Abstract
Recent studies have shown that some coral reef fish larvae return to natal reefs, while others disperse to distant reefs. However, the sensory mechanisms used to find settlement sites are poorly understood. One hypothesis is that larvae use olfactory cues to navigate home or find other suitable reef habitats. Here we show a strong association between the clownfish Amphiprion percula and coral reefs surrounding offshore islands in Papua New Guinea. Host anemones and A. percula are particularly abundant in shallow water beneath overhanging rainforest vegetation. A series of experiments were carried out using paired-choice flumes to evaluate the potential role of water-borne olfactory cues in finding islands. Recently settled A. percula exhibited strong preferences for: (i) water from reefs with islands over water from reefs without islands; (ii) water collected near islands over water collected offshore; and (iii) water treated with either anemones or leaves from rainforest vegetation. Laboratory reared-juveniles exhibited the same positive response to anemones and rainforest vegetation, suggesting that olfactory preferences are innate rather than learned. We hypothesize that A. percula use a suite of olfactory stimuli to locate vegetated islands, which may explain the high levels of self-recruitment on island reefs. This previously unrecognized link between coral reefs and island vegetation argues for the integrated management of these pristine tropical habitats.
Keywords: Amphiprion percula, chemical ecology, clownfish, coral reef ecology, dispersal, habitat selection
1. Introduction
The replenishment and persistence of animal populations are contingent upon dispersing individuals finding and becoming established in a suitable habitat (Rosenzweig 1981; Morris 2003). Habitat selection by dispersing offspring is critical for their future survival and reproduction, and may involve both innate and acquired preferences for their natal habitat (Davis & Stamps 2004; Mabry & Stamps 2008). The majority of marine organisms begin life as larvae that may disperse among isolated adult populations (Scheltema 1986; Caley et al. 1996; Pechenik 1999). The ability to find suitable habitat after a period in open water represents a significant challenge to marine larvae and the mechanisms by which this is achieved are poorly understood. Both passive larval dispersal and behavioural decisions based on a variety of sensory modalities and environmental cues have been implicated (Butman 1987; Pawlik 1992; Rodriguez et al. 1993), but their relative importance is uncertain.
Theory suggests that the persistence of coral reef fish populations requires not only significant connectivity among sub-populations, but also that some juveniles return to natal sub-populations (Armsworth 2002; Hastings & Botsford 2006). Recent empirical research has confirmed a high level of self-recruitment within and connectivity among isolated reef habitats (Jones et al. 1999, 2005; Swearer et al. 1999; Cowen et al. 2000, 2006; Almany et al. 2007; Patterson & Swearer 2007; Hamilton et al. 2008). However, the means by which coral reef fish larvae detect, orient towards and settle onto reefs, either home or away, remain a mystery. In addition, the roles of acquired or innate habitat preferences have seldom been investigated.
Recent advances in our understanding of coral reef fish larvae indicate well-developed sensory and swimming capabilities (Stobutzki & Bellwood 1997; Kingsford et al. 2002; Lecchini et al. 2005a,b; Leis 2006). Evidence suggests that both reef sounds (Leis et al. 2003; Simpson et al. 2005; Montgomery et al. 2006) and olfactory cues (Atema et al. 2002; Arvedlund & Takemura 2006; Døving et al. 2006; Gerlach et al. 2007) could be used as means to locate and orient towards home or a suitable habitat. For example, Gerlach et al. (2007) showed that settling larvae are not only capable of olfactory discrimination among reefs, but also prefer the water-born odours of their home reefs. However, the source of the chemical cues that larvae respond to has not been identified.
It is well known that coral reef fish larvae can use olfactory cues to identify suitable settlement sites, once they are in the vicinity of reef habitat (Sweatman 1988; Lecchini et al. 2005a, b, 2007; Gerlach et al. 2007). Settling larvae have been shown to respond to a variety of olfactory signals, including those that can be traced to resident conspecifics (Sweatman 1988; Booth 1992; Lecchini et al. 2005a, b; Døving et al. 2006), coral tissues (Lecchini et al. 2005a, b, 2007) or symbiotic partners such as anemones (Elliott et al. 1995; Arvedlund & Nielsen 1996; Arvedlund et al. 1999). In the case of anemones, the specific chemical cues that induce the symbiosis have been isolated (Murata et al. 1986). There is also some evidence that larvae can exhibit habitat selection without prior experience (Elliott et al. 1995; Arvedlund & Takemura 2006), while early natal experiences may also influence habitat choices at later developmental stages (Arvedlund & Nielsen 1996). However, in general, the chemical cues that larvae use for finding suitable habitat or returning home remain poorly understood.
Recent work in Papua New Guinea has shown that a high proportion of juvenile clownfish (Amphiprion spp.) recruit to their natal populations (Jones et al. 2005; Almany et al. 2007). Many of these populations appear to be associated with the coral reef habitat that surrounds vegetated islands (Jones et al. 2005; Almany et al. 2007). Some individuals also appear to successfully disperse tens of kilometres between reefs with islands. However, the mechanisms by which larvae discriminate between reefs with and without islands, or identify home islands have not been investigated.
The overall aim of this study was to investigate the role of olfactory cues potentially used by the orange clownfish (Amphiprion percula) larvae to locate island homes. First, we examined the extent to which this species is restricted to the fringing reefs surrounding islands in Kimbe Bay, Papua New Guinea. We then conducted a series of field experiments, using the two-channel choice flumes developed by Gerlach et al. (2007), to test the ability of settled juveniles to discriminate odours from water surrounding islands or treated with either anemones or rainforest leaves. We hypothesized that juveniles would prefer water from reefs with islands over water from reefs without islands; would prefer water from near islands over offshore water; and would positively respond to water containing chemical cues from anemones or island vegetation. Finally, to test whether natal experience influence habitat choices or whether larvae exhibit innate preferences, the ability of naive laboratory-reared larvae to respond to cues from anemones and vegetation was also investigated.
2. Material and methods
(a) Study location and species
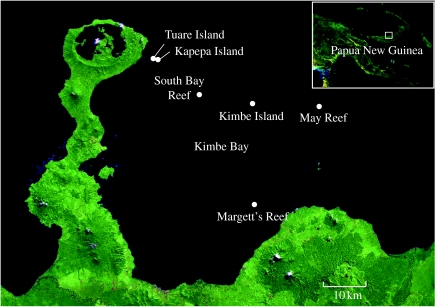
The study was carried out in Kimbe Bay (5°12.530 S, 150°22.801 E) on the island of New Britain, Papua New Guinea (figure 1). The focal clownfish species, A. percula, inhabits two host anemone species, Heteractus magnifica and Stichodactyla haddoni, in this region (Ollerton et al. 2007). Field observations and experiments on A. percula were conducted over two 10 day intervals (20 February–2 March, and 18–28 April 2007). The study encompassed six locations on the western side of Kimbe Bay, including three reefs with islands covered with tropical vegetation (Kapepa Island, Kimbe Island and Tuare Island) and three emergent reefs without islands (Margett's Reef, May Reef and South Bay Reef; figure 1). Supplementary laboratory experiments were conducted at the James Cook University Marine Aquarium Facility in Townsville, Australia.
Figure 1.
Satellite images showing the location of Kimbe Bay (New Britain, Papua New Guinea) and the six study locations within western Kimbe Bay. Three locations were fringing reefs surrounding small islands covered with rainforest vegetation (Kimbe Island, Kapepa Island, Tuare Island) and three were emergent reefs with no islands (Margett's Reef, May Reef, South Bay Reef).
(b) Association with island reefs
Amphiprion percula were counted in four randomly placed 50×4 m visual transects at each of the six study sites. The number of the host anemones of each species (H. magnifica and S. haddoni), and the number of A. percula individuals on each anemone were counted on each transect. All the transects on reefs with islands were laid out 5 m from and parallel to the shore line. On reefs with no islands, transects were laid out 5 m from the inner edge of the reef. A nested ANOVA was used to analyse the square root-transformed anemone transect data, to determine if there was a difference in the abundance of A. percula between reefs with and without islands, and among reefs nested within these categories.
A small boat was used to conduct visual transects to assess the abundance of floating leaf litter on two reefs with islands (Kimbe Island and Tuare Island) and without islands (May Reef and South Bay Reef). In addition, on the two reefs with islands, leaves were counted at four different distances from the shoreline (within 5 m of shoreline, reef crest, 500 m offshore and 1 km offshore). At each location and distance from shore, 10 replicate 100×4 m transects were counted by driving the boat in a straight line parallel to the shoreline and counting the number of floating leaves in a 4 m wide path. Distance offshore was maintained using a hand-held GPS unit.
A detailed map was made of the locations of the two anemone types at the primary study location (Kimbe Island) to determine their proximity to the island and their distribution among reef habitat types.
(c) General protocol for field olfactory choice tests
Field olfactory choice tests were conducted aboard a dive vessel moored near the study locations. To assess the ability of juvenile A. percula to actively choose between water potentially containing different odour stimuli, we used a two-channel choice flume (13 cm×4 cm) developed by Gerlach et al. (2007). This apparatus was designed to conduct pairwise choice experiments, with fish able to freely choose between water flowing from two different sources. The water from the two different sources is gravity fed from buckets and flows through tubes into the choice flume that is partitioned along half of its length. Fish are released at the downstream end where they are free to move to either side or swim towards the preferred water source. Using the protocols outlined in Gerlach et al. (2007), a constant gravity-driven flow of 100 ml min−1 per channel was maintained throughout all trials. The flow rates were measured using a flow metre and dye tests were conducted at each water change to ensure that the rate remained the same for the entire study. The dye test ensured that the two flow channels exhibited distinct and parallel water flow, with no turbulence or eddies.
Newly settled juvenile A. percula were collected from anemones using small hand nets and placed in individual 1 l plastic bags that were two-thirds filled with water. The fish were retained in the bag for a maximum of 24 hours before experiments were conducted, although the majority of the trials took place within 4 hours of collection. For each trial, a single fish was placed into the centre of the downstream end of the choice flume and given 5 min to acclimatize to the two water choices. During this period, the fish was able to swim throughout the chamber. At the end of the acclimation period, the position of the fish on each side of the chamber was recorded at 5 s intervals for a 2 min period. This was followed by a 1 min rest period, during which the water sources were switched, providing a control for potential side preferences that were not associated with the water source. Following the switch of water sources, the entire test, including the acclimation period, was repeated and the total time juveniles were associated with each water source was recorded. Each juvenile was subjected to a single trial, and a minimum of 20 and a maximum of 28 replicates were used for each test. The few fish that did not swim against the water flow during the acclimation period were removed from the study.
Water samples for all tests were stored in buckets for a maximum of 2 days; however, when possible, recruits were tested in water collected within 24 hours. Gerlach et al. (2007) showed in a previous experiment that the age of the water did not affect the choice behaviour. All water was stored in the same location and shaded to ensure similar temperatures throughout the experiment. Chi-squared tests were conducted to detect statistically significant water choices. For each set of experiments, a trial was scored as showing a preference for the water source containing the test stimulus if the fish spent more than 50 per cent of its time on that side of the chamber. A trial was scored as not showing a preference for the test stimulus in the fish that spent 50 per cent or less of its time on the side of the chamber with the water source containing the test stimulus. The total number of trials in which the fish showed a preference for the test stimulus compared with the total number of trials in which the fish did not show a preference were then compared using a Χ2-test and given the null expectation of a 1 : 1 distribution.
(d) Field experiment 1: olfactory discrimination between water samples from reefs with and without islands
Pairwise choice experiments were conduced to test whether juveniles could discriminate water from reefs surrounding islands from water collected at reefs without islands. Beach water (collected from 1 m off the shoreline) was used in the island trials. On non-island reefs, water was collected from the middle of the reef top at its shallowest point. Two separate comparisons were made among island and non-island locations: Kimbe Island versus May Reef (n=28) and Tuare Island versus South Bay Reef (n=22).
(e) Field experiment 2: olfactory discrimination between water samples from different distances from island reefs
Juveniles were given pairwise choices between water from three different positions associated with island reefs: (i) beach water (collected within 1 m off the shoreline), (ii) reef crest water (collected from near the outer edge of the reef crest), and (iii) offshore water (collected 1 km from the island, measured using a GPS). All the three combinations were tested for replicate juveniles collected from each of two islands: Kimbe Island (n=24) and Tuare Island (n=22).
(f) Field experiment 3: olfactory discrimination between water samples treated and not treated with either the anemone S. haddoni or leaves from rainforest trees that overhang water
Juveniles were given pairwise choices between untreated offshore water and offshore water treated with either anemones or rainforest leaves. The anemone trials were conducted to ensure that juveniles were making realistic choices and not just responding to novel chemical cues. Previous studies had shown a strong orientation towards anemones and an ability to detect anemone odours over short distances (e.g. Elliott et al. 1995). For these trials, a single whole anemone was added to the treatment water source.
Rainforest leaves were tested owing to the clear association between A. percula and islands, and the conspicuous presence of floating leaves and leaf litter near islands. The potential response to chemical signals from leaves was tested by adding broken leaves from five common shoreline trees native to Kimbe Island. For each trial, enough leaf matter was added to offshore water samples to cover the surface of the water bucket (9.6 l) and allowed to stand for 2 hours before each trial. Leaves from the five different species were tested both individually (n=20) as well as for a mixture of all the five species (n=24).
(g) Laboratory experiment: olfactory discrimination by naive laboratory reared larvae
To test whether the olfactory preferences observed for field caught settlers were acquired or innate, larvae reared in the laboratory without prior exposure to the stimuli were subjected to similar paired-choice experiments. Larvae were raised from a brood stock of 21 adult breeding pairs of A. percula maintained in 70 l tanks in a closed seawater system at the James Cook University Marine Aquarium Facility for 2 years prior to experimentation. To test whether naive juveniles could discriminate water samples treated with leaves, larvae from different clutches were raised in indoor rearing chambers for 11 days, at which stage the larvae exhibited positive attraction to the sides of the rearing chambers consistent with settlement behaviour. Artificial seawater (Red Sea brand) assumed to contain no biological cues was used for controls and to establish the treatments. At no stage were adults, embryos or larvae allowed to come into contact with terrestrial organic matter, prior to the experimental manipulations. In a manner similar to the field trials, reared larvae were given a choice between water sources treated and not treated with an anemone to test whether laboratory-reared juveniles could make realistic choices consistently with the well-known symbiotic association between A. percula and anemones. To prepare the treatment water, an anemone was placed in a water bucket (9.6 l) for 2 hours and then removed prior to sensory tests being conducted. The same anemone was used for all the trials.
To test responses to leaves, the water samples were treated with leaves from a common coastal rainforest tree (Xanthostemon chrysanthus: Myrtaceae). If olfactory preference for tropical trees is innate, we predicted that the naive larvae would be positively attracted to the steam of water that had been treated with leaves of Xanthostemon. To ensure that larvae were not simply attracted to stimuli from any organic source introduced into the test apparatus, we also tested the response of larvae to water samples treated with leaves from a swamp tree (Melaleuca nervosa: Myrtaceae). Melalueca leaves contain pungent oils that, we expected, would normally be avoided by the larvae. Each treatment source was treated with 20 g of broken leaves (approx. the same amount as used in the field trials). The leaves were cut into 1 cm squared pieces, soaked in 10 l of seawater for 2 hours, and then removed from the water before trials were conducted. A total of 36 and 30 A. percula larvae were tested for their responses to Xanthostemon- and Melaleuca-treated water, respectively. These larvae were offspring from five adult breeding pairs and seven different clutches from each pair.
3. Results
(a) Association with vegetated islands
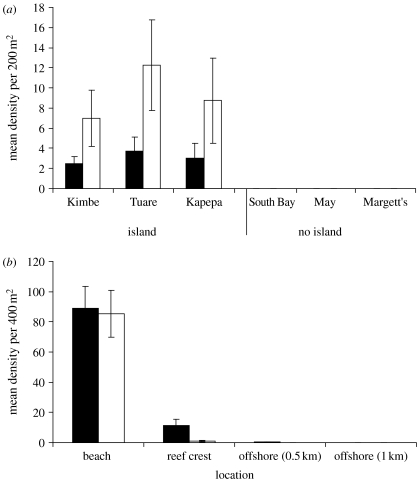
Amphiprion percula, and the anemones they occupy, exhibited a strong association with the fringing reefs surrounding the islands (figure 2a). Amphiprion percula were found at densities of approximately 6–12 per 200 m2 in transects close to islands. A nested ANOVA indicated significantly higher densities near islands (F1,4=27.3, p<0.001) and no significant variation among reefs with islands (F4,18=0.37, p=0.82). The anemones and A. percula were rarely observed on emergent reefs without islands (figure 2a).
Figure 2.
(a) Mean density per 200 m2 of A. percula (open bars) counted along four replicate 50×4 m visual transects at each of six different locations. Reefs are grouped into those with and without vegetated islands. Filled bars, anemones. (b) Mean density per 400 m2 of rainforest leaves at four different distances (beach, reef crest, 0.5 and 1 km) from the two islands (Kimbe Island (filled bar) and Tuare Island (open bar)). On two other reefs without islands (May Reef, South Bay Reef), no leaves were recorded. Error bars are standard errors.
Floating leaves were commonly observed in the beach zone and on reef flats near islands (figure 2b) and were completely absent from reefs without islands. On reefs with islands, the densities of floating leaves declined with distance offshore, and no leaves were observed in the random transects 1 km offshore.
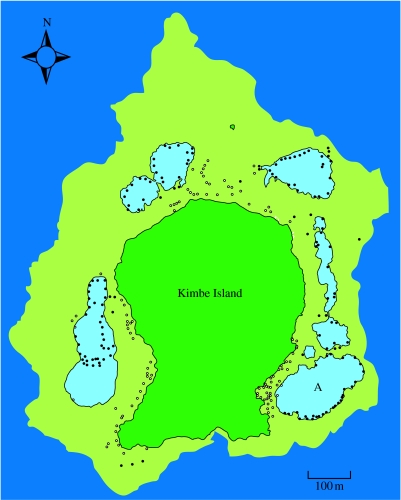
On the island reefs, the two anemones occupied by A. percula are not randomly distributed with respect to the position of the island. At Kimbe Island, S. haddoni was almost always located within 10 m off the shoreline (figure 3), where it was commonly observed beneath overhanging vegetation and in close proximity to leaf litter. The other anemone H. magnifica is more commonly associated with the perimeter of small lagoons, usually 10–30 m from the shoreline, but not on the substantial areas of reef flat or slope further offshore (figure 3).
Figure 3.
Map of Kimbe Island drawn from an IKONOS-2 satellite image with a resolution of 1 m (see Almany et al. 2007). The locations of all the anemones of H. magnifica (filled circles) and S. haddoni (open circles) occupied by the groups of A. percula are marked.
(b) Field experiment 1: olfactory discrimination between water samples from reefs with and without islands
Juvenile A. percula exhibited a strong preference for water samples taken from reefs with islands (table 1a). Juveniles spent over 99 per cent of their time in the choice flume on the side of island water samples and less than 1 per cent of their time associated with water from reefs with no islands. Both the choice of Tuare Island water over South Bay Reef water and that of Kimbe Island water over May Reef were statistically significant (table 1a).
Table 1.
Results of pairwise olfactory choice experiments on field-collected juvenile A. percula, including the choices made between (a) water from reefs with and without islands, (b) water from different distances away from islands and (c) water with and without anemones and rainforest leaves. In addition, (d) shows the pairwise trials for laboratory-reared juveniles and their choices between water with and without anemones and leaves. Data are mean percentage of time spent in water flowing from the two sources ±s.e. Statistic tests are Χ2-tests on the number of trials where larvae exhibited a preference for one water source in the test chamber. n, sample size; p, probability of the data given the null hypothesis that there is no choice.
| pairwise test | choice 1, mean % time±s.e. | choice 2, mean % time±s.e. | Χ2 | n | p | ||
|---|---|---|---|---|---|---|---|
| (a) field experiment 1: reefs with and without islands | Tuare Is. | 99.6±0.04 | South Bay Reef | 0.4±0.04 | 28.0 | 28 | <0.001 |
| Kimbe Is. | 99.5±0.1 | May Reef | 0.5±0.1 | 22.0 | 22 | <0.001 | |
| (b) field experiment 2: distance from island | Tuare Is. beach | 98.0±0.1 | Tuare Is. offshore | 2.0±0.1 | 22.0 | 22 | <0.001 |
| Tuare Is. beach | 95.0±0.3 | Tuare Is. crest | 5.0±0.3 | 22.0 | 22 | <0.001 | |
| Tuare Is. crest | 55.0±0.6 | Tuare Is. offshore | 45.0±0.6 | 0.17 | 22 | 0.683 | |
| Kimbe Is. beach | 93.0±0.4 | Kimbe Is. offshore | 7.0±0.4 | 24.0 | 24 | <0.001 | |
| Kimbe Is. beach | 97.2±0.8 | Kimbe Is. crest | 7.3±0.6 | 20.2 | 24 | <0.001 | |
| Kimbe Is. crest | 57.0±0.8 | Kimbe Is. offshore | 43.0±0.8 | 0.0 | 24 | 1 | |
| (c) field experiment 3: response to anemones and leaves | anemone | 91.0±0.7 | no anemone | 9.0±0.7 | 18.2 | 22 | <0.001 |
| mixed leaves | 89.5±0.7 | no leaves | 10.5±0.7 | 20.2 | 24 | <0.005 | |
| leaves sp. 1 | 90.0±0.6 | no leaves | 10.0±0.7 | 20.0 | 20 | <0.001 | |
| leaves sp. 2 | 92.0±0.4 | no leaves | 8.0±0.4 | 20.0 | 20 | <0.001 | |
| leaves sp. 3 | 90.0±0.7 | no leaves | 10.0±0.7 | 16.2 | 20 | <0.001 | |
| leaves sp. 4 | 92.0±0.6 | no leaves | 8.0±0.6 | 20.0 | 20 | <0.001 | |
| leaves sp. 5 | 94.0±0.4 | no leaves | 6.0±0.4 | 20.0 | 20 | <0.001 | |
| (d) laboratory experiment: response of naive larvae to anemones and leaves | anemone | 98.0±0.2 | no anemone | 2.0±0.2 | 24.0 | 24 | <0.001 |
| rainforest leaves | 96.0±0.2 | no leaves | 4.0±0.2 | 36.0 | 36 | <0.001 | |
| Melaleuca leaves | 0±0 | no leaves | 100±0 | 30.0 | 30 | <0.001 | |
(c) Field experiment 2: olfactory discrimination between water samples from different distances from island reefs
For the two island reefs investigated, juvenile A. percula consistently showed a strong preference for beach water, regardless of whether the choice was between beach water and reef crest water, or between beach water and offshore water (table 1b). The average time in association with beach water was always greater than 90 per cent, and in all the cases, the preference for beach water was statistically significant. The juveniles exhibited no significant discrimination between offshore and reef crest water samples, spending close to 50 per cent of their time in the side of the flow channel of each water source.
(d) Field experiment 3: olfactory discrimination between water samples treated and not treated with either the anemone S. haddoni or leaves from rainforest trees overhanging water
A strong preference was always exhibited for offshore water sources treated with the anemone S. haddoni (table 1c). When given a choice between treated and untreated water, juveniles spent over 90 per cent of their time associated with the anemone-treated water.
The strong field preference for water treated with rainforest leaves was also apparent (table 1c). Given the choice between offshore water treated with rainforest leaves and untreated offshore water, the juveniles spent between 90 and 95 per cent of their time in the treated water flume. The choice was highly significant for each of the five rainforest species tested and the mixed leaf treatment (table 1c).
(e) Laboratory experiment: olfactory discrimination by naive laboratory-reared larvae
Laboratory-reared larvae were strongly attracted to chemical cues from anemones, indicating that 11-day-old larvae that have not settled on reefs are capable of olfactory responses in the water flow chamber. These larvae had no developmental experience with water treated with vegetation. However, when given a choice between artificial seawater and the same water treated with leaves from a rainforest plant, they spent on average 96 per cent of their time in the flow channel receiving leaf-treated water. The preference for rainforest-leaf-treated water was statistically significant and numerically as strong as the attraction to anemone water (table 1c). All the larvae tested exhibited 100 per cent avoidance for water-treated olfactory cues from the plant M. nervosa, confirming that they were not attracted to inappropriate stimuli in the test chamber.
4. Discussion
The clownfish A. percula clearly has a close association with coral reefs surrounding vegetated islands. Both clownfish and host anemone numbers are high on island reefs and sparse on other emergent reefs. Within island reef systems, numbers are the greatest immediately adjacent to the islands themselves, where they are often found beneath overhanging vegetation. Given the strong association between host anemones and island reefs, A. percula larvae can clearly maximize their chances of finding a suitable settlement site by being able to locate and orient towards islands. The islands themselves are a potential source of many olfactory water-borne cues that would not be emanating from reefs without islands. Elevated levels of organic material from the lush tropical rainforest vegetation could clearly extend some distance from the islands. The experimental data presented here strongly suggest that A. percula has an innate olfactory attraction to rainforest vegetation, and once detected, could use this stimulus to find suitable habitat.
One of the striking features of our results is the strength of the preferences, with the juveniles spending in excess of 90 per cent of their time in the flow channel of their choice. They exhibited a strong preference for water coming from reefs surrounding vegetated islands, compared with that from reefs without islands. They exhibited a clear preference for beach water in the flow chamber, when given a choice between this and reef crest or offshore water options. Both the naive and field-experienced juveniles exhibited equally strong preferences for both anemones and rainforest leaves. Furthermore, the naive larvae demonstrated an ability to distinguish between appropriate and inappropriate cues, by avoiding water treated with leaves from a swamp-dwelling tree that contains pungent oils. Given the potential difficulty of finding coral reef habitat after a pelagic larval phase, it is likely that A. percula use a suite of sensory cues to find island reefs and preferred reefs and settlement sites. Our results indicate that leaves from terrestrial plants are one of the cues that guide larvae towards suitable settlement sites.
The high densities of anemones and A. percula on island reefs are almost certainly part of the sensory cocktail used to find a place to settle. Reef fish are clearly capable of responding to chemical signals from anemones (Elliott et al. 1995; Arvedlund & Nielsen 1996; Arvedlund et al. 1999) and conspecifics (Sweatman 1988; Booth 1992; Lecchini et al. 2005b). However, olfactory cues from these sources may be the most important once larvae are in close proximity to reefs. It is likely that the terrestrial signals can be detected at greater distances, given that leaves and organic debris clearly float considerable distances from reefs. These cues could greatly increase the ‘island mass’ effect (sensu Gilmartin & Revelante 1974), essentially making islands a bigger target for larvae searching for suitable habitat. Although the juveniles could not distinguish between offshore and reef crest water in our study, we have noted large amounts of organic debris in island wakes. Elevated ichthyoplankton densities in island wakes and oceanographic features associated with islands are well known (e.g. Leis 1986; Wolanski & Hamner 1988; Heywood et al. 1990; Boehlert et al. 1992). Amphiprion percula larvae that enter island wakes carrying leaves would have a clear olfactory signal that indicates the proximity of an island.
The high levels of natal or self-recruitment on island reefs raise further questions about how larvae find island homes (Jones et al. 1999, 2005; Almany et al. 2007). For example, Almany et al. (2007) have shown that after a planktonic period of 10–12 days, up to 60 per cent of the juvenile A. percula settling at Kimbe Island were the offspring of resident adults. How these juveniles found their way back to the island is unknown, but their ability to respond to olfactory stimuli from islands is clearly implicated. It is possible that larvae use terrestrial cues to remain in sensory contact with home islands in order to find a suitable habitat. Given that juvenile fishes can potentially discriminate home reefs from others (Gerlach et al. 2007), it is also possible that A. percula larvae are homing to their natal island in response to specific vegetation cues. While our experiments suggest that larvae have an innate ability to respond to rainforest vegetation, they may also be capable of imprinting on chemical signals experienced during the embryonic stage (Arvedlund & Nielsen 1996). Alternatively, a general orientation towards vegetated islands, combined with the sparse distribution of reefs surrounding islands in the study site area, could lead to a high probability of natal recruitment, even in the absence of a strict homing mechanism. Further work is required to distinguish among these competing hypotheses.
The study of Almany et al. (2007) also suggests that a significant proportion of juveniles settling at Kimbe Island travel considerable distances, in excess of 20 km among reefs. In Kimbe Bay, island reefs tend to be small and account for less than 1 per cent of the marine habitat (see figure 2 and Beger et al. 2003). Given that fish larvae possess limited physical ability and sensory mechanisms early in development (Egner & Mann 2005; Hogan & Mora 2005), it is likely that they are initially transported away from islands by prevailing currents. However, successful larval migration between such small island targets argues for remarkable sensory and swimming abilities at later developmental stages.
The specific chemical cues that A. percula respond to and the distance over which they can be detected require further investigation. The larvae of many benthic marine organisms either select or avoid chemical signals from marine plants when undergoing metamorphosis or settlement (Pawlik 1992; Forward et al. 2001; Steinberg et al. 2002). Tannins and related compounds produced by marine algae and seagrasses have been implicated (Arnold & Targett 2002). However, there are no other published examples of marine organisms responding to chemical stimuli from terrestrial plants, and the chemical cues themselves are unknown.
The results of our study have important implications for the management of island reef ecosystems. The iconic clownfish A. percula (aka Nemo) provides clear example of a strong link between coral reef and rainforest habitats. Offshore islands are often targets of marine protected area status, because they harbour a diversity of habitats and species, and are the focal point of human activities (Fernandes et al. 2005). Calls for the integrated management of terrestrial and marine habitats (Allison et al. 1998; Jameson et al. 2002; Aronson & Precht 2006) are vital in island environments. Deforestation and development of offshore islands could have an obvious detrimental effect on the ability of these clownfish to find suitable habitat and therefore persist on tropical island reefs. Islands in remote oceanic environments are often associated with increased numbers of endemic species (e.g. Jones et al. 2002; Allen 2007). Mechanisms for homing are likely to be even more important for the persistence of such species.
In conclusion, we hypothesize that A. percula larvae can use olfactory cues from rainforest vegetation for finding island reefs. There are many further questions to be answered including what the chemical cues are, how far from reefs they can be detected and at what developmental stage larvae can respond to them. However, our results demonstrate an important and previously unrecognized link between coral reefs and tropical forests, which may be significant for a variety of island-dwelling reef fishes. The increasing knowledge of how larvae navigate to suitable adult habitats contributes directly to management actions that aim to sustain viable marine populations. Any factors that disrupt this process will have immediate and devastating effects on the ecology of marine organisms.
Acknowledgments
The research accomplished in this project was conducted under ethics approval number A1264 and followed all guidelines for the country in which it took place.
Special thanks are due to Alan Raabe, skipper of MV Febrina. Our ideas germinated from his observation that orange clownfish need to ‘see trees’. We also thank the following people: Michael Berumen, Jen Hodge, Alexandra Sophie Roy, Vanessa Messmer, Kathryn Markey and Jennifer Donelson for assistance in the field and laboratory; Jella Atema, Gabrielle Gerlach and Michael Kingsford for the loan of the two-channel choice flume; Laura Castell and Deborah Dixson for critically reading an early draft of the manuscript and the crew of Febrina, the staff of Mahonia Na Dari Research and Conservation Centre and the staff of Walindi Plantation Resort for logistic support. This work was funded by an ARC Centre of Excellence grant to G.P.J.
References
- Allen G.R. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007;18:541–556. doi:10.1002/aqc.880 [Google Scholar]
- Allison G.W, Lubchenco J, Carr M.H. Marine reserves are necessary but not sufficient for marine conservation. Ecol. Appl. 1998;8:S79–S92. [Google Scholar]
- Almany G.R, Berumen M.L, Thorrold S.R, Planes S, Jones G.P. Local replenishment of coral reef fish population in a marine reserve. Science. 2007;316:742–744. doi: 10.1126/science.1140597. doi:10.1126/science.1140597 [DOI] [PubMed] [Google Scholar]
- Armsworth P.R. Recruitment limitation, population regulation, and larval connectivity in reef–fish metapopulations. Ecology. 2002;83:1092–1104. [Google Scholar]
- Arnold T.M, Targett N.M. Marine tannins: the importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 2002;28:1919–1934. doi: 10.1023/a:1020737609151. doi:10.1023/A:1020737609151 [DOI] [PubMed] [Google Scholar]
- Aronson R.B, Precht W.F. Conservation, precaution, and Caribbean reefs. Coral Reefs. 2006;25:441–450. doi:10.1007/s00338-006-0122-9 [Google Scholar]
- Arvedlund M, Nielsen L.E. Do the anemonefish Amphiprion ocellaris (Pisces: Pomacentridae) imprint themselves to their host sea anemone Heteractis magnifica (Anthozoa: Actinidae)? Ethology. 1996;102:197–211. [Google Scholar]
- Arvedlund M, Takemura A. The importance of chemical environmental cues for juvenile Lethrinus nebulosus Forsskaal (Lethrinidae, Teleostei) when settling into their first benthic habitat. J. Exp. Mar. Biol. Ecol. 2006;338:112–122. doi:10.1016/j.jembe.2006.07.001 [Google Scholar]
- Arvedlund M, McCormick M.I, Fautin D.G, Bildsoe M. Host recognition and possible imprinting in the anemonefish Amphiprion melanopus (Pisces: Pomacentridae) Mar. Ecol. Prog. Ser. 1999;188:207–218. doi:10.1007/s00338-0060122-9 [Google Scholar]
- Atema J, Kingsford M.J, Gerlach G. Larval reef fish could use odour for detection, retention and orientation to reefs. Mar. Ecol. Prog. Ser. 2002;241:151–160. doi:10.3354/meps241151 [Google Scholar]
- Beger M, Jones G.P, Munday P.L. Conservation of coral reef biodiversity: a comparison of reserve selection procedures for corals and fishes. Biol. Conserv. 2003;111:53–62. doi:10.1016/S0006-3207(02)00249-5 [Google Scholar]
- Boehlert G.W, Watson W, Sun L.C. Horizontal and vertical distributions of larval fishes around an isolated oceanic island in the tropical Pacific. Deep-Sea Res. 1992;39:439–466. doi:10.1016/0198-0149(92)90082-5 [Google Scholar]
- Booth D.J. Larval settlement patterns and preferences by domino damselfish, Dascyllus albisella Gill. J. Exp. Mar. Biol. Ecol. 1992;155:85–104. doi:10.1016/00220981(92)90029-A [Google Scholar]
- Butman C.A. Larval settlement of soft-sediment invertebrates: the spatial scales of pattern explained by active habitat selection and the emerging role of hydrodynamical processes. Oceanogr. Mar. Biol. Annu. Rev. 1987;25:113–165. [Google Scholar]
- Caley M.J, Carr M.H, Hixon M.A, Hughes T.P, Jones G.P, Menge B.A. Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 1996;27:477–500. doi:10.1146/annurev.ecolsys.27.1.477 [Google Scholar]
- Cowen R.K, Lwiza K.M.M, Sponaugle S, Paris C.B, Olson D.B. Connectivity of marine populations: open or closed? Science. 2000;287:857–859. doi: 10.1126/science.287.5454.857. doi:10.1126/science.287.5454.857 [DOI] [PubMed] [Google Scholar]
- Cowen R.K, Paris C.B, Srinivasan A. Scaling of connectivity in marine populations. Science. 2006;311:522–527. doi: 10.1126/science.1122039. doi:10.1126/science.1122039 [DOI] [PubMed] [Google Scholar]
- Davis J.M, Stamps J.A. The effect of natal experience on habitat preferences. Trends Ecol. Evol. 2004;19:411–416. doi: 10.1016/j.tree.2004.04.006. doi:10.1016/j.tree.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Døving K.B, Stabell O.B, Östlund-Nilsson S, Fisher R. Fidelity and homing in tropical coral reef cardinalfish: are they using olfactory cues? Chem. Senses. 2006;31:265–272. doi: 10.1093/chemse/bjj028. doi:10.1093/chemse/bjj028 [DOI] [PubMed] [Google Scholar]
- Egner S.A, Mann D.A. Auditory sensitivity of sergeant major damselfish Abudefduf saxatilis from post settlement juvenile to adult. Mar. Ecol. Prog. Ser. 2005;285:213–222. doi:10.3354/meps285213 [Google Scholar]
- Elliott J.K, Elliott J.M, Mariscal R.N. Host selection, location and association behaviours of anemonefishes in field settlement experiments. Mar. Biol. 1995;122:377–389. doi:10.1007/BF00350870 [Google Scholar]
- Fernandes L, et al. Establishing representative no-take areas in the Great Barrier Reef: large-scale implementation of theory on marine protected areas. Conserv. Biol. 2005;19:1733–1744. doi:10.1111/j.1523-1739.2005.00302.x [Google Scholar]
- Forward R.B, Tankersley R.A, Rittschof D. Cues for metamorphosis of brachyuran crabs: an overview. Am. Zool. 2001;41:1108–1122. doi:10.1668/0003-1569(2001)041[1108:CFMOBC]2.0.CO;2 [Google Scholar]
- Gerlach G, Atema J, Kingsford M.J, Black K.P, Miller-Sims V. Smelling home can prevent dispersal of reef fish larvae. Proc. Natl Acad. Sci. USA. 2007;104:858–863. doi: 10.1073/pnas.0606777104. doi:10.1073/pnas.0606777104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin M, Revelante N. The island mass effect on the phytoplankton and primary production of the Hawaiian Islands. J. Exp. Mar. Biol. Ecol. 1974;16:181–204. doi:10.1016/0022-0981(74)90019-7 [Google Scholar]
- Hamilton S.L, Regetz J, Warner R.R. Postsettlement survival linked to larval life in a marine fish. Proc. Natl Acad. Sci. USA. 2008;105:1561–1566. doi: 10.1073/pnas.0707676105. doi:10.1073/pnas.0707676105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings A, Botsford L.J. Persistence of spatial populations depends on returning home. Proc. Natl Acad. Sci. USA. 2006;103:6067–6072. doi: 10.1073/pnas.0506651103. doi:10.1073/pnas.0506651103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood K.J, Barton E.D, Simpson J.H. The effects of flow disturbance by an oceanic island. J. Mar. Res. 1990;48:55–73. doi:10.1357/002224090784984623 [Google Scholar]
- Hogan J.D, Mora C. Experimental analysis of the contribution of swimming and drifting to the displacement of reef fish larvae. Mar. Biol. 2005;147:1213–1220. doi:10.1007/s00227-005-0006-5 [Google Scholar]
- Jameson S.C, Tupper M.H, Ridley J.M. The three screen doors: can marine “protected” areas be effective? Mar. Pollut. Bull. 2002;44:1177–1183. doi: 10.1016/s0025-326x(02)00258-8. doi:10.1016/S0025-326X(02)00258-8 [DOI] [PubMed] [Google Scholar]
- Jones G.P, Milicich M.J, Emslie M.J, Lunow C. Self-recruitment in a coral reef fish population. Nature. 1999;402:802–804. doi:10.1038/45538 [Google Scholar]
- Jones G.P, Caley M.J, Munday P.L. Rarity in coral reef fish communities. In: Sale P.F, editor. Coral reef fishes. Dynamics and diversity in a complex ecosystem. Academic Press; San Diego, CA: 2002. pp. 81–101. [Google Scholar]
- Jones G.P, Planes S, Thorrold S.R. Coral reef fish larvae settle close to home. Curr. Biol. 2005;15:1314–1318. doi: 10.1016/j.cub.2005.06.061. doi:10.1016/j.cub.2005.06.061 [DOI] [PubMed] [Google Scholar]
- Kingsford M.J, Leis J.M, Shanks A, Lindeman K, Morgan S, Pineda J. Sensory environments, larval abilities and local self recruitment. Bull. Mar. Sci. 2002;70:309–340. [Google Scholar]
- Lecchini D, Planes S, Galzin R. Experimental assessment of sensory modalities of coral-reef fish larvae in the recognition of their settlement habitat. Behav. Ecol. Sociobiol. 2005a;58:18–26. doi:10.1007/s00265-004-0905-3 [Google Scholar]
- Lecchini D, Shima J, Banaigs B, Galzin R. Larval sensory abilities and mechanisms of habitat selection of a coral reef fish during settlement. Oecologia. 2005b;143:326–334. doi: 10.1007/s00442-004-1805-y. doi:10.1007/s00442-004-1805-y [DOI] [PubMed] [Google Scholar]
- Lecchini D, Osenberg C.W, Shima J.S, St Mary C.M, Galzin R. Ontogenetic changes in habitat selection during settlement in a coral reef fish: ecological determinants and sensory mechanisms. Coral Reefs. 2007;26:423–432. doi:10.1007/s00338-007-0212-3 [Google Scholar]
- Leis J.M. Vertical and horizontal distributions of fish larvae near coral reefs at Lizard Island, Great Barrier Reef. Mar. Biol. 1986;90:505–516. doi:10.1007/BF00409271 [Google Scholar]
- Leis J.M. Are larvae of demersal fishes plankton or nekton? Adv. Mar. Biol. 2006;51:57–141. doi: 10.1016/S0065-2881(06)51002-8. doi:10.1016/S0065-2881(06)51002-8 [DOI] [PubMed] [Google Scholar]
- Leis J.M, Carson-Ewart B.M, Hay A.C, Cato D.H. Coral reef sounds enable nocturnal navigation by some reef fish larvae in some places at some times. J. Fish Biol. 2003;63:724–737. doi:10.1046/j.1095-8649.2003.00182.x [Google Scholar]
- Mabry K.E, Stamps J.A. Dispersing brush mice prefer habitat like home. Proc. R. Soc. B. 2008;275:543–548. doi: 10.1098/rspb.2007.1541. doi:10.1098/rspb.2007.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J.C, Jeffs A, Simpson S.D, Meekan M, Tindle C. Sounds as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv. Mar. Biol. 2006;51:143–196. doi: 10.1016/S0065-2881(06)51003-X. doi:10.1016/S0065-2881(06)51003-X [DOI] [PubMed] [Google Scholar]
- Morris D.W. Toward an ecological synthesis: a case for habitat selection. Oecologia. 2003;136:1–13. doi: 10.1007/s00442-003-1241-4. doi:10.1007/s00442-003-1241-4 [DOI] [PubMed] [Google Scholar]
- Murata M, Miyagawa-Kohshima K, Nakanishi K, Naya Y. Characterization of compounds that induce symbiosis between sea anemone and anemonefish. Science. 1986;234:585–587. doi: 10.1126/science.234.4776.585. doi:10.1126/science.234.4776.585 [DOI] [PubMed] [Google Scholar]
- Ollerton J, McCollin D, Fauntin D.G, Allen G.R. Finding NEMO: nestedness endangered by mutualistic organization in anemonefish and their hosts. Proc. R. Soc. B. 2007;274:591–598. doi: 10.1098/rspb.2006.3758. doi:10.1098/rspb.2006.3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson H.M, Swearer S.E. Long-distance dispersal and local retention of larvae as mechanisms of recruitment in an island population of a coral reef fish. Austral Ecol. 2007;32:122–130. doi:10.1111/j.1442-9993.2006.01669.x [Google Scholar]
- Pawlik J.R. Chemical ecology of the settlement of benthic marine-invertebrates. Oceangr. Mar. Biol. Annu. Rev. 1992;30:273–335. [Google Scholar]
- Pechenik J.A. On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar. Ecol. Prog. Ser. 1999;177:269–297. doi:10.3354/meps177269 [Google Scholar]
- Rodriguez S.R, Ojeda F.P, Inestrosa N.C. Settlement of benthic marine-invertebrates. Mar. Ecol. Prog. Ser. 1993;97:193–207. doi:10.3354/meps097193 [Google Scholar]
- Rosenzweig M.L. A theory of habitat selection. Ecology. 1981;62:327–335. doi:10.2307/1936707 [Google Scholar]
- Scheltema R.S. On dispersal and planktonic larvae of benthic invertebrates: an eclectic overview and summary of problems. Bull. Mar. Sci. 1986;39:290–322. [Google Scholar]
- Simpson S.D, Meekan M, Montgomery J, McCauley R, Jeffs A. Homeward sound. Science. 2005;308:221. doi: 10.1126/science.1107406. doi:10.1126/science.1107406 [DOI] [PubMed] [Google Scholar]
- Steinberg P.D, De Nys R, Kjelleberg S. Chemical cues for surface colonization. J. Chem. Ecol. 2002;28:1935–1951. doi: 10.1023/a:1020789625989. doi:10.1023/A:1020789625989 [DOI] [PubMed] [Google Scholar]
- Stobutzki I.C, Bellwood D.R. Sustained swimming abilities of the late pelagic stages of coral reef fishes. Mar. Ecol. Prog. Ser. 1997;149:35–41. doi:10.3354/meps149035 [Google Scholar]
- Swearer S.E, Caselle J.E, Lea D.W, Warner R.R. Larval retention and recruitment in an island population of a coral-reef fish. Nature. 1999;402:799–802. doi:10.1038/45533 [Google Scholar]
- Sweatman H.P.A. Field evidence that settling coral reef fish larvae detect resident fishes using dissolved chemical cues. J. Exp. Mar. Biol. Ecol. 1988;124:163–174. doi:10.1016/0022-0981(88)90170-0 [Google Scholar]
- Wolanski E, Hamner W.M. Topographical controlled fronts in the ocean and their biological influences. Science. 1988;241:177–181. doi: 10.1126/science.241.4862.177. doi:10.1126/science.241.4862.177 [DOI] [PubMed] [Google Scholar]