils inside the LIA boundary are undeveloped glacial till with very low organic C and N contents (approx. 0.1 and 0.01%, respectively) and high quartz and calcite contents (Nemergut et al. 2007). Our early successional sites (0–4 years uncovered) are all at least 1 km inside the LIA boundary and therefore have probably been ice-covered for at least the last 700 years. More detailed descriptions of the overall study area have been published elsewhere (Nemergut et al. 2007; Seimon et al. 2007; Schmidt et al. 2008). The age of the youngest sampling sites was determined by ground observations of glacial extent in 2001, 2003 and 2005, and by aerial photos taken in 2003 and 2005 (Seimon et al. 2007). The 4-year-old sites were at the glacial forefront in 2001 and had been uncovered from 4 to 5 years when sampled. Likewise, the 1-year-old sites had been uncovered from 1 to 1.5 years when sampled, and the 0-year-old sites were just being uncovered when sampled. The approximate yearly rate of glacial retreat during this study period (2000–2005) was 20 m yr−1. The age of the oldest sampling sites was determined to be approximately 79 years old by comparing GPS positions with pre-established isolines for glacial coverage in 1931, 1962, 1980 and 2005, as estimated from aerial photographs (Seimon et al. 2007). From 1931 to 1961 the glacier receded at a rate of approximately 14 m yr−1. The 79-year-old sites were located 77 m beyond the 1931 isoline and, assuming the same recession rate of 14 m yr−1, this site was last covered in 1926.
Figure 1.
Photograph of the Puca Glacier taken in March 2005 from a distance of approximately 8 km. Lake Sibinacocha (4900 m.a.s.l.) is in the foreground and the glacier terminus is at an elevation of 5000 m. The highest peak on the horizon is Nevado Chumpe (6106 m.a.s.l.).
(b) Sample collection and transport
Three sets of samples were collected along the chronosequence to analyse microbial community composition and activity. The first set was used for microbiological analyses: at the appropriate distance from the glacier, four soil samples from four randomly selected locations (but all at the same distance from the glacier) were collected from the top 2 cm using sterilized trowels. Soils were placed into sterile 15 ml centrifuge tubes, sealed and kept frozen throughout their return to the US for analysis. The second set of samples was used for N-fixation rate analysis. Eight locations were randomly selected at each distance from the glacier and soils were collected to a 2 cm depth using 2.43 cm diameter, 55 ml clear acrylic tubes. Samples were analysed using the acetylene reduction assay in the field, transferred into sterile plastic bags and kept frozen throughout their return to the US for analysis. These samples were also used for pigment analyses. The third set of samples was used for all other chemical analyses. Ten randomly selected locations at each distance from the glacier were chosen and, using a 2.54 cm soil corer, samples were collected to a depth of 10 cm. Samples were placed in sterile plastic bags and kept frozen throughout their return to the US. All sample analyses were conducted upon return to the laboratory and all samples were kept frozen at all times between collection and analysis.
(c) Microbiological analyses
Heterotrophic microbial biomass was estimated using the substrate-induced growth response approach described by Lipson et al. (1999). This approach was modified for estimating the very low levels of biomass in these soils by using low concentrations of the substrate glutamate (50 μg C g dry soil−1) and incubating for longer time periods (Ley et al. 2004).
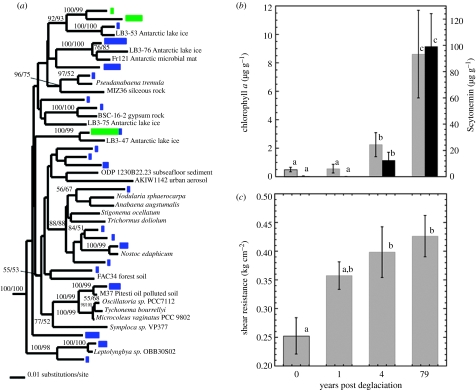
Two replicate bacterial clone libraries were constructed for each of the 0- and 4-year-old soils with PCR primers 515F and 1492R using methods as previously described (Nemergut et al. 2007). Cyanobacteria made up 12 per cent of all clones (n=139) in 0-year-old soils and 31 per cent of clones (n=144) in 4-year-old soils. Sequence alignments were subjected to Bayesian phylogenetic analysis (Huelsenbeck et al. 2001) using the GTR+gamma model of evolution with 1 000 000 generations. Closely related sequences from ARB and BLAST searches were used as reference taxa. Support values for each node of the tree greater than 50 per cent are shown (distance/parsimony). The tree was rooted with Rhodobacter sp. HTCC515 (AY584573) and Sphingomonas oligophenolica (AB018439).
(d) Pigment extraction and high-performance liquid chromatography analyses
Photosynthetic and photoprotective pigment extraction was carried out as described by Bowker et al. (2002). For extracted samples, quantitative and qualitative pigment analyses were performed as described by Karsten et al. (1998) with the following modifications. Pigments were separated on a Waters high-performance liquid chromatography (HPLC) system with a Symmetry C18 column (3.9×150 mm; Waters Corporation, Milford, MA, USA). For each sample, 100 μl of the acetone-pigment solution were injected by the autosampler into the HPLC system and the elution lasted for 23 min. Pigments were identified using known standards by comparative retention times and characteristic absorption maxima obtained from photodiode array and fluorescence detectors.
(e) Nitrogen-fixation measurements
Rates of N fixation in the field were estimated using a modified acetylene reduction assay (Belnap 1996). Soil samples were collected at all sites (eight spatially separate replicates per distance from the glacier) as 2 cm deep intact cores in 2.43 cm diameter, 55 ml clear acrylic tubes. After collection, samples were sealed, injected (through lids fitted with septa) with enough acetylene (generated from calcium carbide) to create a 10 per cent headspace concentration by volume, vented to the atmosphere, and left to incubate in the field for 18 hours in the shade during daylight (12.00 to 18.00, solar time, 19 March 2005) and night time (18.00–06.00). Headspaces were sampled the next morning (20 March 2005) at 06.00. Because we were interested in in situ rates of N fixation, sample moisture content was not manipulated and samples were incubated at the field sites. After incubation, sample headspaces were mixed, sub-sampled, injected into Vacutainer tubes (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) and were returned to the laboratory for immediate gas analysis. Ethylene concentrations were measured using a Shimadzu 14-A Gas Chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a flame ionization detector (330°C) and a Poropak N column (110°C; Supelco, Bellefonte, PA, USA). Parallel acetylene blanks (no soil) were collected during the field incubation and used to assess background levels of ethylene. Controls for ethylene production in the absence of acetylene and for ethylene consumption were also determined and were consistently undetectable.
Using a series of ethylene standards, rates of acetylene reduction were calculated using the units of nanomoles of acetylene reduced per gram dry mass of sample per hour of incubation. However, to facilitate comparison with other estimates and to put N-fixation rates in an ecosystem N-cycle context, rates of acetylene reduction were converted to area-based rates of N fixation. To convert rates to kg N fixed ha−1 yr−1, we (i) applied the theoretical conversion factor of three moles of acetylene reduced for every mole of N fixed (Hardy et al. 1968), (ii) used the surface area of the collection tubes and (iii) assumed N fixation would occur for 10 hours per day. This is an estimate of N fixation calculated from a single analysis and, because free-living N-fixation rates probably vary on seasonal scales (Reed et al. 2007), should not be considered a true annual rate.
(f) Soil nitrogen assays
Total soil N concentrations were measured on ground, oven-dried soils (four spatial replicates of 0–10 cm depth per distance from the glacier) using a Carlo Erba EA 1110 elemental analyser (CE Elantech, Lakewood, NJ, USA). Extractable inorganic soil N concentrations (NH4+ and NO3−) were determined by extracting 10 g of fresh soil with 50 ml of 2 M KCl for 18 hours and then filtering samples (Robertson et al. 1999). Samples were analysed colorimetrically using an Alpkem autoanalyser (OI Analytical, College Station, TX, USA).
(g) Pyrolysis-gas chromatography/mass spectroscopy
We examined the chemical structure of soils and cyanobacterial cultures using pyrolysis-gas chromatography/mass spectroscopy (Pyr-GC/MS; Gleixner et al. 2002; Grandy & Neff 2008). Samples were pyrolysed (10 s) in a GSG Analytical Pyromat Curie-Point pyrolyser (Brechbuhler Scientific, Houston, TX, USA) using a ferromagnetic tube with a Curie-point of 590°C. The pyrolysis products were transferred online to a Trace GC gas chromatograph (ThermoQuest Trace GC, Thermo Finnigan, Waltham, MA, USA). The interface temperature was set to 250°C with a split injection (split ratio 50 : 1, helium flow rate 1.0 ml min−1). Pyrolysis products were separated on a BPX 5 column (60 m×0.25 mm, film thickness 0.25 μm; SGE Analytical Science, Austin, TX, USA) using a temperature programme of 40°C for 5 min, 5°C min−1 to 270°C followed by a jump (30°C min−1) to a final temperature of 300°C. The column outlet was coupled to a Thermo Polaris-Q ion-trap mass spectrometer operated at 70 eV in the EI mode. The transfer line was heated to 270°C and the source temperature was held at 200°C. Pyrolysis products were subsequently identified by comparison with reference spectra after deconvolution and extraction using AMDIS v. 2.64, and then compared with National Institute of Standards and Technology mass spectral libraries and published literature.
(h) Enzyme assays
Enzyme assays were conducted (n=5, 6 and 6 for soil ages 0, 4 and 79 years, respectively) on soils that had been collected in the field and kept frozen during transport, and samples were analysed two weeks after collection. The procedures were as previously described (Weintraub et al. 2007), but with a higher soil : buffer ratio and longer incubation times to increase the sensitivity of the assays. Soil slurries were prepared by shaking 2 g of soil in 125 ml of 50 mM sodium bicarbonate buffer, pH 8.5 (a typical pH for these soils). The slurries were continuously stirred using a magnetic stir plate while 200 μl aliquots were pipetted into 96-well microplates. For each sample, 16 replicate wells were created. These enzyme assays were fluorometric, and were set up in black 96-well microplates. We added 50 μl of 200 mM of the appropriate substrate solution to each sample well. Blank wells were created with 50 μl of buffer and 200 μl of sample slurry. Negative control wells were created with 50 μl of the substrate solution and 200 μl of buffer. Quench standards were created with 50 μl of fluorescence standard (10 mM 4-methylumbelliferone, or 7-amino-4-methylcoumarin in the case of leucine aminopeptidase) and 200 μl of the sample slurry. Reference standards were created with 50 μl of standard and 200 μl of buffer. The assay plates were incubated in the dark at 13°C for up to 24 h. At the end of the incubation, 10 μl aliquots of 1.0 M NaOH were added to each well to raise the pH, as 4-methylumbelliferone and 7-amino-4-methylcoumarin fluoresce more strongly at higher pH. Fluorescence was measured using a Bio-Tek microplate fluorometer (Bio-Tek Instruments, Inc.) with 365 nm excitation and 460 nm emission filters. After correcting for quenching and for the negative controls, enzyme activities were expressed as nmol g dry soil−1 h−1.
(i) Soil stability analyses
To assess the relationship between soil age and soil resistance to erosion, a Torvane Shear Measurement Device (ELE International, Loveland, CO, USA) was used. A Torvane is suggested as the most effective instrument for assessing the resistance of soil to detachment by run-off and erosion (Zimbone et al. 1996), and this technique uses a hand-held vane shear device for rapid determination of shear strength in soils. Briefly, one component of the Torvane has plastic blades that are placed into the surface (top 0.5 cm) of the soil, and the device is twisted such that the soil must resist the movement of the blades. These blades work in conjunction with a meter that measures the soil's resistance and gives a shear strength value in the units kg cm−2.
(j) Statistics
To statistically compare the cyanobacterial community in the 0- and 4-year-old soils we used the Phylo-test (P-test) as previously described (Martin 2002; Schadt et al. 2003). We determined the posterior probability distribution of trees using Bayesian analysis. For each of the Bayesian trees, site was optimized on the tree as a discrete character using parsimony, and the covariation between phylogeny and site was determined as the number of changes between sites needed to explain the observed distribution of cyanobacteria. The significance of the covariation was established by determining the expected number of changes under the null hypothesis that the communities do not covary with phylogeny. The null expectation was estimated by assuming that the identity of sequences across communities remains fixed and that the relationships among sequences are random. If the observed number of transitions from one community to another is less than 95 per cent of the values generated from randomized data, then microbial composition differs significantly between the two communities.
All other data were tested for homoscedasticity (Levene's test for equal variances) and normality (SPSS, Chicago, IL, USA); when data did not meet these assumptions, they were ln-transformed prior to statistical analysis. Differences along the chronosequence were tested with one-way analyses of variances (ANOVAs): for each ecosystem property or rate of a process, an ANOVA was performed using distance from the glacier as the independent factor. To examine finer-scale variation within the chronosequence, multiple comparisons for each ANOVA were performed using Tukey's post hoc analyses. Enzyme data were analysed using one-way multivariate analysis of variance (MANOVA; Data Desk version 6.1, Data Descriptions, Inc., Ithaca, NY, USA) with distance from the glacier as the factor. Multiple comparisons for each MANOVA were performed using LSD post hoc analyses. For all data, significance was determined at α=0.05.
3. Results
Analysis of 16S rDNA sequences from soil genomic DNA libraries revealed marked shifts in the microbial community during the first 4 years of succession following glacier retreat (figure 2a). Statistical comparison of the cyanobacterial communities using the Phylo-test (Martin 2002; Schadt et al. 2003) revealed that the 0- and 4-year-old cyanobacterial communities were significantly different in both diversity and abundance (p<0.001; figure 2a). Additionally, an increase in the concentration of pigments involved in bacterial photosynthesis and photoprotection mirrored the increase in cyanobacterial diversity in the 4-year-old site, providing further evidence that cyanobacteria are abundant in these seemingly barren soils (figure 2b). In addition to the pigments shown in figure 2b, two other cyanobacterial pigments (chlorophyll b and echinenone) went from undetectable levels to 0.60±0.32 and 0.09±0.06 μg g dry soil−1, respectively, during the first 4 years of succession. Plastid sequences from diatoms were also detected in our 16S libraries increasing from approximately 4 per cent relative abundance to 12 per cent over the first 4 years of succession. Although the diversity of diatoms was too low to apply the Phylo-test, the diatom phylotypes in the 0-year-old soils were not related to any known diatoms, whereas those in the 4-year-old soils were 98 per cent related to Nitzschia frustulum, a known Antarctic diatom (Kawecka & Olech 1993). Soil surface stability also increased significantly (F3,16=4.6, p=0.017) along the chronosequence, even during the first 4 years of succession (figure 2c).
Figure 2.
(a) Phylogenetic tree of cyanobacterial sequences from 0-year-old soils (green rectangles) and 4-year-old soils (blue rectangles) compared with known cyanobacterial sequences. The relative length of the rectangle symbolizes the number of sequences of each phylotype. There was a significant change (p>0.001) in cyanobacterial diversity between the 0- and 4 year samples as determined by the Phylo-test (Martin 2002; Schadt et al. 2003). (b) Chlorophyll a (grey bars) and Scytonemin (black bars) pigment concentrations during the first 79 years following deglaciation (n=8 per soil age). Data shown are means±1 s.e. and differing lower-case letters represent significant differences (p<0.05) in a given pigment concentration at different points along the chronosequence (as determined by one-way ANOVAs and Tukey's post hoc analyses). Both chlorophyll a and Scytonemin concentrations were significantly higher in the 4-year-old soils than in the 0- or 1-year-old soils. (c) Means±1 s.e. of soil stability measurements (n=4 for 0- and 1-year-old soil and n=6 for 4- and 79-year-old soils) of soils of varying ages along the chronosequence. Different letters on bars of the same variable indicate significant differences (p<0.05) determined from one-way ANOVA and Tukey's post hoc analysis.
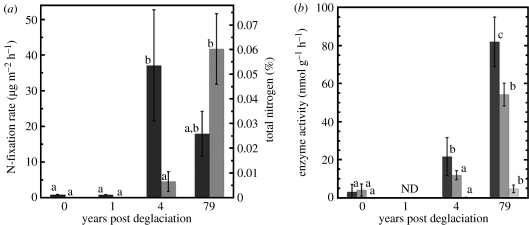
Rates of soil N fixation varied significantly (F3,32=5.2, p=0.005) along the chronosequence (figure 3a). There was a significant increase in N fixation from the low rates at the 0- and 1-year-old sites (0.80 and 0.81 μg m−2 h−1, respectively) to rates that were approximately two orders of magnitude higher in the 4-year-old sites (37 μg m−2 h−1); rates then declined to half that value (although not significantly) in the 79-year-old sites (18 μg m−2 h−1; figure 3a). Activity of three key microbial enzymes increased along the chronosequence, although only phosphatase increased significantly during the first 4 years of succession (F2,14=8.8, p=0.04; figure 3b).
Figure 3.
(a) Means±1 s.e. of N-fixation rates (n=8 per soil age; black bars) and total soil N (n=4 per soil age; grey bars) in soils of varying ages along the chronosequence. Different letters on bars of the same variable indicate significant differences (p<0.05) determined from one-way ANOVA and Tukey's post hoc analysis. (b) Microbial enzyme activities (black bars, phosphatase; grey bars, peptidase; light grey bar, cellulase) in soils of varying ages along the chronosequence. Data shown are means±1 s.e. and differing lower-case letters represent significant differences (p<0.05) in enzyme activity at different points along the chronosequence.
Concentrations of both total and inorganic soil N increased continuously along the chronosequence. Total soil N increased from almost undetectable levels in the 0- and 1-year-old soils to 600 μg g dry soil−1 in the 79-year-old soils (figure 3a). Inorganic N concentrations followed the same pattern (F3,12=11.2, p<0.001) with the lowest concentrations present at the 0- and 1-year-old sites (0.8 and 0 μg NH4+g dry soil−1, respectively, and 0.6 and 0.4 μg NO3− g dry soil−1, respectively), higher concentrations in the 4-year-old soil (2.1 μg NH4+g dry soil−1and 0.6 μg NO3− g dry soil−1), and the highest concentrations found at the 79-year-old soil (4.9 μg NH4+g dry soil−1 and 10.3 μg NO3− g dry soil−1).
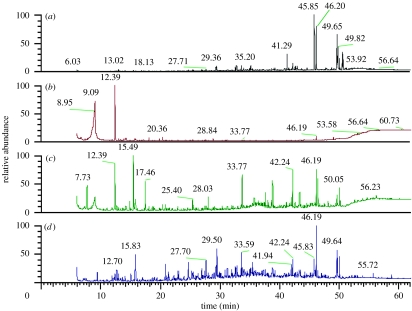
We used Pyr-GC/MS to follow changes in soil organic matter chemistry and to help determine the sources of C for the microbial community. These analyses suggested the presence of only 10 pyrolysis products in 1-year-old soils (figure 4b), despite modification of our methods to detect low C concentrations. By contrast, in the 4-year-old sites we found 17 compounds, all of which indicated a strong microbial signature (table 1), including pentadecanoic acid methyl ester. We also analysed several cultured cyanobacteria that had been detected in our clone libraries of the 4-year-old soil (figure 2a), and we found that pentadecanoic acid methyl ester was the second most abundant compound originating from these organisms.
Figure 4.
Mass spectra for a cyanobacterium ((a) Microcoleus vaginatus PCC 9802) and for soils of differing ages ((b) 1, (c) 4 and (d) 79 years old). Differences among spectra show the increasing diversity of compounds with time since deglaciation and how microbial succession influences the molecular characteristics of soils. Pentadecanoic acid (position 46.19/46.20) was found in both the cyanobacterial cultures and all of the soils tested.
Table 1.
Pyr-GC/MS products detected in soils along the chronosequence of the Puca Glacier. (Cyanobacterial products were determined by comparing the pyrolysis products of cyanobacterial isolates from arid soils (Nostoc punctiforme ATCC 29133 and Microcoleus vaginatus PCC 9802) and soils from the Puca Glacier chronosequence. Results highlight the progression of molecular chemical diversity and the in situ development of microbial communities followed by plant colonization along the gradient.)
| sample origin | number of compounds | microbial lipids (%) | plant bio-markers(%) | cyanobacterial products |
|---|---|---|---|---|
| 1 year | 10 | 14.3 | 0 | slight |
| 4 years | 17 | 91.8 | 0 | yes |
| 79 years | 41 | 62.4 | 5.5 | yes |
4. Discussion
The study of newly developing soils, such as those recently uncovered by glaciers, indicates that heterotrophic organisms play an active role in the biogeochemical cycles of young soils (e.g. Sigler & Zeyer 2004; Bardgett et al. 2007). Yet, in poorly developed mineral soils, levels of soil C increased or remained constant over the first 20 years of primary succession both in our previous studies (Nemergut et al. 2007) and in the study of Bardgett et al. (2007). Thus, although ancient soil C may help fuel the initial stages of succession in recently deglaciated soils, there must be significant inputs of C to keep soil C levels from declining over time. There is evidence to suggest that aeolian inputs of organic matter constitute one source of new C to high-latitude (Hodkinson et al. 2002) and high-altitude soils (Swan 1992), and other work suggests that cyanobacteria are important to C and N inputs very early in succession (reviewed by Hodkinson et al. 2003). Given the high aeolian pollen inputs to the Quelccaya ice cap near our sites (Reese & Liu 2003) and our preliminary evidence for cyanobacterial colonization of our sites, it is likely that both aeolian deposition and cyanobacterial activity contribute to C and N accumulation at our sites. In the present study, we focused on understanding cyanobacterial establishment and C and N cycling during the earliest stages of succession at a very remote, high-elevation and pristine site in the Andes of Southern Peru.
Our soil genomic DNA analyses demonstrated the presence of cyanobacterial sequences even in the youngest soils sampled and revealed a significant increase in the diversity of cyanobacteria during the first 4 years of succession following glacier retreat. The types of cyanobacteria detected along the chronosequence followed a pattern indicating a transition from ice-dwelling cyanobacteria (remnants of cryoconite communities in the surface of the glacier ice) to biological soil crust-like forms over the initial years of succession. For example, only three distinct cyanobacterial phylotypes in two major groups were present in the youngest soils and these were related to cyanobacteria from ice-bound microbial communities in the Dry Valleys of Antarctica (LB3 sequences from Priscu et al. 1998 in figure 2a). By contrast, after 4 years the soils harboured a diverse assemblage of cyanobacteria comprising 20 unique phylotypes in 13 major groups (figure 2a). Some of the phylotypes in the 4-year-old soils fell into previously unknown clades, but many were related to N-fixing species (e.g. Anabaena and Nostoc spp.; Vincent 2000) and to species typically found in desert soil crust communities (e.g. Microcoleus vaginatus; Garcia-Pichel et al. 2001).
In addition, an increase in the abundance of pigments involved in bacterial photosynthesis and photoprotection (Cockell & Knowland 1999) mirrored the increase in cyanobacterial diversity in the 4-year-old soils. Measured pigment concentrations were lower in these high-elevation soils than for other ecosystems (Bowker et al. 2002). Nevertheless, the increased abundance and diversity of cyanobacterial pigments provided corroborating evidence for the development of a diverse photosynthetic community during these earliest stages of succession. Work in Antarctica and the High Arctic has also suggested an important role for cyanobacteria in recently ice-covered barren soils (Wynn-Williams 1990; Kaštovská et al. 2005; Breen & Lévesque 2006), but our work is the first to document the rapid establishment of these versatile organisms in such high-elevation soils.
Our results also indicate that the early successional cyanobacterial community is supporting a broad array of important ecosystem processes. Research in deserts (de Caire et al. 1997), the High Arctic (reviewed in Hodkinson et al. 2003) and Antarctica (reviewed in Wynn-Williams 2000) has shown that cyanobacteria may increase soil stability. Many cyanobacteria produce exopolysaccharides that adhere to soil particles, thus enhancing soil aggregate stability and reducing soil losses from erosion (de Caire et al. 1997). We found that soil stability increased along the chronosequence, paralleling the increase in cyanobacterial diversity and pigment abundance: soil shear strength was nearly doubled in the oldest soil relative to the youngest (figure 2c). Thus, an important role of cyanobacteria (and possibly other microbes including diatoms) in primary succession in extreme environments may be to hold the soil in place. A decrease in soil loss via erosion would allow for the long-term pedogenesis of the extant soil, as well as for a facilitation of the succession of other organisms.
Furthermore, we observed large increases in rates of N fixation between the 1- and 4-year-old soils (figure 3a). Nitrogen-fixation rates in the 4-year-old soils were similar to values reported for well-developed cryptobiotic crusts in low-elevation ecosystems (Cleveland et al. 1999) and to rates in more well-developed, crust-dominated soils at our oldest site (79-year-old soils in figure 3a). Other studies have found biological soil crusts fixing N in young soils (Vitousek 1994; Bliss & Gold 1999), yet to our knowledge these are the first findings of soil N fixation occurring in such recently glaciated soils at high altitudes. These unexpectedly high N-fixation rates indicated the potential for significant microbial N inputs to these soils many years before the establishment of N-fixing lichens or plants. Indeed, total soil N pools increased from almost undetectable levels in the 0- and 1-year-old soils to significantly higher levels in older soils along the gradient (figure 3b). Taken together, these results suggest that N-fixing cyanobacteria (and possibly other unidentified taxa) are active in these soils only 4 years after glacier retreat, and that their activity may drive observed increases in soil N pools.
It is noteworthy that there was high microsite variation in rates of N fixation in both the 4- and 79-year-old soils (note error bars in figure 3a) resulting in a non-significant difference between these soil ages. Such microsite variation has been noted in other studies (e.g. Reed et al. 2007) and in the present case it is probably due to the heterogeneity of crust distribution at the 79-year-old site and the variability in cyanobacterial colonization in the 4-year-old soils. Nonetheless, there is the suggestion that rates of soil N fixation may peak early in this successional sequence, and this is reminiscent of the unimodal pattern of N fixation that occurs during primary plant succession, where N-fixing plants colonize newly deglaciated soil, soil N values increase and the N-fixing organisms are displaced, and N-fixation rates subsequently decline (Matthews 1992; Chapin et al. 1994). Although more data are needed to elucidate this pattern, it is interesting to consider that primary succession may actually sustain two distinct waves of N fixation: a first wave driven by microbial N demand and a second wave driven by plant N demand.
The activity of common microbial enzymes—such as protease, phosphatase and cellulase—increased continuously over the course of succession, reaching a maximum in the 79-year-old soil (figure 3b). The increase in the activity of these enzymes suggests that microbial populations are increasingly accessing organic matter as a source of C and nutrients. Our molecular, pigment and N-fixation rate data suggest that a significant portion of the organic matter may be provided by a diverse cyanobacterial community. The analysis of specific soil enzymes provided further insight into the changing sources of C for the developing microbial community along the soil chronosequence. In contrast to N-fixation rates and phosphatase activity, microbial enzymes involved in plant-derived organic matter decomposition were almost undetectable in both the 0- and 4-year-old soils (cellulase in figure 3b), but were very active in the 79-year-old soils (4.7±2.0 nmol g−1 h−1). These results were validated in a broader sampling of enzyme activities in widely separated plantless soils (n=21) near our site, where King et al. (2008) were also unable to detect cellulase activity. The lack of cellulase activity in the 4-year-old soils suggests that heterotrophic microbes in these soils may not be dependent on aeolian inputs of plant organic matter, but may be relying on microbially derived C sources.
To further elucidate possible relationships between cyanobacteria and C dynamics in these soils we used Pyr-GC/MS, a high-resolution means for identifying the molecular components of soil C compounds (Gleixner et al. 2002; Grandy & Neff 2008). These analyses suggested the presence of only 10 pyrolysis products in the 1-year-old soils, despite modification of our methods to detect low C concentrations (figure 4). By contrast, in the 4-year-old soils we found 17 compounds that indicated a strong microbial signature (table 1), including pentadecanoic acid methyl ester. The increase in complexity of the soil organic matter during the first 4 years after glacial retreat is suggestive of new inputs of organic compounds into these soils, and the fact that the C compounds found were associated with cyanobacterial biomass further suggested the possibility that inputs are dominated by cyanobacteria. For example, we assayed cultured representatives of cyanobacterial species abundant in the 4-year-old soils and found that pentadecanoic acid methyl ester was the second most abundant compound from these cultures (figure 4).
In addition, the 4-year-old soils showed no evidence of allochthonous plant material or any plant-breakdown biomarkers (e.g. furfural, furfural 5-methyl, furan 2,5-dimethyl, methylguaiacol or ethylguaiacol) that would indicate plant decomposition pathways (Grandy & Neff 2008). However, by 79 years the soils were qualitatively similar to a typical well-developed soil, containing plant-derived lignin biomarkers including methylguaiacol and ethylguaiacol. Thus, our enzyme and Pyr-GC/MS results suggest that allochthonous inputs of plant-derived substrates do not become detectable until later in ecosystem development, suggesting that cyanobacterial photosynthesis may be the dominant source of soil C during the earliest stages (0–4 years) of system development.
Overall, our results indicate that a phylogenetically diverse and functioning photosynthetic microbial community develops in the first 4 years following deglaciation at some of the highest elevation (5000 m.a.s.l.) receding glaciers on Earth. Previous work suggested that ecosystem energy and nutrient demands at such extreme sites would be met mainly via allochthonous (e.g. aeolian) inputs of organic matter (Swan 1992; Hodkinson et al. 2002) or ancient organic matter (Bardgett et al. 2007). While heterotrophic communities are active in these newly uncovered soils (and probably use some aeolian and ancient C), the pattern in Pyr-GC/MS products, N-fixation rates and soil N contents seen in the soils correspond with the development of a diverse cyanobacterial community. Thus, these results support a model of early ecosystem development for recently deglaciated soils in which cyanobacteria play a critical role in biogeochemical cycling. Yet the importance of these initial stages of primary succession in affecting subsequent plant establishment remains to be seen, and more work is needed to fully understand how microbial communities become established and are supported during the earliest stages of ecosystem development.
Acknowledgements
We thank J. Belnap for pigment analyses, F. Garcia-Pichel for cyanobacteria cultures and P. Sowell, A. Miller, J. Rosen, K. Doyle, K. Yager, S. Halloy, A. Seimon and T. Seimon for their field and logistical support. This work was supported by a grant from the NSF Microbial Observatories Program (MCB-0455606). Travel and fieldwork were supported by a grant from the National Geographic Society Committee for Research and Exploration.
References
- Bardgett R.D, et al. Heterotrophic microbial communities use ancient carbon following glacial retreat. Biol. Lett. 2007;3:487–490. doi: 10.1098/rsbl.2007.0242. doi:10.1098/rsbl.2007.0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T.P, Adam J.C, Lettenmaier D.P. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature. 2005;438:303–309. doi: 10.1038/nature04141. doi:10.1038/nature04141 [DOI] [PubMed] [Google Scholar]
- Barry R.G. The status of research on glaciers and global glacier recession: a review. Prog. Phys. Geogr. 2006;30:285–306. doi:10.1191/0309133306pp478ra [Google Scholar]
- Belnap J. Soil surface disturbances in cold deserts: effects on nitrogenase activity in cyanobacterial-lichen soil crusts. Biol. Fertil. Soils. 1996;23:362–367. doi:10.1007/BF00335908 [Google Scholar]
- Bliss L.C, Gold W.G. Vascular plant reproduction, establishment, and growth and the effects of cryptogamic crusts within a polar desert ecosystem, Devon Island, NWT, Canada. Can. J. Bot. 1999;77:623–636. doi:10.1139/cjb-77-5-623 [Google Scholar]
- Bormann B.T, Sidle R.C. Changes in productivity and distribution of nutrients in a chronosequence at Glacier Bay National Park, Alaska. J. Ecol. 1990;78:561–578. doi:10.2307/2260884 [Google Scholar]
- Bowker M.A, Reed S.C, Belnap J, Phillips S.L. Temporal variation in community composition, pigmentation, and Fv/Fm of desert cyanobacterial soil crusts. Microb. Ecol. 2002;43:13–25. doi: 10.1007/s00248-001-1013-9. doi:10.1007/s00248-001-1013-9 [DOI] [PubMed] [Google Scholar]
- Bradley R.S, Vuille M, Diaz H.F, Vergara W. Threats to water supplies in the tropical Andes. Science. 2006;312:1755–1756. doi: 10.1126/science.1128087. doi:10.1126/science.1128087 [DOI] [PubMed] [Google Scholar]
- Breen K.B, Lévesque E. Proglacial succession of biological soil crusts and vascular plants: biotic interactions in the High Arctic. Can. J. Bot. 2006;84:1714–1731. doi:10.1139/B06-131 [Google Scholar]
- Chapin F.S, Walker L.R, Fastie C.L, Sharman L.C. Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecol. Monogr. 1994;64:149–175. doi:10.2307/2937039 [Google Scholar]
- Cleveland C.C, et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochem. Cycles. 1999;13:623–645. doi:10.1029/1999GB900014 [Google Scholar]
- Cockell C.S, Knowland J. Ultraviolet radiation screening compounds. Biol. Rev. 1999;74:311–345. doi: 10.1017/s0006323199005356. doi:10.1017/S0006323199005356 [DOI] [PubMed] [Google Scholar]
- Crocker R.L, Major J. Soil development in relation to vegetation and surface age at Glacier Bay, Alaska. J. Ecol. 1955;43:427–448. doi:10.2307/2257005 [Google Scholar]
- de Caire G.Z, de Cano M.S, de Mulé M.C.Z, Palma R.M, Colombo K. Exopolysaccharide of Nostoc muscorum (Cyanobacteria) in the aggregation of soil particles. J. Appl. Phycol. 1997;9:249–253. doi:10.1023/A:1007994425799 [Google Scholar]
- Garcia-Pichel F, Lopez-Cortes A, Nubel U. Phylogenetic and morphological diversity of cyanobacteria in soil desert crusts from the Colorado plateau. Appl. Environ. Microbiol. 2001;67:1902–1910. doi: 10.1128/AEM.67.4.1902-1910.2001. doi:10.1128/AEM.67.4.1902-1910.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleixner G, Poirier N, Bol R, Balesdent J. Molecular dynamics of organic matter in a cultivated soil. Organic Geochem. 2002;33:357–366. doi:10.1016/S0146-6380(01)00166-8 [Google Scholar]
- Grandy A.S, Neff J.C. Molecular C dynamics down stream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci. Total Environ. 2008;404:297–307. doi: 10.1016/j.scitotenv.2007.11.013. doi:10.1016/j.scitotenv.2007.11.013 [DOI] [PubMed] [Google Scholar]
- Hardy R.W.F, Holsten R.D, Jackson E.K, Burns R.C. The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol. 1968;43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson I.D, Webb N.R, Coulson S.J. Primary community assembly on land—the missing stages: why are the heterotrophic organisms always first? J. Ecol. 2002;90:569–577. doi:10.1046/j.1365-2745.2002.00696.x [Google Scholar]
- Hodkinson I.D, Coulson S.D, Webb N.R. Community assembly along proglacial chronosequences in the high Arctic: vegetation and soil development in north-west Svalbard. J. Ecol. 2003;91:651–663. doi:10.1046/j.1365-2745.2003.00786.x [Google Scholar]
- Huelsenbeck J.P, Ronquist F, Nielsen R, Bollback J.P. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2001;294:2310–2314. doi: 10.1126/science.1065889. doi:10.1126/science.1065889 [DOI] [PubMed] [Google Scholar]
- Jumpponen A. Soil fungal community assembly in a primary successional glacier forefront ecosystem as inferred from rDNA sequence analyses. New Phytologist. 2003;158:569–578. doi: 10.1046/j.1469-8137.2003.00767.x. doi:10.1046/j.1469-8137.2003.00767.x [DOI] [PubMed] [Google Scholar]
- Karsten U, Maier J, Garcia-Pichel F. Seasonality in UV-absorbing compounds of cyanobacterial mat communities from an intertidal mangrove flat. Aquat. Microb. Ecol. 1998;16:37–44. doi:10.3354/ame016037 [Google Scholar]
- Kaštovská K, Elster J, Stibal M, Šantrůčková H. Microbial assemblages in soil microbial succession after glacial retreat in Svalbard (high Arctic) Microb. Ecol. 2005;50:396–407. doi: 10.1007/s00248-005-0246-4. doi:10.1007/s00248-005-0246-4 [DOI] [PubMed] [Google Scholar]
- Kawecka B, Olech M. Diatom communities in the Vanishing and Ornithologist Creek, King George Island, South Shetlands, Antarctica. Hydrobiologia. 1993;269/270:327–333. doi:10.1007/BF00028031 [Google Scholar]
- King A.J, Meyer A.F. High levels of microbial biomass and activity in unvegetated tropical and temperate alpine soils. Soil Biol. Biochem. 2008;40:2605–2610. doi:10.1016/j.soilbio.2008.06.026 [Google Scholar]
- Ley R.E, Williams M.W, Schmidt S.K. Microbial population dynamics in an extreme environment: controlling factors in talus soils at 3750 m in the Colorado Rocky Mountains. Biogeochemistry. 2004;68:313–335. doi:10.1023/B:BIOG.0000031032.58611.d0 [Google Scholar]
- Lipson D.A, Schmidt S.K, Monson R.K. Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology. 1999;80:1623–1631. doi:10.2307/176551 [Google Scholar]
- Mark B.G, Seltzer G.O. Evaluation of recent glacier recession in the Cordillera Blanca, Perú (AD 1962–1999): spatial distribution of mass loss and climate forcing. Quat. Sci. Rev. 2005;24:2265–2280. doi:10.1016/j.quascirev.2005.01.003 [Google Scholar]
- Martin A.P. Phylogenetic approaches for describing and comparing the diversity of microbial communities. Appl. Environ. Microbiol. 2002;68:3673–3682. doi: 10.1128/AEM.68.8.3673-3682.2002. doi:10.1128/AEM.68.8.3673-3682.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J.A. Cambridge University Press; Cambridge, UK: 1992. The ecology of recently-deglaciated terrain: a geoecological approach to Glacier Forelands and primary succession. [Google Scholar]
- Nemergut D.R, Anderson S.P, Cleveland C.C, Martin A.P, Miller A.E, Seimon A, Schmidt S.K. Microbial community succession in an unvegetated, recently-deglaciated soil. Microb. Ecol. 2007;53:110–122. doi: 10.1007/s00248-006-9144-7. doi:10.1007/s00248-006-9144-7 [DOI] [PubMed] [Google Scholar]
- Nicol G.W, Tscherko D, Embley T.M, Prosser J.I. Primary succession of soil Crenarchaeota across a receding glacier foreland. Environ. Microbiol. 2005;7:337–347. doi: 10.1111/j.1462-2920.2005.00698.x. doi:10.1111/j.1462-2920.2005.00698.x [DOI] [PubMed] [Google Scholar]
- Priscu J.C, Fristen C.H, Adams E.E, Giovannoni S.J, Paerl H.W, McKay C.P, Doran P.T, Gordan D.A, Lanoil B.D, Pinckney J.L. Perennial Antarctic lake ice: an oasis for life in a polar desert. Science. 1998;280:2095–2098. doi: 10.1126/science.280.5372.2095. doi:10.1126/science.280.5372.2095 [DOI] [PubMed] [Google Scholar]
- Reed S.C, Cleveland C.C, Townsend A.R. Controls over leaf litter and soil nitrogen fixation in two lowland tropical rain forests. Biotropica. 2007;39:585–592. doi:10.1111/j.1744-7429.2007.00310.x [Google Scholar]
- Reese C.A, Liu K.B. Pollen dispersal and deposition on the Quelccaya Ice Cap, Perú. Phys. Geogr. 2003;23:44–58. doi:10.2747/0272-3646.23.1.44 [Google Scholar]
- Reiners W.A, Worley I.A, Lawrence D.B. Plant diversity in a chronosequence at Glacier Bay, Alaska. Ecology. 1971;62:376–386. doi:10.2307/1934737 [Google Scholar]
- Robertson G.P, Wedin D, Groffman P.M, Blair J.M, Holland E.M, Nadelhoffer K.J, Harris D. Soil carbon and nitrogen availability: nitrogen mineralization and soil respiration potentials. In: Robertson G.P, Coleman D.C, Bledsoe C.S, Sollins P, editors. Standard methods of long-term ecological research. Oxford University Press; New York, NY: 1999. pp. 258–271. [Google Scholar]
- Schadt C.W, Martin A.P, Lipson D.A, Schmidt S.K. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science. 2003;301:1359–1361. doi: 10.1126/science.1086940. doi:10.1126/science.1086940 [DOI] [PubMed] [Google Scholar]
- Schmidt S.K, Sobieniak-Wiseman L.C, Kageyama S.A, Halloy S.R.P, Schadt C.W. Mycorrhizal and dark-septate fungi in plant roots above 4270 meters elevation in the Andes and Rocky Mountains. Arctic, Antarctic Alpine Res. 2008;40:576–583. [Google Scholar]
- Seimon T.A, Seimon A, Daszak P, Halloy S.R.P, Schoegel L.M, Aquilar C.A, Sowell P, Hyatt A.D, Konecky B, Simmons J.E. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to deglaciation. Global Change Biol. 2007;13:288–299. doi:10.1111/j.1365-2486.2006.01278.x [Google Scholar]
- Sigler W.V, Zeyer J. Colony-forming analysis of bacterial community succession in deglaciated soils indicates pioneer stress-tolerant opportunists. Microb. Ecol. 2004;48:316–323. doi: 10.1007/s00248-003-0189-6. doi:10.1007/s00248-003-0189-6 [DOI] [PubMed] [Google Scholar]
- Swan L.W. The aeolian biome, ecosystems of the earth's extremes. Bioscience. 1992;42:262–270. doi:10.2307/1311674 [Google Scholar]
- Tscherko D, Rustemeier J, Richter A, Wanek W, Kandeler E. Functional diversity of the soil microflora in primary succession across two glacier forelands in the Central Alps. Eur. J. Soil Sci. 2003;54:685–696. doi:10.1046/j.1351-0754.2003.0570.x [Google Scholar]
- Vincent W.F. Cyanobacterial dominance in the polar regions. In: Whitton B.A, Potts M, editors. The ecology of cyanobacteria. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. pp. 321–340. [Google Scholar]
- Vitousek P.M. Potential nitrogen fixation during primary scuccession in Hawai'i Volcanoes National Park. Biotropica. 1994;26:234–240. doi:10.2307/2388844 [Google Scholar]
- Walker L.R, del Moral R. Cambridge University Press; Cambridge UK: 2003. Primary succession and ecosystem rehabilitation. [Google Scholar]
- Weintraub M.N, Scott-Denton L.E, Schmidt S.K, Monson R.K. The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia. 2007;154:327–338. doi: 10.1007/s00442-007-0804-1. doi:10.1007/s00442-007-0804-1 [DOI] [PubMed] [Google Scholar]
- Wynn-Williams D.D. Ecological aspects of Antarctic microbiology. Adv. Microb. Ecol. 1990;11:71–146. [Google Scholar]
- Wynn-Williams D.D. Cyanobacteria in deserts—life at the limit? In: Whitton B.A, Potts M, editors. The ecology of cyanobacteria. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. pp. 341–366. [Google Scholar]
- Zimbone S.M, Vickers A, Morgan R.P.C, Vella P. Field investigations of different techniques for measuring surface soil shear strength. Soil Technol. 1996;9:101–111. doi:10.1016/0933-3630(96)00002-5 [Google Scholar]