Abstract
The bulbocavernosus (BC) and levator ani (LA) muscles are present in males but absent or severely reduced in females, and the fate of these muscles controls the survival of motoneurons in the sexually dimorphic spinal nucleus of the bulbocavernosus. However, the mechanism underlying the sex difference in BC and LA development has been controversial. We examined the role of cell death in sexual differentiation of the bulbocavernosus BC/LA muscles in mice. Muscle development was mapped from embryonic day 16 (E16) to postnatal day 5 (P5). A sex difference (male > female) first arose on E17 (BC) or E18 (LA), and increased in magnitude postnatally. TUNEL labeling revealed dying cells in the BC and LA muscles of both sexes perinatally. However, females had a significantly higher density of TUNEL-positive cells than did males. A role for the proapoptotic factors, Bax and Bak, in BC/LA development was tested by examining mice lacking one or both of these proteins. In females lacking either Bax or Bak, the BC was absent and the LA rudimentary. Deletion of both bax and bak genes, however, rescued the BC, increased LA size ∼20-fold relative to controls, and virtually eliminated TUNEL-positive cells in both muscles. We conclude that cell death plays an essential role in sexual differentiation of the BC/LA muscles. The presence of either Bax or Bak is sufficient for cell death in the BC/LA, whereas the absence of both prevents sexually dimorphic muscle cell death.
Keywords: bulbocavernosus, cell death, sex difference, bax, bak
Introduction
The striated perineal muscles and the motoneurons that innervate them have been the subjects of intense investigation, owing to their unusual androgen dependence (reviewed in Johansen et al., 2004; Sengelaub and Forger, 2008). The bulbocavernosus (BC) and levator ani (LA) muscles attach to the base of the phallus, and are active during erection and ejaculation (Sachs, 1982; Karacan et al., 1983; Wallach and Hart, 1983). They are innervated by motoneurons in the spinal nucleus of the bulbocavernosus (SNB; Breedlove and Arnold, 1980; Schroder, 1980). Although comparable numbers of SNB motoneurons and functional bulbocavernosus and LA target muscles are present in rats of both sexes prenatally, the persistence of this neuromuscular system requires exposure to androgens (Cihak et al., 1970; Breedlove and Arnold, 1983). As a result, adult females lack a BC and possess only a remnant, or no, LA. Thus, it has been suggested that the BC/LA muscles degenerate in females because of a lack of androgen stimulation (Cihak et al., 1970; Breedlove and Arnold, 1983).
If so, then the BC/LA muscle complex provides the only known example of naturally occurring muscle degeneration in a mammal. Moreover, the fate of the muscles controls the survival of SNB motoneurons (or homologous motoneurons in Onuf's nucleus), which are much more numerous in males than in females of many species (Breedlove and Arnold, 1980; Ueyama et al., 1985; Forger and Breedlove, 1986; Wee and Clemens, 1987; Ulibarri et al., 1995; Forger et al., 1996). The role of cell death in the sexual differentiation of the perineal muscles has been controversial, however, and has not been tested directly. Based on an examination of LA development in rats, Tobin and Joubert (1991) concluded that the sex difference in muscle size seen in adulthood results not from muscle cell death in females, but from a testosterone-dependent increase in the number of LA fibers in males during the first week of life. That is, the muscle does not die in females, it simply fails to grow beyond an embryonic state. Although degeneration of the BC has not been questioned, this is based simply on its absence in females, rather than an examination of cell death, per se.
In contrast to the enormous attention paid to developmental cell death in the nervous system, relatively little is known about cell death in developing muscles. Muscle degeneration has been documented during metamorphosis in moths and frogs (Schwartz et al., 1993; Nishikawa and Hayashi, 1995; Sachs et al., 1997). In the latter case, overexpression of Bax, a pro-death member of the Bcl-2 protein family, accelerates the process of degeneration and antisense to bax blocks regression (Sachs et al., 2004). Bcl-2 family proteins also are crucial regulators of cell death in mammals (Merry and Korsmeyer, 1997). We previously reported that deleting the bax gene rescued SNB motoneurons in female mice, but did not rescue the BC muscle and had only a modest effect on LA muscle fiber number (Jacob et al., 2005). One possible interpretation of this result is that cell death does not contribute importantly to the sexually dimorphic development of the perineal muscles. Alternatively, Bax may be functionally redundant with another member of the Bcl-2 family, Bak, in BC/LA muscles. Bax and Bak are the two full-length, prodeath members of the family capable of releasing cytochrome c from the mitochondrial membrane, and they play overlapping roles in many cells types (Lindsten et al., 2000; Wei et al., 2001). Both Bax and Bak are expressed in developing mouse skeletal muscle (Dominov et al., 2001).
Here we mapped the ontogeny of the sex difference in BC/LA muscle size in mice and assessed the rate of apoptosis in the perinatal BC/LA by terminal deoxynucleotidyl transferase-mediated dUTP nickend labeling (TUNEL). We also examined BC/LA morphology in bax or bak single knockouts, and bax/bak double knockout mice, to test the contribution of cell death to the sexual differentiation of these muscles and to identify specific genes involved.
Methods
Ontogeny of Sex Differences in BC and LA Muscle Volume
The development of sex differences in BC/LA muscle size was examined in wild-type C57BL/6 offspring of timed-pregnant dams. Breeding females were checked daily for copulatory plugs, and the day a plug was detected was designated E1. Most litters in our colonies are born on E20 (= P1). Five to seven mice of each sex were collected during the morning hours on embryonic days 16 (E16), E17, E18, and E19, and on postnatal days 1 (P1), P3, and P5. Animals were decapitated and immersion fixed in Bouin's solution. Perineums were dissected out, embedded in paraffin wax, sectioned at 7 μm, mounted on slides, and stained with Gomori's trichrome.
BC and LA muscles were traced in every seventh section using StereoInvestigator imaging software (MBF Bioscience, Williston, Vermont). Muscle areas were calculated, summed, and multiplied by the distance between traced sections (49 μm) to obtain the total muscle volume in each animal.
TUNEL-Labeling in Perineal Muscles
The lower torsos of embryonic and neonatal mice were fixed in 4% paraformaldehyde for at least 1 week; perineums and surrounding tissues were then dissected out, embedded in paraffin, sectioned at 8 μm, and mounted on slides. TUNEL labeling was performed using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA) according to the manufacturer's instructions. Briefly, slides were deparaffinized, pretreated with proteinase K (10 μg/mL), and incubated with 3% hydrogen peroxide to quench endogenous peroxidase. Following a short incubation in equilibration buffer, slides were incubated in terminal deoxynucleotidyl transferase (TdT) enzyme, rinsed, and incubated with anti-digoxigenin anti-body conjugated to a peroxidase reporter. Color was developed using the peroxidase substrate diaminobenzadine. Slides were then counterstained with 0.5% methyl green and coverslipped with Permount. Negative controls were processed in the absence of TdT.
For quantification, the BC and LA were traced in every third section, and TUNEL-positive cells were counted using the “meander scan” function of StereoInvestigator to systematically move through the muscles. The density of TUNEL-positive cells was calculated by dividing the number of labeled cells in each muscle by the total area of the traced muscle. We also quantified TUNEL cell density on E18 in two control muscles: the smooth muscle circling the rectum and a striated hamstring muscle. Portions of these muscles appeared in the same sections as the BC/LA, and were analyzed as described for the BC/LA.
BC/LA Muscle Development in the Absence of bax, bak, or Both bax and bak
BC/LA muscle volume was compared in bax−/− females and their wild-type bax +/+ siblings on P6. For this analysis, we established breeding pairs of mice heterozygous for the bax gene deletion (bax+/−) on a C57BL/6 background (The Jackson Labs, Bar Harbor, Maine). Offspring were genotyped by PCR amplification of tail DNA using a set of three primers as previously described (White et al., 1998).
The BC and LA were also examined in bax/bak DKO mice. Heterozygous bax (bax+/−) and bak (bak+/−) mutant mice were generated as described (Lindsten et al., 2000), and mated to obtain mice heterozygous for both genes (bax+/−bak+/−). To generate bax/bak DKO animals, breeding pairs of bax+/−/bak+/− mice were established, and offspring genotyped using PCR amplification of tail DNA (Lindsten et al., 2000). Sex was determined by anogenital distance and confirmed by the presence or absence of a vagina in sections through the perineum.
bax/bak DKO animals have a severely reduced survival rate and typically die during perinatal life (Lindsten et al., 2000). We examined four bax/bak DKO young adult females. For two of these animals, the muscles were sectioned as described for analyses of BC/LA volume, mentioned earlier. In the remaining two, the LA was cut in cross-section, enabling analyses of fiber number. LA fibers were counted in two sections from each animal and these counts were averaged to estimate total fiber number. bax/bak DKO females were compared to female littermates (when possible), or to more distant female relatives of the same age. Genotypes of controls included bak knockout (bax+/+bak−/−, N = 3) and bax+/−bak+/− (N = 1) mice. Wild-type (bax+/+/bak+/+) and bax knock-out (bax−/−bak+/+) females collected previously (Jacob et al., 2005) were also used as comparison controls.
Data Analyses
BC/LA muscle volumes and TUNEL cell density in wild-type animals were analyzed by two-way ANOVAs (sex-by-age). Significant main effects or interactions were followed by planned comparisons tests using Fisher's least significant difference. Paired t-tests were used to compare muscle volume and number of TUNEL-positive cells in wild-type and bax−/−neonates. Means are reported ± SEMs.
Results
BC/LA Muscle Development
The BC and LA had a similar appearance in males and females during prenatal development (apart from overall volume differences described below). The muscles were composed primarily of myocytes and early myotubes on E16 and E17 (see Fig. 1). By E19, many more myotubes with centralized nuclei were identified as well as some early myofibers in which nuclei were more peripheral. Some faint cross-striations were also observable by E19 in most animals. Postnatally, the appearance of the BC/LA muscles diverged in males and females. Individual fibers were striated in females but appeared less organized, with much of the “muscle” volume taken up by connective tissue or empty space (see Fig. 1). The proximal ends of the LA attached to the base of the penis in males, but had no obvious attachment site in females and appeared to float in space. BC and LA muscle fibers in perinatal mice were smaller in diameter than those of surrounding skeletal muscles, which appeared considerably more mature with clearer cross striations. This is in accord with observations in the rat suggesting that the perineal muscles develop late relative to other striated muscles (Cihak et al., 1970; Jordan et al., 1988).
Figure 1.
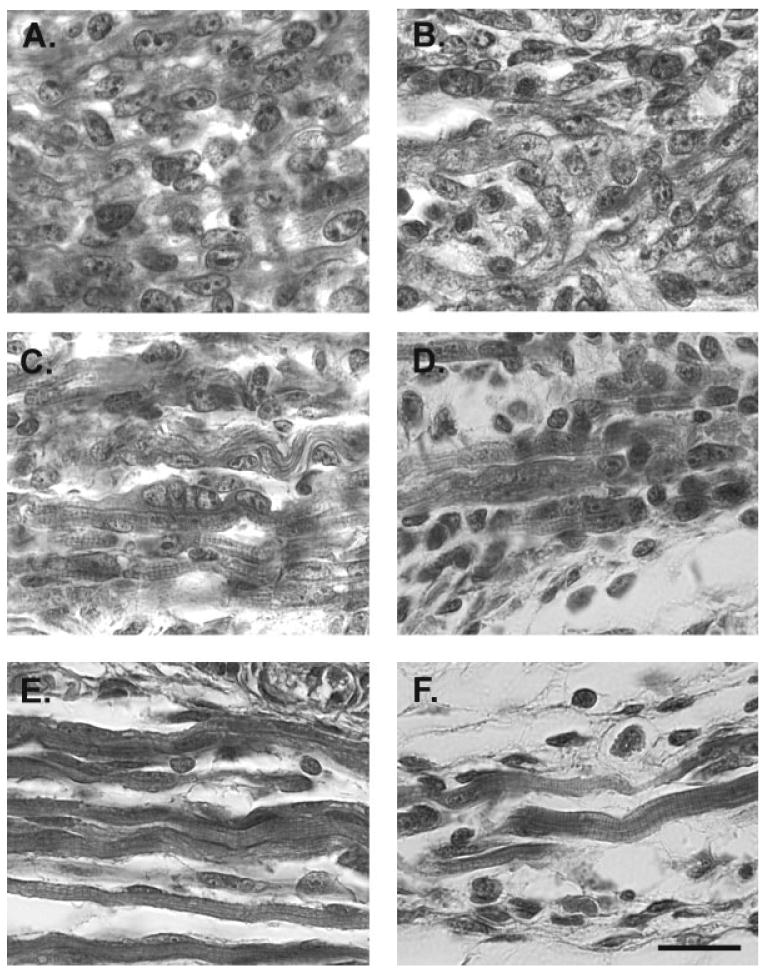
Photomicrographs of trichrome-stained LA muscles in male (left) and female (right) mice on E16 (A, B), P1 (C, D), and P5 (E, F). (A, B) At E16, the LA in both sexes is composed primarily of myocytes and a few early myotubes. (C, D) Myotubes with centralized nuclei are seen at P1. Striations can also be seen at this age. (E, F) By P5, LA muscle fibers in both sexes have a mature appearance with peripheral nuclei and clear striations. Fibers are sparse in females, however, and some appear to be truncated. Nonetheless, those fibers that are present in females are similar in size and appearance to those in males. Scale bar = 20 μm.
Quantitative analyses revealed no sex difference in BC muscle volume at E16 [Fig. 2(A)]. The BC grew markedly in males, quadrupling in size between E16 and E19. A significant sex difference was observed at every age examined from E17 through P5 [Fig. 2(A)]. BC muscle volume significantly increased in males between the ages of E16 and E17, E17 and E18, P1 and P3 (p < 0.05 for each comparison), and again between P3 and P5 (p < 0.005). The BC was found in all embryonic females. Small BC fragments were also found in females on the day of birth, but no BC was seen in any female on P3 or P5. ANOVA revealed main effects of age (p < 0.0005) and sex (p < 0.0005), as well as a significant sex-by-age interaction on BC volume (p < 0.0005).
Figure 2.
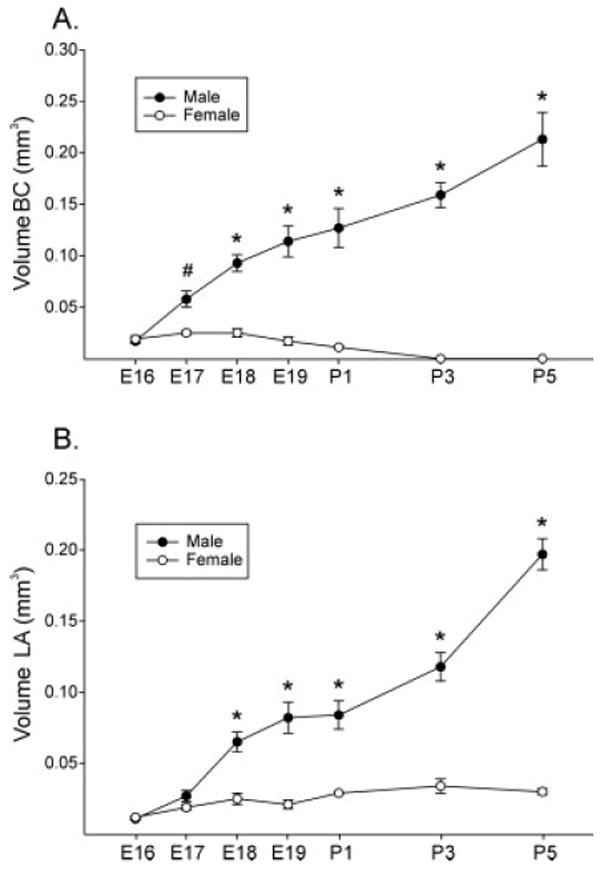
Mean volume (± SEM) of the BC and LA muscles in perinatal mice. Muscle volumes are similar in both sexes on E16. (A) A significant sex difference in BC size is first seen on E17, and the magnitude of the difference increases postnatally. No BC was found in any female on P3 or P5. (B) A sex difference in LA size emerges on E18 and increases at later ages. Females retained a small LA at all ages examined. #, p < 0.05; *, p < 0.0005.
The pattern of LA development was similar to that of the BC except that females retained a small LA at all ages examined. ANOVA revealed main effects of sex (p < 0.0005) and age (p < 0.0005), and a sex-by-age interaction (p < 0.0005) on LA volume. There was no sex difference in LA muscle volume on E16 or E17, but the LA was significantly larger in males than in females at each age from E18 to P5 [Fig. 2(B)]. LA muscle volume increased significantly in males between the ages of E16 and E18 (p < 0.0005) as well as between P1 and P3 (p < 0.001) and again between P3 and P5 (p < 0.0005). LA muscle volume did not significantly change in females during the time points examined (p > 0.3 for all comparisons).
These muscle volume data are consistent with the interpretation that the BC degenerates in females, but that the LA may simply fail to grow (Tobin and Joubert, 1991). To more directly assess the role of cell death, we quantified TUNEL labeling in the BC/LA and examined muscle development in mice with mutations in cell death genes.
Cell Death in the BC/LA Muscles
TUNEL-labeled cells were found in the BC and the LA of both sexes on E18 and P1 (Figs. 3 and 4). However, females had a higher density of TUNEL labeling than did males in the BC (p < 0.0005; Fig. 4), with no effect of age and no sex-by-age interaction. In the LA, we saw significant effects of both sex and age on TUNEL cell density. Females again had a higher number of dying cells per unit area of muscle than did males (p < 0.0005; Fig. 4). The density of TUNEL-positive cells also was higher on E18 than on P1 (p < 0.0005), due primarily to a decrease in TUNEL labeling in the female LA between E18 and P1 (p < 0.005).
Figure 3.
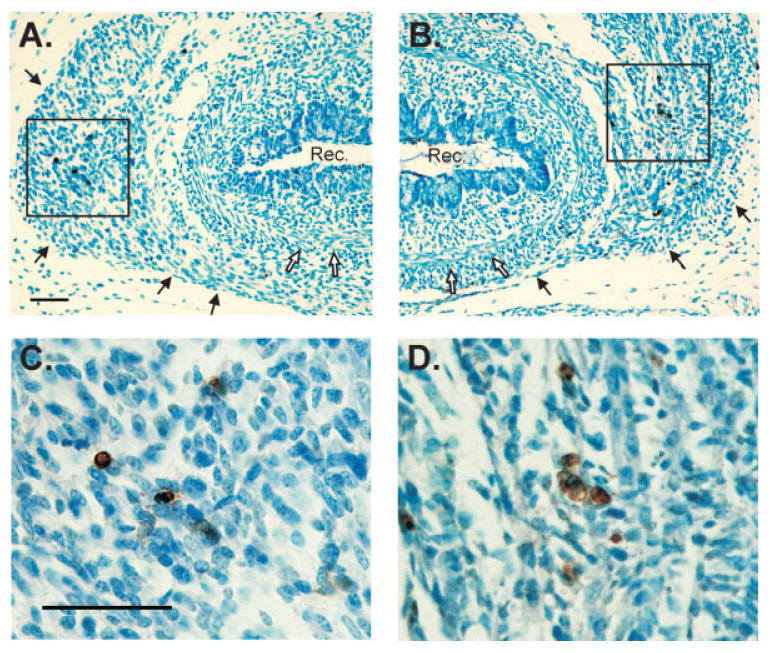
(A, B) Low magnification photomicrographs of TUNEL-labeled sections through the perineum of an E18 male (A) and E18 female (B). Sections were counterstained with methyl green. Black arrows indicate the LA muscle; white arrows indicate smooth muscle of the rectum. Dorsal is down. (C, D) Higher magnifications of boxed areas in (A) and (B), respectively. A higher density of TUNEL-positive cells (brown) is seen in the LA of females. Scale bars = 50 μm. Rec, rectum. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 4.
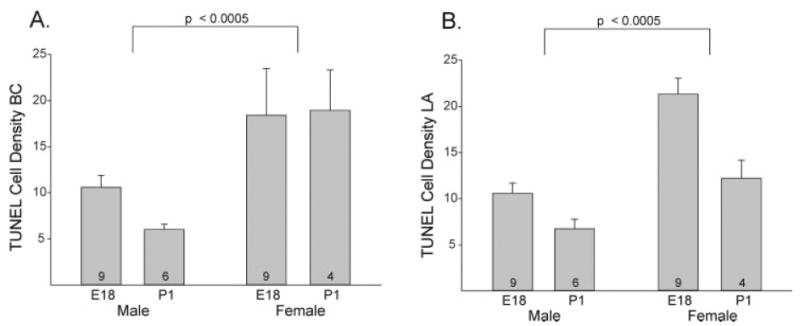
Mean (+SEM) number of TUNEL-labeled cells (per 105μm2) in the BC (A) and LA (B) muscles of perinatal mice. Females had a higher density of TUNEL labeling than males (p < 0.0005 in both cases). There was also a significant effect of age on the density of TUNEL-labeled cells in the LA, with a reduction in the rate of cell death between E18 and P1 (p < 0.0005). The number of animals per group is indicated at the base of each bar.
In contrast to observations in the BC and LA, the density of TUNEL labeling did not differ between males and females in the muscle circling the rectum (8.3 ± 0.7 and 8.9 ± 1.7 TUNEL-positive cells per 105μm2 for males and females, respectively; p > 0.7). Rates of cell death in the hamstring muscle were quite low and also did not differ by sex (2.5 ± 0.3 versus 3.4 ± 0.6 TUNEL-positive cells per 105μm2 for males and females, respectively; p > 0.2).
Muscle Development and Cell Death in Knockout Animals
We previously reported that bax deletion did not rescue the BC muscle and caused only a modest increase in LA fiber number in adult female mice (Jacob et al., 2005). Similarly, in the present study, no BC could be identified in any of the wild-type (N = 5) or bax−/− (N = 4) females examined perinatally. Although mean LA volume was nearly twice as large in bax−/− females compared to wild-type females on P6, this was not significant (p = 0.063; not shown). We also compared the rate of apoptosis in the BC and LA in bax−/− and wild-type newborns. The density of TUNEL-positive cells on P1 did not differ between wild-type and bax−/− females in the BC (18.9 ± 4.4 versus 14.4 ± 8.1 TUNEL-positive cells per 105μm2 for wild-type and bax−/− females, respectively; p > 0.6) or the LA (12.2 ± 2 versus 10.7 ± 2.1 TUNEL-positive cells per 105μm2 for wild-type and bax−/− females, respectively; p > 0.6). Thus, deletion of the bax gene alone did not significantly alter any of the parameters of BC/LA muscle development measured here.
To determine whether Bax may be redundant with the related protein, Bak, in the perineal muscles, we also examined the BC/LA in bak knockout (bak−/−) and bax/bak DKO females in adulthood. As was the case for wild-type and bax−/− mice, the BC could not be identified in bak−/− females. Similarly, although we did not perform a quantitative comparison, the LA of bak−/− animals was rudimentary, and resembled that in wild-type females. In contrast, the perineums of bax/bak DKO females exhibited several remarkable features. Externally, the perineal region had a bulging appearance, primarily around the rectum. Sections through the perineum suggest that this is due at least in part to the size of the LA. The LA of bax/bak DKO females was many times larger than that of females of any other genotype examined (see Fig. 5). The LA of bax/bak DKO females also lacked the midline raphe that normally forms prenatally to join the left and right muscle halves just dorsal to the rectum (Cihak et al., 1967). LA halves of bax/bak DKO female mice were joined by a connective tissue bridge, or by no visible connection at all, and a similar observation was made in one male bax/bak DKO examined (not shown). This suggests that cell death controls size of the LA and may also be required for fusion of the left and right muscle halves.
Figure 5.
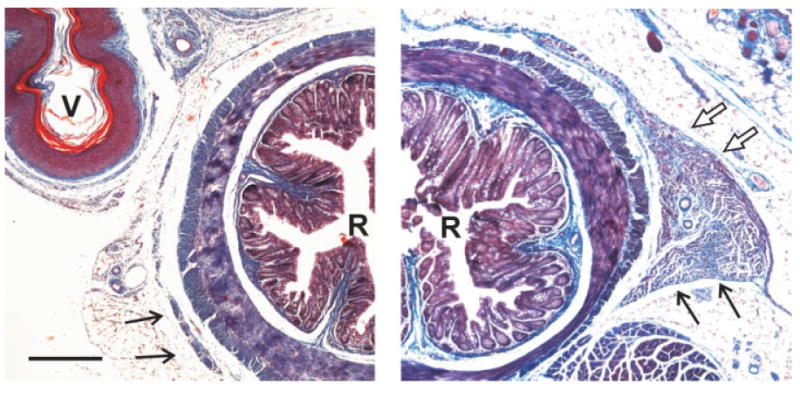
Low-power photomicrographs of trichrome-stained sections through the perineum of an adult wild-type female (left), and a bax/bak DKO female (right). Black arrows point to the LA muscle, which is much larger in bax/bak DKO females than in wild type. White arrows (right) indicate a portion of the BC muscle in the bax/bak DKO. At this level of the perineum, the vagina (V) was present in wild-type females, but absent in bax/bak DKO females (see Lindsten et al., 2000). R, rectum. Scale bar = 300 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In two bax/bak DKO females, the LA was cut in near-perfect cross section, which allowed us to estimate fiber number (see Fig. 6). We found 660 muscle fibers in one of these females and 928 in the other. Thus, bax/bak DKO adult females had an average of roughly 800 LA fibers, in comparison to ∼40 fibers in wild-type females and ∼130 in bax−/− females (Jacob et al., 2005).
Figure 6.
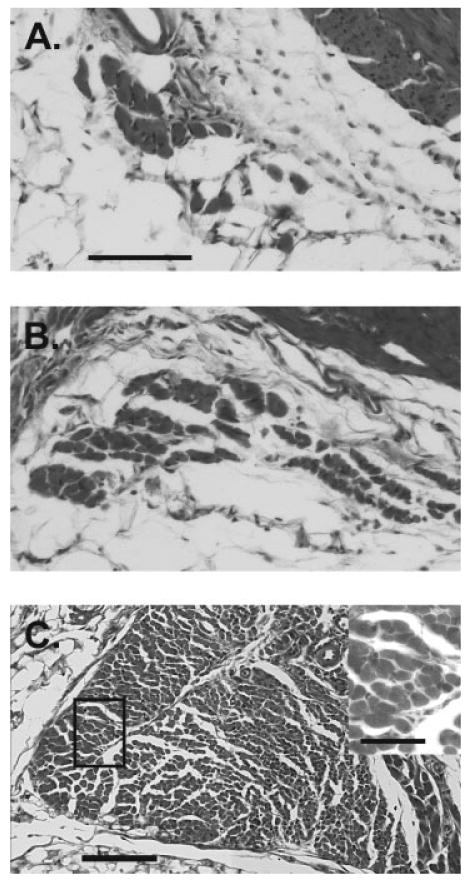
High-power photomicrographs of cross-sections through the LA of a wild-type (A), bax−/− (B), and bax/bak DKO (C) adult female. LA fiber number in bax/bak DKO females was increased markedly over that of wild-type or bax−/− females. Inset in (C) is a higher magnification view of boxed area. Scale bar in A = 100 μm for A and B; scale bars in C = 200 μm for main view and 50 μm for inset.
In contrast to all other genotypes examined, a BC muscle was also present in bax/bak DKO females. In males, the BC encircles the base of the penis and wraps around the ventral ends of the LA; attachment sites are exclusively to the penile bulb (Sachs, 1982). bax/bak DKO females do not have a penile bulb. Nonetheless, we identified the BC as a thick cluster of striated muscle fibers that join up with the LA as the LA runs lateral and ventral to the rectum (see Fig. 7). These fibers meet the LA at an oblique angle, as is seen for the portion of the BC that wraps around the LA in males. BC fibers of bax/bak DKO females also appeared to continue more ventrally, up the sides of the urethra [Fig. 7(A)]. A marked waviness in these fibers was apparent, perhaps because they lacked an attachment site; apart from this, the BC fibers of bax/bak DKO females appeared normal, with clear striations [Fig. 7(B)].
Figure 7.
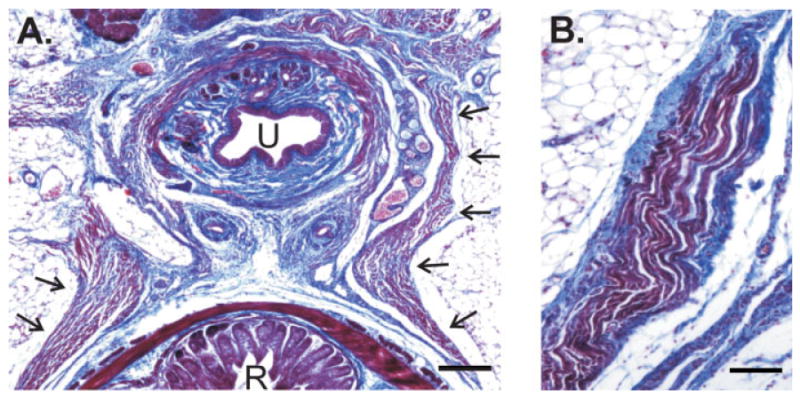
Photomicrographs of trichrome stained sections through the perineums of adult bax/bak DKO females. (A) The BC muscle (arrows) can be seen running along the side of the urethra and between the urethra and rectum. U, urethra; R, rectum. Scale bar = 100 μm. (B) Higher-power view of BC muscle fibers in a bax/bak DKO female. This view was taken at the level of the urethra. There is no obvious attachment site and fibers exhibit a marked waviness. Dorsal is down in both views. Scale bar = 50 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
These observations led us to predict that cell death would be markedly reduced in the perineal muscles of bax/bak DKO females during early development. We were able to test this prediction in a single bax/bak DKO female and her bax+/−/bak−/− female littermate, which were processed for TUNEL as described earlier. A large number of TUNEL-positive cells were seen in the BC/LA muscles of the bax+/−/bak−/− littermate control (see Fig. 8), suggesting that even a single copy of bax in the absence of bak is sufficient to mediate BC/LA muscle cell death. In contrast, we could not identify a single definitive TUNEL-positive cell in any section through the BC or LA of the bax/bak DKO. The muscles were also notably larger in the bax/bak DKO newborn than in the control.
Figure 8.
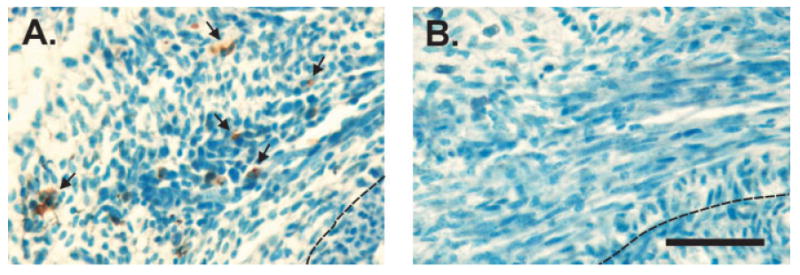
Photomicrographs of TUNEL-stained sections through the BC/LA of a bax+/−/bak−/− female (A) and a bax/bak DKO female (B) on the second day of life. Many TUNEL-positive cells are seen in the bax+/−/bak−/− female (arrows), whereas cell death is abrogated by bax/bak double gene deletion. Dashed line indicates the edge of the rectum. Scale bar = 20 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
We find that the BC and LA muscles form in embryonic mice of both sexes, and that muscle volumes do not differ between males and females on E16. A sex difference in both muscles emerges just before birth and increases in magnitude postnatally. TUNEL-positive cells are found in the BC and the LA of wild-type mice at E18 and P1, and the density of these cells is higher in females. In female mice with targeted deletions of two pro-apoptotic genes, bax and bak, a BC muscle is retained and the LA is many times larger than that of wild-type mice. In addition, TUNEL-positive cells are drastically reduced or eliminated in the BC/LA muscles of a bax/bak DKO. Taken together, we conclude that apoptotic cell death plays an essential role in the sexually dimorphic development of the BC and LA muscles.
In contrast to the remarkable effects of deleting both bax and bak, the elimination of either gene alone had little effect on the development of the BC/LA muscles in female mice. This differs from what is seen in the SNB motoneurons which innervate the BC/LA muscles: we observed the complete rescue of SNB motoneurons in bax−/− mice (Jacob et al., 2005). This disparity is likely related to the fact that developing neurons lack Bak, or express only a neuron-specific Bak variant with altered function (Sun et al., 2001; Uo et al., 2005). Thus, while Bax and Bak play overlapping roles in many cell types, including the BC/LA muscles, Bax alone is singularly important for developmental cell death in neurons, including SNB motoneurons.
The overall pattern of perineal muscle development seen here resembles that previously reported for rats. The BC and LA are present and innervated in rats of both sexes at E22 (i.e., 1 day before birth, Cihak et al., 1970; Rand and Breedlove, 1987). Signs of degeneration such as the appearance of vacuoles and irregularly spaced myofibrils (Cihak et al., 1970; Varela et al., 2000) led Cihak et al. to suggest that the LA “involutes” in females soon after birth. Some years later, however, Tobin and Joubert (1988) reported that a small LA persists in adult female rats. In addition, based on counts of muscle units (clusters of two or more myotubes enclosed by a common membrane) from E22 to P6, they concluded that the sex difference in LA muscle size seen in adults results not from degeneration of the LA in females, but rather from a lack of muscle fiber addition in females during development (Tobin and Joubert, 1991).
Our results support the interpretation that cell death contributes importantly to the sexual differentiation of the BC and LA, but do not rule out a role for other mechanisms. Despite the striking increase in size compared to control females, the perineal muscles of bax/bak DKO females are not completely masculinized. Based on simple visual inspection, muscle volume is reduced compared to that of males. In addition, although the number of LA fibers seen in female bax/bak DKOs (∼800) far exceeds that of control females, or of females lacking only bax or bak, it nonetheless falls short of LA fiber number in males (∼2000, as reported in Jacob et al., 2005). The BC and LA muscles are sensitive to circulating androgens in adulthood (Wainman and Shipounoff, 1941; Venable, 1966), and we would expect perineal muscle volume to significantly increase in testosterone-treated female bax/bak DKOs. Nonetheless, circulating androgen levels are unlikely to account for the difference in fiber number between males and bax/bak DKO females, because androgen treatments after the perinatal period increase LA muscle fiber size without a concomitant increase in fiber number (Venable, 1966; Tobin and Joubert, 1991).
It is possible that cell death proteins other than Bax and Bak are involved in the sexual differentiation of the BC/LA muscles and that, in females with deletions of all relevant cell death genes, muscle fiber numbers would match those of males. However, the virtual elimination of TUNEL-labeled cells in the perineal muscles of a newborn bax/bak DKO female, as seen here, suggests that there is little to no death independent of Bax and Bak. We favor the alternative suggestion that, in addition to cell death, androgen-dependent myogenesis occurs in the BC and LA during perinatal life. In male rats, LA muscle unit number increases sharply during the first postnatal week (Tobin and Joubert, 1991). The marked increase in muscle volume seen here in male mice between P1 and P5 is also consistent with this suggestion.
Interestingly, despite the fact that the neonatal period is a time of rapid perineal muscle growth in males, dying cells are evident in both the LA and BC. In fact, although the density of TUNEL-labeled cells in the BC and LA is greater in females, the absolute number is greater in males, because of much larger muscle sizes in males. This suggests that a baseline rate of cell turnover is a normal part of BC/LA muscle development, compatible with muscle growth. Some cell death is also seen during normal development in other mammalian striated muscles. TUNEL-positive or pyknotic nuclei are present during the first postnatal week in hindlimb and scalp muscles of rats (Trachtenberg, 1998; de Torres et al., 2002). Denervation increases the number of these dying cells (Trachtenberg, 1998), and leads to a significant decrease in muscle fiber number (Soileau et al., 1987). Similarly, the elevated rate of cell death in the female BC/LA likely contributes to the sex difference in muscle fiber number. We also note that the density of dying cells in the LA decreased significantly between E18 and P1, while remaining elevated in the BC at both ages. This could help to explain why the BC completely degenerates, but a small LA persists, in females.
It is also possible that the cell types undergoing apoptosis may differ between males and females. Striated muscle tissue is composed of multiple cell types, including muscle fibers, fibroblasts, satellite cells, and terminal Schwann cells, and the identity of individual dying cells cannot be determined by the techniques used here. We presume that some of the TUNEL-positive cells in females are BC muscle fibers (or myocytes and myotubes at earlier stages in development), because BC fibers are present prenatally, but absent after P1. Similarly, although volume of the LA did not significantly change in females between E16 and P1, fibers likely die in this muscle as well, because volume measurements at later ages included much more empty space and connective tissue (and, hence, presumably fewer fibers) than was seen prenatally.
Some of the TUNEL-positive cells in the BC and LA may also be satellite cells, which can divide and fuse to existing muscle fibers to form new fibers (Schultz and McCormick, 1994). If so, then cell death and myogenesis might interact. Testosterone treatment of castrated adult male and intact female rats causes proliferation of satellite cells in the LA muscle (Joubert and Tobin, 1989; Joubert et al., 1994). Thus, it is possible that perinatal testosterone programs differential muscle growth in males and females by controlling the survival of satellite cells. A recent study of satellite cells in the LA of developing rats reports a greater absolute number of satellite cells in males, although satellite cell density did not vary by sex (Niel et al., 2007).
The BC may be the only example of a mammalian striated muscle that forms and then completely degenerates in one sex as a normal part of development. This death is Bak and Bak dependent (current report) and is normally controlled by androgens (Breedlove and Arnold, 1983). This suggests that androgens may regulate the expression of Bak or Bax, or of proteins that interact with Bak or Bax, in the perineal muscles. Striated muscle fiber loss also occurs in several neuromuscular diseases (Miller and Girgenrath, 2006). Evidence from mutant mice indicates that muscle may be the primary site of action for the toxic effects of the SOD1 mutation implicated in the familial form of amyotrophic lateral sclerosis (Pramatarova et al., 2001; Lino et al., 2002; Dobrowolny et al., 2005). In addition, mouse models of congenital muscular dystrophy type 1A exhibit severe skeletal muscle loss (Miyagoe et al., 1997), and either the muscle-specific overexpression of bcl-2 or the targeted deletion of bax improve muscle pathology (Girgenrath et al., 2004; Dominov et al., 2005). Study of the mechanisms controlling the sexually dimorphic development of the BC/LA muscles may therefore be of relevance for understanding cell death in neuromuscular disease.
Acknowledgments
Contract grant sponsor: NIH; contract grant numbers: MH07285, MH068482.
Contract grant sponsor: NCI.
References
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihak R, Gutmann E, Hanzilikova V. Morphological, physiological characteristics, development and homology of the m. levator ani of the rat. Anat Anz. 1967;120:492–506. [PubMed] [Google Scholar]
- Cihak R, Gutmann E, Hanzlikova V. Involution and hormone-induced persistence of the M. sphincter (levator) ani in female rats. J Anat. 1970;106:93–110. [PMC free article] [PubMed] [Google Scholar]
- de Torres C, Munell F, Roig M, Reventos J, Macaya A. Naturally occurring cell death during postnatal development of rat skeletal muscle. Muscle Nerve. 2002;26:777–783. doi: 10.1002/mus.10268. [DOI] [PubMed] [Google Scholar]
- Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, Molinaro M, et al. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominov JA, Houlihan-Kawamoto CA, Swap CJ, Miller JB. Pro- and anti-apoptotic members of the Bcl-2 family in skeletal muscle: A distinct role for Bcl-2 in later stages of myogenesis. Dev Dyn. 2001;220:18–26. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1088>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dominov JA, Kravetz AJ, Ardelt M, Kostek CA, Beermann ML, Miller JB. Muscle-specific BCL2 expression ameliorates muscle disease in laminin α2-deficient, but not in dystrophin-deficient, mice. Hum Mol Genet. 2005;14:1029–1040. doi: 10.1093/hmg/ddi095. [DOI] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Sexual dimorphism in human and canine spinal cord: role of early androgen. Proc Natl Acad Sci USA. 1986;83:7527–7531. doi: 10.1073/pnas.83.19.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Frank LG, Breedlove SM, Glickman SE. Sexual dimorphism of perineal muscles and motoneurons in spotted hyenas. J Comp Neurol. 1996;375:333–343. doi: 10.1002/(SICI)1096-9861(19961111)375:2<333::AID-CNE11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Girgenrath M, Dominov JA, Kostek CA, Miller JB. Inhibition of apoptosis improves outcome in a model of congenital muscular dystrophy. J Clin Invest. 2004;114:1635–1639. doi: 10.1172/JCI22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob DA, Bengston CL, Forger NG. Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J Neurosci. 2005;25:5638–5644. doi: 10.1523/JNEUROSCI.1200-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JA, Jordan CL, Breedlove SM. Steroid hormone masculinization of neural structure in rats: A tale of two nuclei. Physiol Behav. 2004;83:271–277. doi: 10.1016/j.physbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP. Synapse elimination occurs late in the hormone-sensitive levator ani muscle of the rat. J Neurobiol. 1988;19:335–356. doi: 10.1002/neu.480190403. [DOI] [PubMed] [Google Scholar]
- Joubert Y, Tobin C. Satellite cell proliferation and increase in the number of myonuclei induced by testosterone in the levator ani muscle of the adult female rat. Dev Biol. 1989;131:550–557. doi: 10.1016/s0012-1606(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Joubert Y, Tobin C, Lebart MC. Testosterone-induced masculinization of the rat levator ani muscle during puberty. Dev Biol. 1994;162:104–110. doi: 10.1006/dbio.1994.1070. [DOI] [PubMed] [Google Scholar]
- Karacan I, Aslan C, Hirshkowitz M. Erectile mechanisms in man. Science. 1983;220:1080–1082. doi: 10.1126/science.6844930. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino MM, Schneider C, Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 2002;22:4825–4832. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- Miller JB, Girgenrath M. The role of apoptosis in neuromuscular diseases and prospects for anti-apoptosis therapy. Trends Mol Med. 2006;12:279–286. doi: 10.1016/j.molmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, et al. Laminin α2 chain-null mutant mice by targeted disruption of the Lama2 gene: A new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- Niel L, Willemsen KR, Volante SN, Monks DA. Sexual dimorphism and androgen regulation of satellite cell population in differentiating rat levator ani muscle. Dev Neurobiol. 2007;68:115–122. doi: 10.1002/dneu.20580. [DOI] [PubMed] [Google Scholar]
- Nishikawa A, Hayashi H. Spatial, temporal and hormonal regulation of programmed muscle cell death during metamorphosis of the frog Xenopus laevis. Differentiation. 1995;59:207–214. doi: 10.1046/j.1432-0436.1995.5940207.x. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Ontogeny of functional innervation of bulbocavernosus muscles in male and female rats. Brain Res. 1987;430:150–152. doi: 10.1016/0165-3806(87)90186-6. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Abdallah B, Hassan A, Levi G, de Luze A, Reed JC, Demeneix BA. Apoptosis in Xenopus tadpole tail muscles involves Bax-dependent pathways. FASEB J. 1997;11:801–807. doi: 10.1096/fasebj.11.10.9271365. [DOI] [PubMed] [Google Scholar]
- Sachs LM, Le Mevel S, Demeneix BA. Implication of bax in Xenopus laevis tail regression at metamorphosis. Dev Dyn. 2004;231:671–682. doi: 10.1002/dvdy.20166. [DOI] [PubMed] [Google Scholar]
- Schroder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Jones MEE, Kosz L, Kuah H. Selective repression of actin and myosin heavy chain expression during the programmed cell death of insect skeletal muscle. Dev Biol. 1993;158:448–455. doi: 10.1006/dbio.1993.1202. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Forger NG. The spinal nucleus of the bulbocavernosus: Firsts in androgen-dependent neural sex differences. Horm Behav. 53:596–612. doi: 10.1016/j.yhbeh.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soileau LC, Silberstein L, Blau HM, Thompson WJ. Reinnervation of muscle fiber types in the newborn rat soleus. J Neurosci. 1987;7:4176–4194. doi: 10.1523/JNEUROSCI.07-12-04176.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YF, Yu LY, Saarma M, Timmusk T, Arumae U. Neuron-specific Bcl-2 homology 3 domain-only splice variant of Bak is anti-apoptotic in neurons, but pro-apoptotic in non-neuronal cells. J Biol Chem. 2001;276:16240–16247. doi: 10.1074/jbc.M010419200. [DOI] [PubMed] [Google Scholar]
- Tobin C, Joubert Y. The levator ani of the female rat: A suitable model for studying the effects of testosterone on the development of mammalian muscles. Biol Struct Morphog. 1988;1:28–33. [PubMed] [Google Scholar]
- Tobin C, Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev Biol. 1991;146:131–138. doi: 10.1016/0012-1606(91)90453-a. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT. Fiber apoptosis in developing rat muscles is regulated by activity, neuregulin. Dev Biol. 1998;196:193–203. doi: 10.1006/dbio.1998.8871. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Mizuno N, Takahashi O, Nomura S, Arakawa H, Matsushima R. Central distribution of efferent and afferent components of the pudendal nerve in macaque monkeys. J Comp Neurol. 1985;232:548–556. doi: 10.1002/cne.902320411. [DOI] [PubMed] [Google Scholar]
- Ulibarri C, Popper P, Micevych PE. Motoneurons dorsolateral to the central canal innervate perineal muscles in the Mongolian gerbil. J Comp Neurol. 1995;356:225–237. doi: 10.1002/cne.903560207. [DOI] [PubMed] [Google Scholar]
- Uo T, Kinoshita Y, Morrison RS. Neurons exclusively express N-Bak, a BH3 domain-only Bak isoform that promotes neuronal apoptosis. J Biol Chem. 2005;280:9065–9073. doi: 10.1074/jbc.M413030200. [DOI] [PubMed] [Google Scholar]
- Varela CR, Bengston L, Xu J, MacLennan AJ, Forger NG. Additive effects of ciliary neurotrophic factor and testosterone on motoneuron survival; differential effects on motoneuron size and muscle morphology. Exp Neurol. 2000;165:384–393. doi: 10.1006/exnr.2000.7475. [DOI] [PubMed] [Google Scholar]
- Venable JH. Constant cell populations in normal, testosterone-deprived and testosterone-stimulated levator ani muscles. Am J Anat. 1966;119:263–270. doi: 10.1002/aja.1001190205. [DOI] [PubMed] [Google Scholar]
- Wainman P, Shipounoff GC. The effects of castration and testosterone propionate on the striated perineal musclulature in the rat. Endocrinology. 1941;29:975–978. [Google Scholar]
- Wallach SJ, Hart BL. The role of the striated penile muscles of the rat in seminal plug dislodgement and deposition. Physiol Behav. 1983;31:815–821. doi: 10.1016/0031-9384(83)90278-0. [DOI] [PubMed] [Google Scholar]
- Wee BE, Clemens LG. Characteristics of the spinal nucleus of the bulbocavernosus are influenced by genotype in the house mouse. Brain Res. 1987;424:305–310. doi: 10.1016/0006-8993(87)91475-2. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


